Photo-Induced Vertical Alignment of Liquid Crystals via In Situ Polymerization Initiated by Polyimide Containing Benzophenone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(Amic Acid)
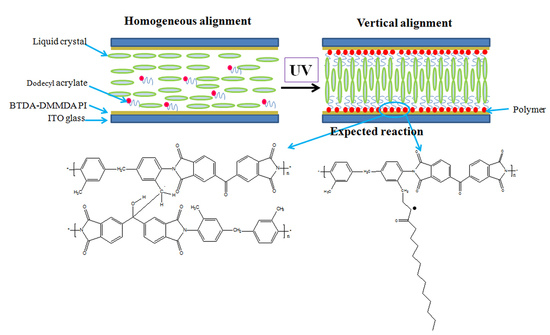
2.3. Preparation of Liquid Crystal Cells
2.4. Characterization
3. Results and Discussion
3.1. Analysis of Chemical Structures of Polyimide
3.2. Alignment Behavior of Liquid Crystals
3.3. Thermal Stability of Alignment
3.4. Surface Morphology of Alignment Layers
3.5. Contact Angles of Polyimide Alignment Layers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, S.G.; Kim, S.M.; Kim, Y.S.; Lee, H.K.; Lee, S.H.; Lee, G.D.; Lyu, J.J.; Kim, K.H. Stabilization of the liquid crystal director in the patterned vertical alignment mode through formation of pretilt angle by reactive mesogen. Appl. Phys. Lett. 2007, 90, 261910. [Google Scholar] [CrossRef]
- Yoshida, H. Vertically aligned nematic (VAN) LCD technology. Handb. Vis. Disp. Technol. 2014, 1–4, 1485–1505. [Google Scholar]
- Yao, J.; Brenizer, J.; Hui, R.; Yin, S. Photonic fiber and crystal devices: Advances in materials and innovations in device applications VI. Proceeding of the SPIE, San Diego, CA, USA, 12–13 August 2012. [Google Scholar]
- Lee, S.W.; Kim, S.I.; Park, Y.H.; Ree, M.; Rim, Y.N.; Yoon, H.J.; Kim, H.C.; Kim, Y.B. Liquid-crystal alignment on the rubbed film surface of semi-flexible copolyimides containing n-alkyl side groups. Mol. Cryst. Liq. Cryst. 2000, 349, 279–282. [Google Scholar] [CrossRef]
- Seo, D.S.; Muroi, K.I.; Kobayashi, S. Generation of pretilt angles in nematic liquid-crystal, 5CB, media aligned on polyimide films prepared by spin-coating and LB techniques: Effect of rubbing. Mol. Cryst. Liq. Cryst. 1992, 213, 223–228. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, J.G.; Song, I.K.; Oh, J.M.; Yi, M.H. Effect of side chain structure of polyimides on a pretilt angle of liquid crystal cells. Polymer 2006, 47, 1555–1562. [Google Scholar] [CrossRef]
- Liu, Z.J.; Yu, F.F.; Zhang, Q.; Zeng, Y.; Wang, Y.G. Preparation and characterization of a novel polyimide liquid crystal vertical alignment layer. Eur. Polym. J. 2008, 44, 2718–2727. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zeng, Y.; Fang, Y.Q.; Zhang, Q.; Wang, Y.H. A study of the transition of liquid-crystal alignment from homeotropic to planar on a polyimide layer. Liq. Cryst. 2010, 37, 271–278. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, X.G.; Gong, S.M.; Liu, L.L.; Sun, Z.; Shao, L.S.; Wang, Y.H. Effect of the functional diamine structure on the properties of a polyimide liquid crystal alignment film. RSC Adv. 2015, 5, 25348–25356. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.K.; In, S.; Yoon, A.R.; Yu, C.J.; Kim, J.H. Liquid Crystal Alignment Control Using Reactive Mesogen Mixed with Alignment Layers; IDW: San Diego, CA, USA, 2009. [Google Scholar]
- Hsu, C.J.; Chen, B.L.; Huang, C.Y. Controlling liquid crystal pretilt angle with photocurable prepolymer and vertically aligned substrate. Opt. Express 2016, 24, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Mizusaki, M.; Nakamura, K. Morphology analysis for effect of the polymer cluster produced from adamantyl-bearing trifunctional monomer on the stability of liquid crystal alignment. Chem. Lett. 2014, 43, 119–121. [Google Scholar] [CrossRef]
- Iimura, Y.; Akiyama, H.; Li, X.T.; Kobayashi, S. Photoalignment control of LC and its applications to LCD fabrication. In Proceedings of the SPIE, San Jose, CA, USA, 1 April 1998. [Google Scholar]
- Usami, K.; Sakamoto, K.; Yokota, J.; Uehara, Y.; Ushioda, S. Polyimide photo-alignment layers for inclined homeotropic alignment of liquid crystal molecules. Thin Solid Films 2008, 516, 2652–2655. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Huang, W.; Cao, H.; Zheng, Y.D.; Wang, G.J.; Yang, Z.; Yang, H. Homeotropic alignment of nematic liquid crystals by a photocross-linkable organic monomer containing dual photofunctional groups. J. Phys. Chem. B 2009, 113, 2961–2965. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Lee, M.H.; Lee, S.H.; Kang, S.W. In situ homeotropic alignment of nematic liquid crystals based on photoisomerization of azo-dye, physical adsorption of aggregates, and consequent topographical modification. Adv. Mater. 2013, 25, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.M.; Xiang, J.; Nemati, H.; Bisoyi, H.K.; Gutierrez-Cuevas, K.; Wang, L.; Gao, M.; Zhou, S.; Yang, D.K.; Lavrentovich, O.D.; et al. Light-driven reversible alignment switching of liquid crystals enabled by azo thiol grafted gold nanoparticles. Chemphyschem 2015, 16, 1852–1856. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, K.; Kim, H.S.; Wu, S.T. Novel surface-stabilized vertical alignment mode for fast-response liquid crystal display. J. Disp. Technol. 2012, 8, 296–298. [Google Scholar] [CrossRef]
- Lu, L.; Sergan, V.; Bos, P.J. Mechanism of electric-field-induced segregation of additives in a liquid-crystal host. Phys. Rev. E 2012, 86, 051706. [Google Scholar] [CrossRef] [PubMed]
- Goetz, A.; Taugerbeck, A.; Bernatz, G.; Tarumi, K. 48.1: Advanced liquid-crystal materials for the polymer-sustained vertically aligned (PS-VA) mode. SID Symp. Dig. Tech. Pap. 2010, 41, 718–720. [Google Scholar] [CrossRef]
- Lyu, J.J.; Kikuchi, H.; Kim, D.H.; Lee, J.H.; Kim, K.H.; Higuchi, H.; Lee, S.H. Phase separation of monomer in liquid crystal mixtures and surface morphology in polymer-stabilized vertical alignment liquid crystal displays. J. Phys. D 2011, 44, 325104. [Google Scholar] [CrossRef]
- Liu, B.Y.; Chen, L.J. Role of surface hydrophobicity in pretilt angle control of polymer-stabilized liquid crystal alignment systems. J. Phys. Chem. C 2013, 117, 13474–13478. [Google Scholar] [CrossRef]
- Mizusaki, M.; Nakanishi, Y. Improvement of image sticking on liquid crystal displays with polymer layers produced from mixed monomers. Liq. Cryst. 2016, 43, 704–710. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Okamoto, K. Relationship between concentration of initiator and image-sticking phenomenon of polymer-sustained-alignment liquid crystal displays. Jpn. J. Appl. Phys. 2012, 51, 041701. [Google Scholar] [CrossRef]
- Mizusaki, M.; Nakanishi, Y.; Enomoto, S.; Hara, Y. Evaluation of image sticking property on liquid crystal displays with polymer layers produced from phenanthrene-carrying monomers. Liq. Cryst. 2016, 43, 1208–1214. [Google Scholar] [CrossRef]
- Kang, H.; Lee, J.H.; Kim, D.G.; Kang, D. Control of pretilt angle in liquid crystal and photocurable monomer system. Mol. Cryst. Liq. Cryst. 2015, 607, 94–103. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wei, J.; Jiang, X.S.; Yin, J. Highly efficient sulfur-containing polymeric photoinitiators bearing side-chain benzophenone and coinitiator amine for photopolymerization. J. Photochem. Photobiol. A 2007, 186, 106–114. [Google Scholar] [CrossRef]
- Ma, H.M.; Davis, R.H.; Bowman, C.N. A novel sequential photoinduced living graft polymerization. Macromolecules 2000, 33, 331–335. [Google Scholar] [CrossRef]
- Stachowiak, T.B.; Svec, F.; Frechet, J.M.J. Patternable protein resistant surfaces for multifunctional microfluidic devices via surface hydrophilization of porous polymer monoliths using photografting. Chem. Mater. 2006, 18, 5950–5957. [Google Scholar] [CrossRef]
- Tao, Y.U.; Peng, Z.H.; Rina, W.U.; Ping, R.S.; Zhang, L.; Xuan, L. Photo-alignment of nematic liquid crystal on photo-sensitive polyimide (btda-tmmda). Chin. J. Liq. Cryst. Disp. 2003, 18, 93–96. [Google Scholar]
- Pryde, C.A. IR studies of polyimides. I. Effects of chemical and physical changes during cure. J. Polym. Sci. Polym. Chem. 1989, 27, 711–724. [Google Scholar] [CrossRef]
- Yuan, H.J.; Zhang, S.C.; Lu, C.X.; He, S.Q.; An, F. Improved interfacial adhesion in carbon fiber/polyether sulfone composites through an organic solvent-free polyamic acid sizing. Appl. Surf. Sci. 2013, 279, 279–284. [Google Scholar] [CrossRef]
- Murakami, K.; Ando, S. Effects of UV crosslinking under high temperature on the refractive indices and aggregation structures of benzophenone-containing polyimides. J. Photopolym. Sci. Technol. 2011, 24, 277–282. [Google Scholar] [CrossRef]
- Kang, H.; Lee, J.M.; Kim, J.H.; Lee, J.H.; Park, J.S.; Seo, J.G.; Kang, D. Homeotropic alignment properties of liquid crystal and photocurable monomer system via UV irradiation. Mol. Cryst. Liq. Cryst. 2015, 606, 101–110. [Google Scholar] [CrossRef]








| Samples | DA-0 | DA-0.5 | DA-1 | DA-2 |
|---|---|---|---|---|
| PI | BTDA-DMMDA PI | |||
| Weight ratios of DA (wt %) | 0.0 | 0.5 | 1.0 | 2.0 |
| S1720/S1505 a | 1.48 | 2.83 | 2.88 | 3.17 |
| S1670/S1505 | 2.69 | 1.99 | 1.68 | 1.13 |
| Contrast ratios | ND b | 412:1 | 423:1 | 380:1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Shao, L.; Bai, Q.; Che, X.; Liu, B.; Wang, Y. Photo-Induced Vertical Alignment of Liquid Crystals via In Situ Polymerization Initiated by Polyimide Containing Benzophenone. Polymers 2017, 9, 233. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9060233
Wang F, Shao L, Bai Q, Che X, Liu B, Wang Y. Photo-Induced Vertical Alignment of Liquid Crystals via In Situ Polymerization Initiated by Polyimide Containing Benzophenone. Polymers. 2017; 9(6):233. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9060233
Chicago/Turabian StyleWang, Fei, Leishan Shao, Qiyao Bai, Xinyuan Che, Bin Liu, and Yinghan Wang. 2017. "Photo-Induced Vertical Alignment of Liquid Crystals via In Situ Polymerization Initiated by Polyimide Containing Benzophenone" Polymers 9, no. 6: 233. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9060233