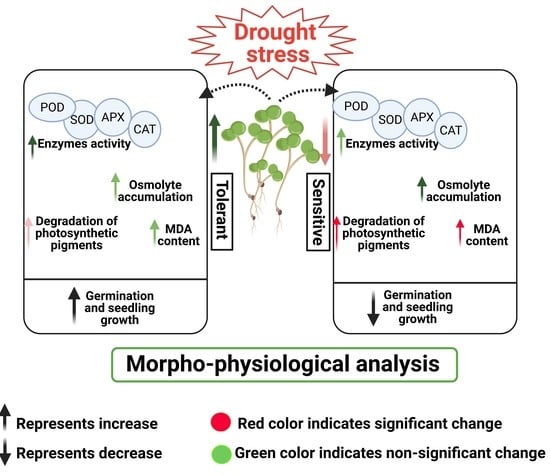
Rapeseed Morpho-Physio-Biochemical Responses to Drought Stress Induced by PEG-6000
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Materials and Growth Conditions
2.2. Germination Trails
2.3. Assessment of Morphological Traits
2.4. Determination of Photosynthetic Pigments
2.5. Determination of Relative Water Content (RWC)
2.6. Determination of Total Soluble Sugar, Total Soluble Protein, Proline, and MDA Contents
2.7. Measurement of Antioxidant Enzyme Activities
2.8. Microstructural Analysis
2.9. Statistical Analysis
3. Results
3.1. Variation in Seed Germination Traits under Drought Stress
3.2. Variation in Seedling Growth Traits under Drought Stress
3.3. Correlations of Traits under Control and PEG-6000 Stress
3.4. Variation in Growth-Related Traits of Rapeseed Seedlings
3.5. Variations in Photosynthetic Pigments under Drought Stress
3.6. Variation of Osmo-Protectants, MDA, Proline and RWC Contents in Rapeseed Seedlings
3.7. Activities of Enzymatic Antioxidants under PEG-6000 Induced Drought Stress
3.8. Microstructural Variation in Rapeseed Seedlings under Drought Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, L.; Shah, T.; Cheng, Y.; LÜ, Y.; Zhang, X.K.; Zou, X.L. Physiological and molecular responses to cold stress in rapeseed (Brassica napus L.). J. Integr. Agric. 2019, 18, 2742–2752. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.L.L.; Whalen, J.K. Enhancing Rapeseed Tolerance to Heat and Drought Stresses in a Changing Climate: Perspectives for Stress Adaptation from Root System Architecture. Adv. Agron. 2018, 151, 87–157. [Google Scholar]
- Channaoui, S.; Kahkahi, R.E.; Charafi, J.; Mazouz, H.; Fechtali, M.E.; Nabloussi, A. Germination and Seedling Growth of a Set of Rapeseed (Brassica napus) Varieties under Drought Stress Conditions. Int. J. Environ. Agric. Biotechnol. 2017, 2, 487–494. [Google Scholar] [CrossRef]
- Zhu, M.; Monroe, J.G.; Suhail, Y.; Villiers, F.; Mullen, J.; Pater, D.; Hauser, F.; Jeon, B.W.; Bader, J.S.; Kwak, J.M.; et al. Molecular and systems approaches towards drought-tolerant canola crops. New Phytol. 2016, 210, 1169–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandzi, L.N.; Bradley, G.; Mutengwa, C. Morphological Responses of Maize to Drought, Heat and Combined Stresses at Seedling Stage. J. Biol. Sci. 2018, 19, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Song, S.; Wang, W.; Wang, C.; Li, H.; Wang, F.; Li, S.; Sun, X. Screening diverse soybean genotypes for drought tolerance by membership function value based on multiple traits and drought-tolerant coefficient of yield. BMC Plant Biol. 2020, 20, 321. [Google Scholar] [CrossRef]
- Dani, A.R.H.; Siswoyo, T.A. Impact of Drought Stress during Germination on Antioxidant Capacities and Antioxidant Enzymes Activities of Madura Local Maize (Zea mays) Seeds. Agric. Sci. 2019, 10, 1506–1516. [Google Scholar] [CrossRef] [Green Version]
- Razaji, A.; Farzanian, M.; Sayfzadeh, S. The effects of seed priming by ascorbic acid on some morphological and biochemical aspects of rapeseed (Brassica napus L.) under drought stress condition. Int. J. Biosci. 2014, 4, 432–442. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Khan, M.I.R.; Fujita, M. Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. S. Afr. J. Bot. 2018, 115, 50–57. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; AA Mohamed, I.; Wang, Z.; Khatab, A.; Sherif, A.; Ahmad, H.; Khan, M.N.; Hassan, H.M.; Elrewainy, I.M. Antioxidative and Metabolic Contribution to Salinity Stress Responses in Two Rapeseed Cultivars during the Early Seedling Stage. Antioxidants 2021, 10, 1227. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Ni, F.; Rizwan, M.; Fahad, S.; Hu, L. Morpho-physiological and biochemical responses of tolerant and sensitive rapeseed cultivars to drought stress during early seedling growth stage. Acta Physiol. Plant. 2019, 41, 25. [Google Scholar] [CrossRef]
- Basal, O.; Szabó, A.; Veres, S. PEG-induced drought stress effects on soybean germination parameters. J. Plant Nutr. 2020, 43, 1768–1779. [Google Scholar] [CrossRef]
- Kaydan, D.; Yagmur, M. Germination, seedling growth and relative water content of shoot in different seed sizes of triticale under osmotic stress of water and NaCI. Afr. J. Biotechnol. 2008, 7, 2862–2868. [Google Scholar] [CrossRef]
- Abdel-Ghani, A.H.; Neumann, K.; Wabila, C.; Sharma, R.; Dhanagond, S.; Owais, S.J.; Börner, A.; Graner, A.; Kilian, B. Diversity of germination and seedling traits in a spring barley (Hordeum vulgare L.) collection under drought simulated conditions. Genet. Resour. Crop Evol. 2015, 62, 275–292. [Google Scholar] [CrossRef]
- Mamnabi, S.; Nasrollahzadeh, S.; Ghassemi-Golezani, K.; Raei, Y. Improving yield-related physiological characteristics of spring rapeseed by integrated fertilizer management under water deficit conditions. Saudi J. Biol. Sci. 2020, 27, 797–804. [Google Scholar] [CrossRef]
- Hellal, F.A.; El-Shabrawi, H.M.; Abd El-Hady, M.; Khatab, I.A.; El-Sayed, S.A.A.; Abdelly, C. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. J. Genet. Eng. Biotechnol. 2018, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-J.; Zhang, K.-K.; Du, C.-Z.; Li, J.; Xing, Y.-X.; Yang, L.-T.; Li, Y.-R. Effect of drought stress on anatomical structure and chloroplast ultrastructure in leaves of sugarcane. Sugar Tech 2015, 17, 41–48. [Google Scholar] [CrossRef]
- Cheng, L.; Han, M.; Yang, L.m.; Li, Y.; Sun, Z.; Zhang, T. Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis Georgi under drought stress. Ind. Crops Prod. 2018, 122, 473–482. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Kocsy, G.; Tari, I.; Vanková, R.; Zechmann, B.; Gulyás, Z.; Poór, P.; Galiba, G. Redox control of plant growth and development. Plant Sci. 2013, 211, 77–91. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Brestič, M.; Afrin, S.; Sakil, M.A.; Hossain, M.T.; Hossain, M.A.; Hossain, M.A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Liu, L.; Wang, H.; Li, D.; Bai, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Li, C. Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 2018, 14, e0216575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahammed, G.J.; Wang, Y.; Mao, Q.; Wu, M.; Yan, Y.; Ren, J.; Wang, X.; Liu, A.; Chen, S. Dopamine alleviates bisphenol A-induced phytotoxicity by enhancing antioxidant and detoxification potential in cucumber. Environ. Pollut. 2020, 259, 113957. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, Y.; Yu, S.; Huang, Y.; Liu, H.; Chen, W.; He, X. Effects of drought stress on growth, physiology and secondary metabolites of Two Adonis species in Northeast China. Sci. Hortic. 2020, 259, 108795. [Google Scholar] [CrossRef]
- Kumari, N.; Yadav, M.; Sharma, V. Differential response of Brassica juncea cultivars to al; consequences for chlorophyll a fluorescence, antioxidants and psb a gene. J. Plant Interact. 2018, 13, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Ju, Y.-L.; Yue, X.-F.; Zhao, X.-F.; Zhao, H.; Fang, Y.-L. Physiological, micro-morphological and metabolomic analysis of grapevine (Vitis vinifera L.) leaf of plants under water stress. Plant Physiol. Biochem. 2018, 130, 501–510. [Google Scholar] [CrossRef]
- Meneses, C.H.S.G.; Bruno, R.D.L.A.; Fernandes, P.D.; Pereira, W.E.; Lima, L.H.G.D.M.; Lima, M.M.D.A.; Vidal, M.S. Germination of cotton cultivar seeds under water stress induced by polyethyleneglycol-6000. Sci. Agric. 2011, 68, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Badr, A.; El-Shazly, H.H.; Tarawneh, R.A.; Börner, A. Screening for drought tolerance in maize (Zea mays L.) germplasm using germination and seedling traits under simulated drought conditions. Plants 2020, 9, 565. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.; Khatab, A.; Sherif, A.; Wang, Z.K.; Salah, A.; Nishawy, E.; Ayaad, M.; Kuai, J. Modulation of salinity impact on early seedling stage via nano-priming application of Zinc oxide on rapeseed (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 376–392. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Li, H.Z.; Zhou, W.J.; Takeuchi, Y.; Yoneyama, K. Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regul. 2006, 49, 27–34. [Google Scholar] [CrossRef]
- Yasmeen, A.; Basra, S.M.A.; Wahid, A.; Nouman, W.; Rehman, H.U. Exploring the potential of Moringa oleifera leaf extract (MLE) as a seed priming agent in improving wheat performance. Turk. J. Bot. 2013, 37, 512–520. [Google Scholar] [CrossRef]
- Subramanyam, K.; Laing, G.D.; Van Damme, E.J.M. Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Front. Plant Sci. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Kong, Y.; Xu, X.; Zhu, L. Cyanobactericidal Effect of Streptomyces sp. HJC-D1 on Microcystis auruginosa. PLoS ONE 2013, 8, e57654. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Jian, H.; Wang, J.; Wang, T.; Wei, L.; Li, J.; Liu, L. Identification of Rapeseed MicroRNAs Involved in Early Stage Seed Germination under Salt and Drought Stresses. Front. Plant Sci. 2016, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Finch-Savage, W.E.; Clay, H.A.; Lynn, J.R.; Morris, K. Towards a genetic understanding of seed vigour in small-seeded crops using natural variation in Brassica oleracea. Plant Sci. 2010, 179, 582–589. [Google Scholar] [CrossRef]
- Shahverdikandi, M.A.; Tobeh, A.; Godehkahriz, S.J.; Rastegar, Z. The study of germination index of canola cultivars for drought resistance. Int. J. Agron. Plant Prod 2011, 2, 89–95. [Google Scholar]
- Okçu, G.; Kaya, M.D.; Atak, M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242. [Google Scholar] [CrossRef]
- Ajirloo, A.R.; Mohammadi, G.R.; Ghobadi, M. The Effect of Priming on Seed Performance of Chickpea (Cicer arietinum L.) under Drought Stress. J. Agric. Environ. Sci. 2011, 1, 1349–1351. [Google Scholar]
- Boureima, S.; Eyletters, M.; Diouf, M.; Diop, T.A.; Damme, P.V. Sensitivity of Seed Germination and Seedling Radicle Growth to Drought Stress in Sesame (Sesamum indicum L.). Res. J. Environ. Sci. 2011, 5, 557–564. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kadioglu, A.; Turgut, R. Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthe setosa (Rosc.) Eichler. Can. J. Plant Sci. 2000, 80, 373–378. [Google Scholar] [CrossRef]
- Li, J.; Zeng, L.; Cheng, Y.; Lu, G.; Fu, G.; Ma, H.; Liu, Q.; Zhang, X.; Zou, X.; Li, C. Exogenous melatonin alleviates damage from drought stress in Brassica napus L. (rapeseed) seedlings. Acta Physiol. Plant. 2018, 40, 43. [Google Scholar] [CrossRef]
- Sharif, P.; Seyedsalehi, M.; Paladino, O.; Van Damme, P.; Sillanpää, M.; Sharifi, A.A. Effect of drought and salinity stresses on morphological and physiological characteristics of canola. Int. J. Environ. Sci. Technol. 2018, 15, 1859–1866. [Google Scholar] [CrossRef]
- Hasibeder, R.; Fuchslueger, L.; Richter, A.; Bahn, M. Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol. 2015, 205, 1117–1127. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Shahbaz, M.; Ali, Q. Drought-induced modulation in growth and mineral nutrients in canola (Brassica napus L.). Pak. J. Bot. 2013, 45, 93–98. [Google Scholar]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The Critical Role of Zinc in Plants Facing the Drought Stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Batool, M.; El-Badri, A.M.; Hassan, M.U.; Haiyun, Y.; Chunyun, W.; Zhenkun, Y.; Jie, K.; Wang, B.; Zhou, G. Drought Stress in Brassica napus: Effects, Tolerance Mechanisms, and Management Strategies. J. Plant Growth Regul. 2022, 1–25. [Google Scholar] [CrossRef]
- Hassan, M.; Aamer, M.; Chattha, M.; Ullah, M.; Suleman, S.; Nawaz, M.; Yanqin, M.; Huang, G. The role or potassium in plants under drought stress; Mini review. J. Basic Appl. Sci. 2017, 13, 268–271. [Google Scholar] [CrossRef]
- Rasheed, A.; Hassan, M.U.; Aamer, M.; Batool, M.; Fang, S.; WU, Z.; LI, H. A Critical Review on the Improvement of Drought Stress Tolerance in Rice (Oryza sativa L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1756–1788. [Google Scholar] [CrossRef]
- Jing, D.W.; Xing, S.J.; Du, Z.Y.; Liu, F.C. Effects of drought stress on the growth, photosynthetic characteristics, and active oxygen metabolism of poplar seedlings. Chin. J. Appl. Ecol. 2013, 24, 1809–1816. [Google Scholar]
- Zhang, J.; Mason, A.S.; Wu, J.; Liu, S.; Zhang, X.; Luo, T.; Redden, R.; Batley, J.; Hu, L.; Yan, G. Identification of putative candidate genes for water stress tolerance in canola (Brassica napus). Front. Plant Sci. 2015, 6, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragab, M.E.; Helal, N.A.S.; Sawan, O.M.; Fawzy, Z.F.; El Sawy, S.M. Foliar application of glycine betaine for alleviating water stress of tomato plants grown under sandy soil conditions. Int. J. ChemTech Res. 2015, 8, 52–67. [Google Scholar]
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant. 2016, 158, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.P.; Kang, H.J.; Ouyang, Z.; Zhuang, W.; Yu, Q. Photosynthetic parameter estimations by considering interactive effects of light, temperature and CO2 concentration. Int. J. Plant Prod. 2015, 9, 321–346. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, G.; Long, W.; Zou, X.; Li, F.; Nishio, T. Recent progress in drought and salt tolerance studies in Brassica crops. Breed. Sci. 2014, 64, 60–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Bai, Z.; Li, C.; Qu, P. Effect of drought stress on ultrastructure of flag leaves in wheat chromosome substitution lines. J. Chin. Electron Microsc. Soc. 2009, 28, 68–73. [Google Scholar]
- Chen, J.; Li, R.; Guo, P.; Xia, Y.; Tian, C.; Miao, S. Impact of drought stress on the ultrastructure of leaf cells in three barley genotypes differing in level of drought tolerance. Chin. Bull. Bot. 2011, 46, 28. [Google Scholar]
- Zhang, Y.-B.; Yang, S.-L.; Dao, J.-M.; Deng, J.; Shahzad, A.N.; Fan, X.; Li, R.-D.; Quan, Y.-J.; Bukhari, S.A.H.; Zeng, Z.-H. Drought-induced alterations in photosynthetic, ultrastructural and biochemical traits of contrasting sugarcane genotypes. PLoS ONE 2020, 15, e0235845. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Harmens, H.; Zheng, X.; Zhang, C. Melatonin enhances drought resistance by regulating leaf stomatal behaviour, root growth and catalase activity in two contrasting rapeseed (Brassica napus L.) genotypes. Plant Physiol. Biochem. 2020, 149, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abass Ahanger, M.; Nasser Alyemeni, M.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.; Lentzsch, P.; Müller, M.E.H. Carbohydrate Dynamics in Leaves of Rapeseed (Brassica napus) Under Drought. J. Agron. Crop Sci. 2012, 198, 207–217. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, Q.; Tian, Y.; Meng, F. Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yü et Lu roots. BMC Plant Biol. 2017, 17, 53. [Google Scholar] [CrossRef] [Green Version]









| Variety | FG% | GR | VI (I) | VI (II) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 10% | 15% | CK | 10% | 15% | CK | 10% | 15% | CK | 10% | 15% | |
| CY 81 | 91.67 b–e | 90.55 cde | 57.77 j | 20.76 m | 20.76 j | 9.840 l | 976.7 de | 1045 fgh | 592.3 hi | 33.33 h–k | 27.38 d–h | 10.33 k |
| YYZ 3 | 99.33 a | 98.88 ab | 99.44 a | 43.42 cd | 32.47 c | 28.28 bc | 1061 cd | 1120 c-f | 878.7 cd | 31.66 klm | 20.55 j | 16.54 efg |
| YY 28 | 97.77 abc | 96.11 abc | 78.33 h | 35.49 f | 27.69 efg | 14.28 k | 347.3 j | 520.3 l | 336.7 j | 31.33 lm | 24.68 hi | 13.67 hij |
| CY 36 | 99.33 a | 98.88 ab | 89.44 def | 30.95 hi | 28.16 ef | 23.44 ef | 804.3 gh | 1102 d-g | 820.4 de | 32.66 jkl | 27.32 e–h | 18.33 b |
| JYZ 158 | 96.67 a–d | 98.33 ab | 97.77 ab | 51.33 a | 39.24 a | 30.82 ab | 1243 ab | 1361 a | 1041 a | 45.66 ab | 34.67 a | 22.33 a |
| ZY 50 | 98.67 a | 99.44 ab | 96.66 abc | 28.29 ij | 27.64 efg | 22.53 fg | 942.1 d-g | 1245 bc | 813.3 de | 32.66 jkl | 26.66 e–h | 14.80 f–i |
| QY 33 | 96.67 a–d | 100.0 a | 85.56 efg | 42.54 cd | 33.48 c | 20.36 ghi | 1133 c | 1388 a | 881.7 efg | 37.66 d–h | 23.68 l | 14.67 f-i |
| ZY 51 | 96.67 a-d | 96.11 a–d | 67.67 i | 25.22 k | 24.72 hi | 13.99 k | 873.7 efg | 976.5 hi | 509.6 i | 40.33 cd | 26.89 e–h | 13.67 ij |
| XZY 518 | 99.33 a | 99.44 ab | 91.30 b-e | 41.25 d | 31.56 cd | 22.36 fg | 973.7 def | 1087 gh | 712.3 fg | 37.66 d–i | 27.01 f–h | 18.46 bc |
| GHY 8 | 89.33 e | 89.44 def | 88.88 def | 24.94 kl | 24.95 hi | 20.83 gh | 746.3 h | 788.1 j | 687.5 gh | 35.86 i–l | 23.60 l | 13.66 ij |
| ZYZ 108 | 91.11 cde | 88.33 ef | 79.33 hg | 33.28 fg | 24.79 hi | 17.63 j | 595.1 i | 976.6 hi | 617.3 h | 37.58 e–i | 29.38 cde | 15.79 e-h |
| NZ 1838 | 90.00 de | 83.88 f | 55.67 j | 16.93 n | 15.26 k | 7.251 m | 839.1 fgh | 921.1 i | 259.3 j | 32.64 jkl | 20.55 j | 7.336 l |
| XZY 553 | 98.67 ab | 99.44 ab | 96.67 abc | 43.86 c | 32.37 c | 25.47 de | 987.7 de | 1070 e-h | 922.3 bc | 41.66 bc | 32.28 bc | 18.51 bc |
| YY 9 | 99.33 a | 96.66 abc | 78.33 h | 31.69 gh | 22.32 il | 14.99 k | 810.7 gh | 710.6 jk | 352.7 j | 38.66 def | 20.33 j | 12.67 j |
| HYZ 62 | 96.67 a–d | 97.77 ab | 88.33 def | 41.83 d | 29.98 de | 19.90 hij | 973.7 de | 985.7 ghi | 607.3 hi | 48.43 a | 32.28 ab | 17.33 cde |
| QY 3 | 95.67 a–e | 94.44 a–d | 82.67 fgh | 38.62 e | 28.58 ef | 14.10 k | 1127 c | 1298 ab | 818.2 de | 38.66 def | 26.67 e–h | 16.67 cde |
| QY 7 | 90.00 de | 90.55 cde | 90.00 cde | 33.35 fg | 25.61 gh | 22.65 fg | 822.7 gh | 976.0 hi | 883.3 bcd | 28.66 m | 20.66 j | 16.33 cde |
| ZS 11 | 96.11 a–e | 97.77 b | 93.67 a–d | 32.70 gh | 26.60 fgh | 26.67 cd | 956.3 d–g | 1072 fgh | 1033 a | 35.47 g–j | 29.33 def | 16.33 cde |
| YG 2009 | 66.67 f | 66.66 g | 28.67 k | 9.352 o | 10.51 l | 4.661 n | 540.3 i | 606.4 kl | 162.4 k | 21.69 n | 14.36 k | 3.330 m |
| HYZ 72 | 93.33 a–e | 96.66 abc | 88.33 def | 27.65 j | 25.31 gh | 17.84 ij | 1046 cd | 1351 ab | 787.7 ef | 37.69 d-g | 27.59 fgh | 15.67 f–i |
| QY 1 | 98.67 ab | 96.11 a-d | 88.33 fgh | 31.31 gh | 27.68 efg | 20.64 gh | 1133 c | 1176 cd | 972.3 a | 36.39 e–i | 26.33 ghi | 17.33 cde |
| TYZ 283 | 96.67 a–e | 97.77 ab | 82.67 cde | 22.64 lm | 20.90 j | 13.92 k | 1036 cd | 1137 cde | 587.7 hi | 35.68 f–j | 27.51 d–g | 14.51 ghi |
| GZ 1 | 93.67 a–e | 93.88 b–e | 90.00 cde | 38.35 e | 31.55 cd | 22.59 fg | 1136 bc | 1325 ab | 974.3 ab | 38.77 de | 29.30 cd | 17.68 bcd |
| FY 520 | 98.88 a | 100.0 a | 98.30 ab | 46.36 b | 36.43 b | 32.28 a | 1281 a | 1088 d–g | 896.7 bcd | 47.44 a | 32.67 ab | 23.78 a |
| Mean | 94.67 | 94.49 | 83.07 | 33.00 | 27.02 | 19.44 | 921.3 | 1056 | 706.01 | 36.87 | 26.49 | 15.65 |
| Variety | ShFW | RFW | ShDW | RDW | ShL | RL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 10% | 15% | CK | 10% | 15% | CK | 10% | 15% | CK | 10% | 15% | CK | 10% | 15% | CK | 10% | 15% | |
| CY 81 | 0.235 f–i | 0.188 efg | 0.135 g–j | 0.133 c–f | 0.117 a | 0.055 cd | 0.025 e–h | 0.030 f–l | 0.026 g | 0.0084 d–g | 0.0098 c–f | 0.0087 b | 1.965 hij | 1.873 cde | 1.620 a | 8.691 bcd | 9.681 d–g | 8.469 a–d |
| YYZ 3 | 0.205 i | 0.129 m | 0.114 kl | 0.117 f–i | 0.075 h–k | 0.050 def | 0.024 ghi | 0.021 n | 0.025 h | 0.0082 fgh | 0.0087 fg | 0.0046 jk | 2.160 f–j | 1.473 klm | 1.186 b–f | 8.450 bcd | 10.18 def | 7.597 c–f |
| YY 28 | 0.270 c–f | 0.196 def | 0.142 d–g | 0.054 m | 0.064 i–l | 0.040 ghi | 0.032 b | 0.034 d–j | 0.033 cd | 0.0050 l | 0.0081 g | 0.0057 hij | 2.236 d-h | 1.993 a | 1.486 abc | 1.320 j | 3.427 l | 2.817 l |
| CY 36 | 0.225 hi | 0.182 f-i | 0.155 d–f | 0.106 h-k | 0.094 efg | 0.059 bc | 0.027 cde | 0.032 ge–k | 0.033 cd | 0.0091 def | 0.0096 efg | 0.0075 bc | 2.007 g-j | 1.729 def | 1.482 ab | 5.987 fgh | 9.250 fgh | 7.689 b–e |
| JYZ 158 | 0.323 ab | 0.235 ab | 0.177 a | 0.154 a | 0.117 a | 0.054 cd | 0.037 a | 0.035 b–f | 0.034 cd | 0.0113 ab | 0.0154 a | 0.0098 a | 2.505 a–d | 1.887 bcd | 1.463 abc | 10.46 a | 11.89 abc | 9.183 abc |
| ZY 50 | 0.228 ghi | 0.176 g-j | 0.118 h–l | 0.106 ijk | 0.098 cde | 0.035 ij | 0.032 b | 0.031 f–l | 0.033 cd | 0.0068 hij | 0.0128 b | 0.0046 kl | 1.883 ij | 1.613 f-j | 1.450 a–d | 7.563 c–f | 10.91 bcd | 6.853 d–h |
| QY 33 | 0.274 cde | 0.159 jkl | 0.115 jkl | 0.124 f-i | 0.081 fgh | 0.061 abc | 0.024 ghi | 0.024 mn | 0.019 i | 0.0081 e–h | 0.0107 cd | 0.0063 gh | 2.363 a–d | 1.397 mn | 1.226 a–f | 9.020 abc | 12.62 a | 8.063 b–e |
| ZY 51 | 0.274 cde | 0.183 fgh | 0.158 b–e | 0.147 ab | 0.098 de | 0.049 def | 0.030 bc | 0.036 b–e | 0.038 ab | 0.0107 bc | 0.0095 c-g | 0.0054 ij | 2.353 a–e | 1.733 d–h | 1.536 ab | 6.587 e-h | 8.481 hi | 5.984 f-i |
| XZY 518 | 0.243 e–h | 0.165 i-l | 0.147 c–g | 0.138 b–e | 0.105 a-e | 0.054 cd | 0.032 b | 0.030 h–l | 0.034 ab | 0.009 def | 0.0109 cde | 0.0074 cd | 1.877 jk | 1.346 n | 1.193 b–f | 7.621 cde | 9.353 fgh | 6.637 e–h |
| GHY 8 | 0.288 bc | 0.182 f-i | 0.118 i–l | 0.098 kl | 0.077 ghi | 0.039 ghi | 0.026 cde | 0.029 jkl | 0.027 c | 0.0099 cd | 0.0058 h | 0.0046 lm | 2.576 a | 1.886 bcd | 1.087 f | 5.563 h | 6.857 jk | 6.657 e–h |
| ZYZ 108 | 0.342 a | 0.238 a | 0.158 a–d | 0.067 m | 0.098 de | 0.039 ghi | 0.037 a | 0.041 a | 0.026 fg | 0.0050 kl | 0.0094 c–g | 0.0046 lm | 2.566 a | 1.686 e–i | 1.203 b–f | 3.911 i | 9.371 fgh | 6.517 e–h |
| NZ 1838 | 0.262 c–g | 0.165 i-l | 0.112 kl | 0.101 jkl | 0.079 gh | 0.029 j | 0.031 b | 0.030 i–l | 0.031 gh | 0.0062 ijk | 0.0055 h | 0.0033 n | 2.003 g–j | 1.566 jkl | 0.966 ef | 7.357 d-g | 9.317 fgh | 3.717 jkl |
| XZY 553 | 0.288 c | 0.222 bcd | 0.147 c–g | 0.147 ab | 0.102 a–e | 0.044 fg | 0.033 b | 0.034 c–g | 0.034 c | 0.0108 ab | 0.0083 fg | 0.0056 ghi | 2.316 c–g | 1.663 e–j | 1.516 ab | 7.560 def | 9.073 fgh | 8.003 b–e |
| YY 9 | 0.262 c–g | 0.158 kl | 0.129 g–k | 0.129 def | 0.054 l | 0.035 hij | 0.031 b | 0.033 d–i | 0.032 cde | 0.0073 hij | 0.0054 h | 0.0037 mn | 2.166 d–h | 1.473 lm | 1.049 c–f | 5.993 gh | 5.877 k | 3.463 kl |
| HYZ 62 | 0.354 a | 0.227 ab | 0.164 abc | 0.147 ab | 0.105 a–e | 0.035 ij | 0.029 bcd | 0.036 bcd | 0.039 a | 0.0123 a | 0.0098 c–f | 0.0057 ghi | 2.316 b–g | 1.573 h–l | 1.286 a–e | 7.583 b–e | 8.597 ghi | 5.573 hij |
| QY 3 | 0.261 c–g | 0.175 g-k | 0.142 d–g | 0.149 ab | 0.113 abc | 0.064 ab | 0.028 def | 0.028 lm | 0.030 ef | 0.0091 def | 0.0097 c–f | 0.0064 ef | 2.226 d–h | 1.566 jkl | 1.407 a–e | 9.251 ab | 12.19 a | 8.483 a–d |
| QY 7 | 0.206 i | 0.156 l | 0.142 e–h | 0.113 g–j | 0.074 h–k | 0.044 fg | 0.022 i | 0.029 klm | 0.027 fg | 0.0080 e–h | 0.0091 d–g | 0.0051 ijk | 1.576 k | 1.333 n | 1.193 a–f | 7.567 def | 9.260 fgh | 8.491 a–d |
| ZS 11 | 0.244 d–h | 0.188 efg | 0.135 f–i | 0.125 d–g | 0.115 ab | 0.041 gh | 0.025 fgh | 0.030 g–l | 0.031 de | 0.0087 d–g | 0.0097 c–f | 0.0068 de | 2.353 a–f | 1.580 g–k | 1.653 ab | 7.397 d–g | 9.387 e-h | 9.070 abc |
| YG 2009 | 0.238 f–i | 0.158 jkl | 0.108 l | 0.086 l | 0.062 jkl | 0.028 j | 0.027 def | 0.031 f–l | 0.032 cde | 0.0062 jk | 0.0054 h | 0.0024 o | 2.241 d–g | 1.581 i–l | 1.007 def | 5.876 gh | 7.437 ij | 4.613 ijk |
| HYZ 72 | 0.285 c | 0.226 abc | 0.142 f–i | 0.124 e–h | 0.059 kl | 0.035 hi | 0.031 b | 0.038 ab | 0.036 b | 0.0075 ghi | 0.0107 c | 0.0052 sjk | 2.493 abc | 1.961 ab | 1.399 a-e | 8.460 bcd | 12.097 ab | 7.393 d-g |
| QY 1 | 0.221 hi | 0.168 h–l | 0.143 d–g | 0.152 ab | 0.102 b–e | 0.054 de | 0.025 efg | 0.029 i–l | 0.033 cd | 0.009 def | 0.0127 b | 0.0063 fg | 2.156 f–i | 1.678 e–j | 1.573 ab | 9.103 ab | 10.65 cde | 9.357 a |
| TYZ 283 | 0.248 d–h | 0.206 sde | 0.147 c–g | 0.128 d–g | 0.075 hij | 0.029 j | 0.032 b | 0.037 abc | 0.037 ab | 0.0081 e–h | 0.0098 c–f | 0.0038 mn | 2.166 e–i | 1.837 cde | 1.317 a-e | 8.481 bcd | 9.993 def | 5.707 ghi |
| GZ 1 | 0.277 cd | 0.206 cde | 0.156 b–f | 0.141 a–d | 0.113 a-s | 0.044 efg | 0.023 hi | 0.030 g–l | 0.031 de | 0.0095 cde | 0.0124 b | 0.0067 de | 2.576 a | 1.967 abc | 1.350 a-d | 9.169 ab | 12.08 ab | 9.323 ab |
| FY 520 | 0.332 a | 0.237 a | 0.181 ab | 0.148 abc | 0.095 ef | 0.076 a | 0.032 b | 0.034 c–h | 0.037 ab | 0.0086 d–h | 0.0082 g | 0.0065 ef | 2.580 a | 1.746 d–g | 1.466 abc | 10.32 a | 9.147 fgh | 8.143 c-f |
| Mean | 0.266 | 0.188 | 0.140 | 0.122 | 0.090 | 0.045 | 0.089 | 0.032 | 0.031 | 0.0084 | 0.0094 | 0.0056 | 2.235 | 1.672 | 1.337 | 7.470 | 9.463 | 6.968 |
| Variety | FG% | GR | VI (II) | VI (I) | ShFW | RFW | ShDW | RDW | ShL | RL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 15% | 10% | 15% | 10% | 15% | 10% | 15% | 10% | 15% | 10% | 15% | 10% | 15% | 10% | 15% | 10% | 15% | 10% | 15% | |
| CY 81 | 0.98 cd | 0.63 k | 0.99 b | 0.47 kl | 0.82 a | 0.32 m | 1.07 gh | 0.59 i | 0.80 ab | 0.57 cde | 0.88 c | 0.41 de | 1.18 cde | 1.06 fg | 1.16 d | 0.95 b | 0.95 a | 0.82 a | 1.11 i | 0.97 ef |
| YYZ 3 | 0.99 bcd | 1.00 a | 0.74 ghi | 0.65 cd | 0.62 i | 0.50 b | 1.09 e–h | 0.82 ef | 0.63 jkl | 0.56 def | 0.64 ij | 0.42 de | 0.89 k | 1.05 fg | 1.06 d | 0.56 jk | 0.68 f-i | 0.55 ij | 1.20 ghi | 0.89 fg |
| YY 28 | 0.98 cd | 0.80 i | 0.78 efg | 0.40 mn | 0.78 ab | 0.45 efg | 1.49 b | 0.96 bc | 0.72 d–h | 0.52 f–j | 1.19 b | 0.74 a | 1.07 fgh | 1.06 gh | 1.61 b | 1.12 a | 0.89 b | 0.66 de | 2.59 a | 2.13 a |
| CY 36 | 0.99 bcd | 0.89 efg | 0.90 d | 0.75 b | 0.82 a | 0.58 a | 1.36 c | 1.03 ab | 0.81 ab | 0.68 a | 0.88 c | 0.55 b | 1.20 bcd | 1.23 cde | 0.98 ef | 0.82 de | 0.86 b | 0.74 bc | 1.54 c | 1.28 c |
| JYZ 158 | 1.02 abc | 1.01 a | 0.76 fgh | 0.60 efg | 0.75 bcd | 0.49 bc | 1.08 fgh | 0.83 ef | 0.73 d–g | 0.55 e-h | 0.76 def | 0.35 fg | 0.93 jk | 0.90 i | 1.37 c | 0.86 cd | 0.74 cde | 0.58 ghi | 1.13 i | 0.87 gh |
| ZY 50 | 1.00 a–d | 0.98 abc | 0.97 bc | 0.79 ab | 0.82 a | 0.44 efg | 1.33 c | 0.85 e | 0.77 bcd | 0.52 g-j | 0.92 c | 0.33 ghi | 0.97 h–k | 1.04 gh | 1.89 a | 0.68 jk | 0.85 b | 0.76 b | 1.44 cd | 0.90 fg |
| QY 33 | 1.03 ab | 0.88 e-h | 0.78 efg | 0.48 ghi | 0.62 i | 0.39 jk | 1.27 cd | 0.72 g | 0.58 l | 0.42 mn | 0.65 hij | 0.49 c | 0.99 g-j | 0.81 i | 1.32 c | 0.77 hi | 0.59 j | 0.52 jk | 1.39 def | 0.89 fg |
| ZY 51 | 0.99 bcd | 0.70 j | 0.98 b | 0.55 hij | 0.66 hi | 0.34 lm | 1.13 e–h | 0.58 i | 0.66 ij | 0.57 cde | 0.66 hi | 0.33 gh | 1.23 bcd | 1.27 bcd | 0.89 fgh | 0.50 lm | 0.73 c-f | 0.65 def | 1.28 fgh | 0.90 fg |
| XZY 518 | 1.00 a–d | 0.92 de | 0.76 fgh | 0.54 hij | 0.71 c-h | 0.48 bcd | 1.12 e–h | 0.75 g | 0.68 g–j | 0.60 c | 0.76 d-g | 0.39 ef | 0.95 ijk | 1.08 fg | 1.21 d | 0.82 ef | 0.72 d-h | 0.63 efg | 1.22 ghi | 0.87 gh |
| GHY 8 | 1.00 a–d | 0.99 ab | 1.00 b | 0.83 a | 0.67 ghi | 0.40 hij | 1.07 fgh | 0.94 c | 0.63 jkl | 0.41 n | 0.78 def | 0.39 ef | 1.09 fg | 1.03 gh | 0.58 j | 0.46 n | 0.73 c-g | 0.42 n | 1.23 gh | 1.19 c |
| ZYZ 108 | 0.96 de | 0.87 fgh | 0.74 ghi | 0.52 ij | 0.79 ab | 0.42 g–j | 1.65 a | 1.04 a | 0.69 f–i | 0.46 kl | 1.46 a | 0.59 b | 1.09 fg | 0.70 j | 1.87 a | 0.91 bc | 0.66 i | 0.47 lmn | 2.39 b | 1.66 b |
| NZ 1838 | 0.93 e | 0.61 k | 0.90 d | 0.42 lm | 0.62 i | 0.24 n | 1.08 fgh | 0.30 k | 0.63 jkl | 0.43 lmn | 0.78 de | 0.28 ij | 0.94 jk | 0.99 h | 0.88 gh | 0.54 l | 0.78 c | 0.48 klm | 1.26 gh | 0.50 k |
| XZY 553 | 1.00 a–d | 0.98 abc | 0.74 ghi | 0.58 fgh | 0.74 b–f | 0.42 g–j | 1.09 fgh | 0.94 cd | 0.77 bcd | 0.51 hij | 0.69 ghi | 0.29 hij | 1.03 fgh | 1.03 fg | 0.76 i | 0.51 lm | 0.71 d-h | 0.65 def | 1.20 hi | 1.05 de |
| YY 9 | 0.97 de | 0.78 i | 0.70 i | 0.47 kl | 0.52 j | 0.33 m | 0.87 i | 0.43 j | 0.60 kl | 0.49 jk | 0.41 k | 0.27 jkl | 1.07 fgh | 1.01 gh | 0.74 i | 0.50 l | 0.68 f–i | 0.48 klm | 0.98 j | 0.57 k |
| HYZ 62 | 1.01 a–d | 0.91 def | 0.72 hi | 0.47 kl | 0.67 ghi | 0.36 kl | 1.04 h | 0.63 hi | 0.64 jk | 0.46 kl | 0.71 f-i | 0.24 kl | 1.25 bc | 1.34 a | 0.79 hi | 0.46 n | 0.68 ghi | 0.55 hij | 1.13 i | 0.73 ij |
| QY 3 | 0.98 cd | 0.86 gh | 0.74 ghi | 0.36 n | 0.69 fgh | 0.44 e–h | 1.18 def | 0.74 g | 0.67 hij | 0.54 e–i | 0.75 d–g | 0.43 de | 0.97 ghi | 1.05 ef | 1.08 de | 0.71 i | 0.70 e–i | 0.63 efg | 1.32 efg | 0.92 fg |
| QY 7 | 1.00 a–d | 1.00 a | 0.76 fgh | 0.68 c | 0.72 c–g | 0.58 a | 1.16 efg | 1.05 a | 0.75 b-e | 0.68 a | 0.65 hig | 0.39 ef | 1.32 a | 1.25 ab | 1.12 d | 0.63 k | 0.84 b | 0.75 b | 1.22 ghi | 1.12 d |
| ZS 11 | 1.02 abc | 0.97 abc | 0.81 ef | 0.81 a | 0.83 a | 0.46 cde | 1.14 efg | 1.07 a | 0.77 bcd | 0.55 efg | 0.91 c | 0.32 ghi | 1.23 ab | 1.26 ab | 1.12 d | 0.78 fg | 0.67 hi | 0.70 cd | 1.26 gh | 1.22 c |
| YG 2009 | 1.00 a–d | 0.43 l | 1.12 a | 0.49 jk | 0.67 ghi | 0.18 o | 1.11 e–h | 0.30 k | 0.66 ij | 0.45 lm | 0.71 e-h | 0.32 ghi | 1.17 cde | 1.20 c-e | 0.80 hi | 0.39 o | 0.70 d–i | 0.44 mn | 1.26 gh | 0.78 hi |
| HYZ 72 | 1.04 a | 0.94 cd | 0.91 d | 0.64 cde | 0.72 c–h | 0.41 hij | 1.32 c | 0.76 fg | 0.78 abc | 0.49 jk | 0.47 k | 0.28 ijk | 1.23 bc | 1.18 de | 1.43 c | 0.68 jk | 0.78 c | 0.56 hij | 1.43 cde | 0.87 gh |
| QY 1 | 0.97 de | 0.89 e | 0.88 d | 0.65 cd | 0.70 c–h | 0.47 cde | 1.06 gh | 0.86 e | 0.76 b–e | 0.64 b | 0.66 hi | 0.35 fg | 1.16 def | 1.32 bc | 1.41 c | 0.69 jk | 0.78 c | 0.73 bc | 1.17 hi | 1.03 de |
| TYZ 283 | 1.01 a–d | 0.85 h | 0.92 cd | 0.62 def | 0.75 d–h | 0.40 ij | 1.12 e-h | 0.56 i | 0.83 a | 0.59 cd | 0.59 j | 0.22 l | 1.18 bcd | 1.17 e | 1.20 d | 0.47 mn | 0.84 b | 0.60 fgh | 1.17 hi | 0.67 j |
| GZ 1 | 1.00 a–d | 0.95 bc | 0.82 e | 0.59 fgh | 0.76 bc | 0.46 def | 1.19 de | 0.87 de | 0.74 c–f | 0.56 def | 0.80 d | 0.31 g–j | 1.31 a | 1.34 a | 1.30 c | 0.70 ghi | 0.76 cd | 0.52 jk | 1.32 efg | 1.01 e |
| FY 520 | 1.01 a–d | 0.99 ab | 0.78 efg | 0.68 c | 0.70 e-h | 0.48 bcd | 0.85 i | 0.69 gh | 0.71 e–i | 0.54 e–h | 0.64 ij | 0.51 cd | 1.06 fgh | 1.16 e | 0.94 fg | 0.74 fg | 0.67 ghi | 0.56 hij | 0.88 j | 0.78 ij |
| Mean | 0.99 | 0.87 | 0.84 | 0.58 | 0.72 | 0.42 | 1.16 | 0.76 | 0.71 | 0.53 | 0.76 | 0.38 | 1.10 | 1.10 | 1.15 | 0.68 | 0.75 | 0.60 | 1.34 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, M.; El-Badri, A.M.; Wang, Z.; Mohamed, I.A.A.; Yang, H.; Ai, X.; Salah, A.; Hassan, M.U.; Sami, R.; Kuai, J.; et al. Rapeseed Morpho-Physio-Biochemical Responses to Drought Stress Induced by PEG-6000. Agronomy 2022, 12, 579. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12030579
Batool M, El-Badri AM, Wang Z, Mohamed IAA, Yang H, Ai X, Salah A, Hassan MU, Sami R, Kuai J, et al. Rapeseed Morpho-Physio-Biochemical Responses to Drought Stress Induced by PEG-6000. Agronomy. 2022; 12(3):579. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12030579
Chicago/Turabian StyleBatool, Maria, Ali Mahmoud El-Badri, Zongkai Wang, Ibrahim A. A. Mohamed, Haiyun Yang, Xueying Ai, Akram Salah, Muhammad Umair Hassan, Rokayya Sami, Jie Kuai, and et al. 2022. "Rapeseed Morpho-Physio-Biochemical Responses to Drought Stress Induced by PEG-6000" Agronomy 12, no. 3: 579. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12030579