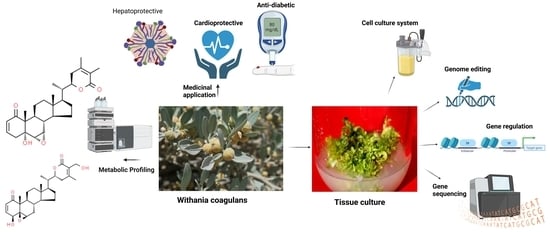
Biotechnological Intervention and Withanolide Production in Withania coagulans
Abstract
:1. Introduction
2. Ethnobotanical Profile
3. Phytochemical Profile
4. Breeding and Cultivation Potential of W. coagulans
5. Biotechnological Intervention for the Conservation and Withanolide Production
5.1. In Vitro Propagation in Withania coagulans
5.2. Synthetic Seed Production
6. Withanolide Production In Vitro
6.1. Thin Cell Layer (TCL) Culture and Elicitation
6.2. Cell Suspension and Root Culture
6.3. Application of Genetic Engineering and Omics Techniques in Secondary Metabolites Production
7. Safety Evaluation
8. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, I.; Nama, K.S. Withania coagulans (Stocks) Dunal: A Rare Ethnomedicinal Plant of the Western Rajasthan Desert. Int. J. Pharmaceut. Biomed. Res. 2015, 2, 34–40. [Google Scholar]
- Barad, R.; Soni, P.; Upadhyay, S.; Upadhyay, U. Withania coagulans and Psidium guajava: An Overview. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 42–47. [Google Scholar]
- Rathore, M.S.; Khatri, K.; Kheni, J.; Shekhawat, N.S. Biotechnological Advancement in an Important Medicinal Plant, Withania coagulans: An Overview and Recent Updates. In Biotechnological Approaches for Medicinal and Aromatic Plants; Kumar, N., Ed.; Springer: Singapore, 2018; pp. 445–465. [Google Scholar]
- Bhandari, M.M. Flora of Indian Desert; MPS Reports: Jodhpur, India, 1990. [Google Scholar]
- Gilani, S.A.; Kikuchi, A.; Watanabe, K.N. Genetic Variation within and among Fragmented Populations of Endangered Medicinal Plant, Withania coagulans (Solanaceae) from Pakistan and Its Implications for Conservation. Afr. J. Biotechnol. 2009, 8, 2948–2958. [Google Scholar]
- Gupta, R.; Sonawane, T.; Pai, S. An Overview on Pharmaceutical Properties and Biotechnological Advancement of Withania coagulans. Adv. Tradit. Med. 2021, 22, 673–683. [Google Scholar] [CrossRef]
- Khan, M.I.; Maqsood, M.; Saeed, R.A.; Alam, A.; Sahar, A.; Kieliszek, M.; Miecznikowski, A.; Muzammil, H.S.; Aadil, R.M. Phytochemistry, Food Application, and Therapeutic Potential of the Medicinal Plant (Withania coagulans): A Review. Molecules 2021, 26, 6881. [Google Scholar] [CrossRef]
- Gupta, P.C. Withania coagulans Dunal—An Overview. Int. J. Pharm. Sci. 2012, 12, 68–71. [Google Scholar]
- Srivastav, A.K.; Das, P. Phytochemical Extraction and Characterization of Roots of Withania somnifera for Its Anti-Bacterial, Antioxidant, Anti-Inflammation and Analgesic Activity. Int. J. Innov. Res. Dev. 2014, 7, 22–33. [Google Scholar]
- Hemalatha, S.; Kumar, R.; Kumar, M. Withania coagulans Dunal: A Review. Pharmacogn. Rev. 2008, 2, 351–358. [Google Scholar]
- Ciddi, V. Withaferin A from Cell Cultures of Withania somnifera. Indian J. Pharm. Sci. 2010, 68, 490–492. [Google Scholar] [CrossRef] [Green Version]
- Jilani, K.; Adrian, L.; Mohanad, Z.; Nazneen, S.; Florian, L. Withaferin A-stimulated Ca2+ Entry, Ceramide Formation and Suicidal Death of Erythrocytes. Toxicol. Vitr. 2013, 27, 52–58. [Google Scholar] [CrossRef]
- Subramanian, S.S.; Sethi, P.D.; Glotter, E.; Kirson, I.; Lavie, D. 5, 20α(R)-Dihydroxy-6α,7α-Epoxy-1-Oxo-(5α) with a-2,24-Dienolide, A new steroidal lactone from Withania coagulans. Phytochemistry 1971, 10, 685–688. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Prabu, P.C.; Panchapakesan, S.; Raj, C.D. Acute and Sub-Acute Oral Toxicity Assessment of the Hydroalcoholic Extract of Withania somnifera Roots in Wistar Rats. Phytother. Res. 2013, 27, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.; Qureshi, R.; Ikram, M.; Ahmad, M.S.; Jabeen, B.; Asi, M.R.; Khan, J.A.; Ali, S.; Lilge, L. In Vitro Anticancer Activities of Withania coagulans against HeLa, MCF-7, RD, RG2, and INS-1 Cancer Cells and Phytochemical Analysis. Integr. Med. Res. 2018, 7, 184–191. [Google Scholar] [CrossRef]
- Adnan, M.; Bibi, R.; Azizullah, A.; Andaleeb, R.; Mussarat, S.; Tariq, A.; Ullah, R.; Elsalam, N.M.; Khan, A.L.; Begum, S. Ethnomedicinal Plants Used against Common Digestive Problems. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 99–117. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, A.; Abbas, S.; Dur-e-Shawar, N.A.; Jamal, A.S.; Choudhary, M.I. New Withanolides from Withania spp. J. Nat. Prod. 1993, 56, 1000–1006. [Google Scholar] [CrossRef]
- Neogi, P.; Kawai, M.; Butsugan, Y.; Mori, Y.; Suzuki, M. Withacoagin, a New Withanolide from Withania coagulans Roots. Bull. Chem. Soc. Jpn. 1988, 61, 4479–4481. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, L. Handbook of Ayurvedic Medicinal Plants: Herbal Reference Library, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001; Volume 2. [Google Scholar]
- Jain, R.; Kachhwaha, S.; Kothari, S. Phytochemistry, Pharmacology, and Biotechnology of Withania somnifera and Withania coagulans: A Review. J. Med. Plants Res. 2012, 6, 5388–5399. [Google Scholar]
- Mishra, J.; Dash, A.K.; Mishra, S.N.; Gupta, A.K. Withania coagulans in Treatment of Diabetics and Some Other Diseases: A Review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1251. [Google Scholar]
- Gupta, V.; Keshari, B.B. Withania coagulans Dunal (Paneer Doda): A Review. Int. J. Ayurvedic Herb. Med. 2013, 3, 1330–1336. [Google Scholar]
- Atta-ur-Rahman, A.; Choudhary, M.I.; Yousaf, M.; Gul, W.; Qureshi, S. New Withanolides from Withania coagulans. Chem. Pharm. Bull. 1998, 46, 1853–1856. [Google Scholar] [CrossRef] [Green Version]
- Maryam, K.; Mehrana; Mitra, N. Remedial Use of Withanolides from Withania Coagolans (Stocks). Adv. Life Sci. 2012, 2, 6–19. [Google Scholar]
- Adebajo, A.; Ayoola, O.; Iwadewa, E.; Akindahunsi, A.; Omisore, N.; Adewunmi, C.; Adenowo, T. Antitrichomonal, Biochemical and Toxicological Activities of Methanolic Extract and Some Carbazole Alkaloids Isolated from the Leaves of Murraya koenigii Growing in Nigeria. Phytomedicine 2006, 13, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.K.; Tong, X.; Mukerji, R.; Zhang, H.; Timmermann, B.N.; Cohen, M.S. Withaferin A, a Cytotoxic Steroid from Vassobia brewiflora, Induces Apoptosis in Human Head and Neck Squamous Cell Carcinoma. J. Nat. Prod. 2010, 73, 1476–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, S.S.; Sethi, P.D. Withaferin-A from the Roots of Withania coagulans. Curr. Sci. 1969, 38, 267–268. [Google Scholar]
- Atta-ur-Rahman, A.; Dur-e-Shahwar, N.A.; Choudhary, M.I. Withanolides from Withania coagulans. Phytochemistry 2003, 63, 387–390. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, A.; Shabbir, M.; Yousaf, M.; Qureshi, S.; Dur-e-Shahwar, N.A.; Naz, A.; Choudhary, M.I. Three Withanolides from Withania coagulans. Phytochemistry 1999, 52, 1361–1364. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, M.; Shabbir, D.-E.-S.; Choudhary, M.I.; Voelter, W.; Hohnholz, D. New Steroidal Lactones from Withania coagulans. Heterocycles 1998, 47, 1005–1012. [Google Scholar]
- Velde, V.V.; Lavie, D.; Budhiraja, R.D.; Sudhir, S.; Garg, K.N. Potential Biogenetic Precursors of Withanolides from Withania coagulans. Phytochemistry 1983, 22, 2253–2257. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Dur-e-Shahwar, N.A.; Parveen, Z.; Jabbar, A.; Ali, I.; Atta-ur-Rahman, A. Antifungal Steroidal Lactones from Withania coagulanc. Phytochemistry 1995, 40, 1243–1246. [Google Scholar] [CrossRef]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazon, J. Steroidal Lactones from Withania somnifera, an Ancient Plant for Novel Medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.-M.; Gao, S.; Bunting, D.P.; Gunatilaka, A.A.L. Unusual Withanolides from Aeroponically Grown Withania somnifera. Phytochemistry 2011, 72, 518–522. [Google Scholar] [CrossRef]
- Nur-e-Alam, M.; Yousaf, M.; Qureshi, S.; Baig, I.; Nasim, S. A Novel Dimeric Podophyllotoxin-Type Lignan and a New Withanolide from Withania coagulans. Helv. Chim. Acta 2003, 86, 607–614. [Google Scholar] [CrossRef]
- Maurya, R.; Singh, A.B.; Srivastava, A.K. Coagulanolide, a Withanolide from Withania coagulans Fruits and Antihyperglycemic Activity. Bioorg. Med. Chem. Lett. 2008, 18, 6534–6537. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, S.; Wahi, A.K.; Singh, P.N.; Chansouria, J.P. Hypoglycemic Activity of Withania coagulans Dunal in Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2004, 93, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-F.; Ma, L.; Sun, L.-J.; Ali, M.; Arfan, M.; Liu, J.-W.; Hu, L.-H. Immunosuppressive Withanolides from Withania coagulans. Chem. Biodivers. 2009, 6, 141. [Google Scholar] [CrossRef]
- Maher, S.; Choudhary, M.I.; Saleem, F.; Rasheed, S.; Waheed, I.; Halim, S.A.; Azeem, M.; Abdullah, I.B.; Froeyen, M.; Mirza, M.U.; et al. Isolation of Antidiabetic Withanolides from Withania coagulans Dunal and Their In Vitro and In Silico Validation. Biology 2020, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Srivastava, N. In Vitro Studies on Organophosphate Pesticides Induced Oxidative DNA Damage in Rat Lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 761, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Jogee, P.S.; Agarkar, G.; Santos, C.A. Anticancer Activities of Withania somnifera: Current Research, Formulations, and Future Perspectives. Pharm. Biol. 2016, 54, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Budhiraja, R.D.; Sudhir, S.; Garg, K.N. Cardiovascular Effects of a Withanolide from Withania coagulans, Dunal Fruits. Indian J. Physiol. Pharmacol. 1983, 27, 129–134. [Google Scholar]
- Budhiraja, R.D.; Garg, K.N.; Sudhir, S.; Arora, B. Protective effect of 3-ss-hydroxy-2, 3-dihydrowithanolide F against CCl4-induced hepatotoxicity. Planta Medica 1986, 52, 28–29. [Google Scholar] [CrossRef]
- Budhiraja, R.D.; Sudhir, S.; Garg, K.N. Anti-inflammatory activity of 3 β-Hydroxy-2, 3-dihydro-withanolide F. Planta Med. 1984, 50, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Bahr, V.; Hansel, R. Immunomodulating properties of 5, 20a (R)-dihydroxy-6a,7a-epoxy-1-oxo(5a)-witha-2,24-dienolide and solasodine. Planta Med. 1982, 44, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, P.A.; Lavie, D.; Budhiraja, R.D.; Sudhir, S.; Garg, K.N. Spectroscopic Studies on a Withanolide from Withania coagulans. Phytochemistry 1984, 23, 143–149. [Google Scholar] [CrossRef]
- Sethi, P.D.; Subramanian, S. Steroidal Constituents of Withania coagulans Roots. Indian J. Pharm. 1971, 38, 22–23. [Google Scholar]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L. Consequences of Changing Biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Jain, R.; Sinha, A.; Jain, D.; Kachhwaha, S.; Kothari, S.L. Adventitious Shoot Regeneration and In Vitro Biosynthesis of Steroidal Lactones in Withania coagulans (Stocks) Dunal. Plant Cell Tissue Organ Cult. 2010, 105, 135–140. [Google Scholar] [CrossRef]
- Rathore, M.S.; Shekhawat, S.; Kaur, G.; Singh, R.P.; Shekhawat, N.S. Micropropagation of Vegetable Rennet (Withania coagulans [Stocks] Dunal)-A Critically Endangered Medicinal Plant. J. Sustain. For. 2012, 31, 727–746. [Google Scholar] [CrossRef]
- Shahzad, A.; Sharma, S.; Parveen, S.; Saeed, T. Historical Perspective and Basic Principles of Plant Tissue Culture. In Plant Biotechnology: Principles and Applications; Abdin, M., Kiran, U., Kamaluddin, A., Eds.; Springer: Singapore, 2017; pp. 3–13. ISBN 978-981-10-2961-5. [Google Scholar]
- Shahzad, A.; Parveen, S.; Sharma, S.; Shaheen, A.; Saeed, T. Plant Tissue Culture: Applications in Plant Improvement and Conservation. In Plant Biotechnology: Principles and Applications; Abdin, M., Kiran, U., Kamaluddin, A., Eds.; Springer: Singapore, 2017; pp. 15–35. ISBN 978-981-10-2961-5. [Google Scholar]
- Jain, R.; Sinha, A.; Kachhwaha, S.; Kothari, S.L. Micropropagation of Withania coagulans (Stocks) Dunal: A Critically Endangered Medicinal Herb. J. Plant Biochem. Biotechnol. 2009, 18, 249–252. [Google Scholar] [CrossRef]
- Valizadeh, J.; Valizadeh, M. Development of Efficient Micropropagation Protocol for Withania coagulans (Stocks) Dunal. Afr. J. Biotechnol. 2011, 10, 7611–7616. [Google Scholar]
- Castilla, A.M. Micropropagation of Withania frutescens: One Way to Recover Reduced or Extinct Plant Populations in the Balearic Islands. Bolletí De La Soc. D’història Nat. De Les Balear. 2011, 54, 67–74. [Google Scholar]
- Sharma, N.; Sachdeva, P.; Dhiman, M.; Koshy, E.P. Comparative Evaluation of In Vitro Regeneration Potential of Seeds of W. somnifera and W. coagulans. Biotechnol. Int. 2015, 8, 21–33. [Google Scholar]
- Joshi, H.; Nekkala, S.; Soner, D.; Kher, M.M.; Nataraj, M. In Vitro Shoot Multiplication of Withania coagulans (stocks) Dunal. Plant Tissue Cult. Biotechnol. 2016, 26, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Rathore, M.S.; Mastan, S.G.; Yadav, P.; Bhatt, V.D.; Shekhawat, N.S.; Chikara, J. Shoot Regeneration from Leaf Explants of Withania coagulans (Stocks) Dunal and Genetic Stability Evaluation of Regenerates with RAPD and ISSR Markers. S. Afr. J. Bot. 2016, 102, 12–17. [Google Scholar] [CrossRef]
- Tripathi, D.; Rai, K.K.; Rai, S.K.; Rai, S.P.J. An Improved Thin Cell Layer Culture System for Efficient Clonal Propagation and In Vitro Withanolide Production in a Medicinal Plant Withania coagulans Dunal. Ind. Crops Prod. 2018, 119, 172–182. [Google Scholar] [CrossRef]
- Ghahremani, A.; Ganji Moghadam, E.; Tatari, M.; Khosroyar, S. Effect of Type and Concentration of Growth Regulators on the Proliferation and Marcotting of Withania coagulans Medical Plant. Iran. J. Hortic. Sci. 2020, 51, 2. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hoque, A.; Islam, S. pH management in plant tissue culture: A review. J. Plant Biotechnol. 2021, 48, 241–250. [Google Scholar]
- Padmavathy, S.; Rajeswari, S. Role of pH in Plant Tissue Culture. In Plant Tissue Culture; Springer: Singapore, 2021; pp. 61–81. ISBN 978-981-15-8641-0. [Google Scholar]
- Jahan, M.S.; Rabbani, M.G. The Effect of Sucrose Concentration on Shoot Induction and Multiplication of Cucumis melo var. Dudaim via Tissue Culture Technique. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 24–31. [Google Scholar]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Sharma, A.; Prasad, B.C.N. Role of Carbon Sources on In Vitro Plant Growth and Development. In Plant Growth and Health Promoting Bacteria; Springer: Singapore, 2017; pp. 33–47. [Google Scholar]
- Zhou, X.; Yu, Q.; Yao, Y.; Liu, X.; Zhang, M.; Zhang, Y. Effects of Agar Concentration and Plant Growth Regulators on In Vitro Shoot Regeneration of Sweet Cherry (Prunus avium L.). Horticulturae 2020, 55, 352–356. [Google Scholar]
- He, L.; Huang, X.; Li, X.; Li, Y.; Liu, Y.; Xu, Z. Agar and Activated Charcoal Concentrations Affect Shoot Regeneration from Hypocotyl Explants of Nitraria tangutorum Bobr. J. For. Res. 2021, 32, 1119–1128. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture: Volume 1. The Background; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-1-4020-5005-7. [Google Scholar]
- Li, Y.; Wang, J.; Li, S.; Li, J.; Du, H.; Zhao, C. Effects of Light Quality and Photoperiod on In Vitro Regeneration and Growth of Lonicera macranthoides. Plant Cell Tissue Organ Cult. 2021, 10, 315–324. [Google Scholar]
- Ahmad, Z.; Teixeira da Silva, J.A.; Shahzad, A. Biotechnological Interventions in Bamboo Plants. Plant Cell Tissue Organ Cult. 2023, 153, 459–487. [Google Scholar] [CrossRef]
- Rahimi, S.; Bahmani, R.; Tabarzad, M. Temperature Effect on Callus Induction, Regeneration, and Antioxidant Enzymes Activity of Stevia rebaudiana Bertoni. Plants 2021, 10, 375–382. [Google Scholar]
- Jiang, Y.; Song, X.; Li, J.; Dai, X. Light Quality and Photoperiod Affect Shoot Proliferation and Rooting of Two Pear Rootstocks In Vitro. Front. Plant Sci. 2021, 12, 721311. [Google Scholar]
- Dehvari, N.P.; Abbaspour, H.H.; Asare, M.H.; Saadatmand, S. An Efficient Protocol for In Vitro Regeneration from the Nodal Explants of Withania coagulans (Stocks) Dunal: A Valuable Medicinal Herb. Acta Agric. Slov. 2021, 117, 1–7. [Google Scholar]
- Mirjalili, M.H.; Esmaeili, H. Callus Induction and Withanolides Production through Cell Suspension Culture of Withania coagulans (Stocks) Dunal. J. Med. Plants 2022, 21, 79–91. [Google Scholar] [CrossRef]
- Sharma, S.; Shahzad, A.; Teixeira da Silva, J.A. Synseed Technology—A Complete Synthesis. Biotechnol. Adv. 2013, 31, 186–207. [Google Scholar] [CrossRef]
- Van, K.T.T. Thin Cell Layer Concept. In Thin Cell Layer Culture System: Regeneration and Transformation Applications; Nhut, D.T., Van Le, B., Tran Thanh Van, K., Thorpe, T., Eds.; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Azad, M.O.K.; Al-Forkan, M.; Rana, M.M. Thin Cell Layer Culture: Features and Applications in Plant Biotechnology. Plant Cell Tissue Organ Cult. 2021, 10, 485–510. [Google Scholar]
- Chamkhi, I.; Benali, T.; Aanniz, T.; El Menyiy, N.; Guaouguaou, F.; El Omari, N.; El-Shazly, M.; Zengin, G.; Bouyahya, A. Plant-Microbial Interaction: The Mechanism and the Application of Microbial Elicitor Induced Secondary Metabolites Biosynthesis in Medicinal Plants. Plants 2021, 67, 269–295. [Google Scholar] [CrossRef]
- Ganie, I.B.; Ahmad, Z.; Shahzad, A.; Zaushintsena, A.; Neverova, O.; Ivanova, S.; Wasi, A.; Tahseen, S. Biotechnological Intervention and Secondary Metabolite Production in Centella asiatica L. Plants 2022, 11, 2928. [Google Scholar] [CrossRef] [PubMed]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K. Methyl Jasmonate and Salicylic Acid as Powerful Elicitors for Enhancing the Production of Secondary Metabolites in Medicinal Plants: An Updated Review. Plant Cell Tissue Organ Cult. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Maurya, B.; Rai, K.K.; Pandey, N.; Sharma, L.; Goswami, N.K.; Rai, S.P. Influence of Salicylic Acid Elicitation on Secondary Metabolites and Biomass Production in In-Vitro Cultured Withania coagulans (L.) Dunal. Plant Arch. 2019, 19, 1308–1316. [Google Scholar]
- Tripathi, D.; Meena, R.P.; Pandey-Rai, S. Short term UV-B radiation mediated modulation of physiological traits and withanolides production in Withania coagulans (L.) Dunal under in-vitro condition. Physiol. Mol. Biol. Plants 2021, 27, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal Plant Cell Suspension Cultures: Pharmaceutical Applications and High-Yielding Strategies for the Desired Secondary Metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Rao, S.R.; Ravishankar, G.A. Plant Cell Cultures: Chemical Factories of Secondary Metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar]
- Bagheri, A.; Valizadeh, M.; Sharifi, A.; Senthil, K. Evaluation of Withaferin a Content in Different Accessions and in In Vitro Cultures of Withania coagulans (Stocks) Dunal. Iran. J. Med. Aromat. Plants 2016, 16, 231–243. [Google Scholar]
- Singh, R.; Kalia, R.K.; Sharma, R.; Singh, R.; Dhawan, A.K. Hairy Root Culture: A Biotechnological Approach for Secondary Metabolite Production. Eng. Life Sci. 2015, 15, 1–11. [Google Scholar]
- Murugesan, P.A.; Senthil, K.A. Influence of Auxin on Biomass Production and Withanolide Accumulation in Adventitious Root Culture of Indian Rennet: Withania coagulans. Asian J. Pharm. Clin. Res. 2017, 10, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Murthy, H.N.; Dandin, V.S.; Zhong, J.J.; Paek, K.Y. Strategies for Enhanced Production of Plant Secondary Metabolites from Cell and Organ Cultures. Prod. Biomass Bioact. Compd. Using Bioreact. Technol. 2014, 118, 471–508. [Google Scholar]
- Sharma, M.; Kaur, C.; Katnoria, J.K.; Nagpal, A.K. Hairy Root Cultures: An Efficient Plant-Based System for the Production of Secondary Metabolites. Crit. Rev. Biotechnol. 2021, 41, 28–48. [Google Scholar]
- Flores, H.E.; Vivanco, J.M.; Loyola-Vargas, V.M. “Radicle” Biochemistry: The Biology of Root Specific Metabolism. Trends Plant Sci. 1999, 4, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sangwan, R.S.; Bansal, S.; Sangwan, N.S. Efficient genetic transformation of Withania coagulans (Stocks) Dunal mediated by Agrobacterium tumefaciens from leaf explants of In Vitro multiple shoot culture. Protoplasma 2013, 250, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M. Overexpression of the Arabidopsis thaliana Squalene Synthase Gene in Withania coagulans Hairy Root Cultures. Biol. Plant. 2011, 55, 357–360. [Google Scholar] [CrossRef]
- Jadaun, J.S.; Sangwan, N.S.; Narnoliya, L.K.; Tripathi, S.; Sangwan, R.S. Withania coagulans Tryptophan Decarboxylase Gene Cloning, Heterologous Expression, and Catalytic Characteristics of the Recombinant Enzyme. Protoplasma 2017, 254, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bohra, M.; Sharma, K.; Kachhwaha, S.; Kothari, S.; Jain, R. Leaves and roots of In Vitro cultured Withania coagulans adopt different pathways for withanolide biosynthesis: A comparative transcriptome study. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A Review on Phytochemicals, Pharmacological Activities, Drug Interactions, and Associated Toxicities of Licorice (Glycyrrhiza sp.). Food Front. 2021, 2, 449–485. [Google Scholar] [CrossRef]
- Raizner, A.E.; Cooke, J.P. Dietary Supplements: Facts and Fallacies. Methodist Debakey Cardiovasc. J. 2019, 15, 169–170. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Bouic, P.J.; Rosenkranz, B. An Overview of the Evidence and Mechanisms of Herb-Drug Interactions. Front. Pharmacol. 2012, 3, 69. [Google Scholar] [CrossRef] [Green Version]
- Yasir, M.; Shrivastava, R.; Jain, P.; Das, D. Hypoglycemic and Antihyperglycemic Effects of Different Extracts and Combinations of Withania coagulans Dunal and Acacia arabica Lamk in Normal and Alloxan-Induced Diabetic Rats. Phcog. Commun. 2012, 2, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Husain, I.; Dale, O.R.; Martin, K.; Gurley, B.J.; Adams, S.J.; Avula, B.; Chittiboyina, A.G.; Khan, I.A.; Khan, S.I. Screening of Medicinal Plants for Possible Herb-Drug Interactions through Modulating Nuclear Receptors, Drug-Metabolizing Enzymes and Transporters. J. Ethnopharm. 2023, 301, 115822. [Google Scholar] [CrossRef] [PubMed]





| Plant Part Used | Traditional Uses | References |
|---|---|---|
| Leaves | Used for curdling or curdling the milk | [12] |
| Fruits | Blood purifier, asthma, biliosity, intestinal infection, sedative, emetic, alterative, diuretic, and applied to wounds | [8,13] |
| Dried fruits | Useful in intestinal diseases, dyspepsia, and flatulent colic | [14] |
| Ripened fruits | Useful in chronic liver problems, diuretic and with sedative properties | [6] |
| Twigs | For cleaning teeth | [15] |
| Smoke from the plant | Toothache relief by inhaling the smoke of the plant and headaches relied on | [15] |
| Flowers | Used in the treatment of diabetes | [15,16] |
| Seeds | Useful in lumbago, ophthalmia, and to reduce inflammation in hemorrhoids | [6,17] |
| Roots | Used as an immunosuppressant | [18] |
| Phytochemical Compounds | Part of the Plant | Biological Activity | Reference |
|---|---|---|---|
| Coagulin C, Coagulin B, Coagulin D, Coagulin E Coagulin F, Coagulin, Coagulin H, Coagulin I, Coagulin J, Coagulin K, Coagulin L Coagulin M, Coagulin N, Coagulin O, Coagulin P, Coagulin Q, Coagulin R, Coagulin H | Aerial parts (leaves and stem), whole plant | Antihyperglycaemic, immunosuppressant, immunosuppressive | [30,31,37] |
| Coagulin L | Antihyperglycemic | [38] | |
| Coagulanolide | Fruit | [37] | |
| Withanolide F | [37] | ||
| Withanolide L | Aerial parts (leaves and stem) | Immunosuppressive | [39] |
| Withanolide J, 27-hydroxywithanolide I, Coagulin E, ajugnin E, withaperuvin C | Whole plant | Antidiabetic | [40] |
| Withaferin A | Root | Antibacterial, immunomodulating, antispasmodic, antifungal, sedative antitumor, cytotoxic | [23] |
| Withacoagulin H | Aerial parts (leaves and stem) | Inhibit the proliferation of lymphocyte | [41] |
| Withacoagulin G, Withacoagulin H, Withacoagulin I | Anticancerous, inhibits tumor cells proliferation | [42] | |
| 3β-hydroxy-2,3-dihydrowithanolide F), Withanolide D | Fruit | Hepatoprotective, anti-inflammatory, blood pressure lowering, central nervous system depressant | [32,43] |
| 17β-Hydroxywithanolide K | Fruit | Antihyperglycaemic, antimicrobial | [33,37] |
| 3β,14α,17β,20αF-Tetrahydroxy-1-oxo-20S,22R-witha-5,24-dienolide (or 3β-hydroxy-2,3-dihydrowithanolide F) | Fruit | Hepatoprotective, anti-inflammatory, blood pressure lowering, central nervous system depressant | [32,42,43,44,45] |
| (20S,22R)-6α,7α-Epoxy-5α-dihydroxy-1-oxowitha-2,24- Dienolide | Whole plant | Immunosuppressant | [19,46] |
| 20β-Hydroxy-1-oxo-(22R)-witha-2,5,24-trienolide, 17β-Hydroxy-14α, 20α-epoxy-1-oxo-(22R)-witha-3,5,24-trienolide, Withacoagulin | Immunomodulatory | [29] | |
| Coagulin S, Bispicropodophyllin glucoside | Immunomodulatory | [36] | |
| Ergosta-5,25-diene-3β,24ξ-diol | Fruit | Precursor for withanolide synthesis | [32] |
| 3β-hydroxy-2,3-dihydrowithanolide H, Sitosterol-β-D-glucoside | Whole plant | Immunosuppressive | [47] |
| 5,27-Dihydroxy-6α,7α-epoxy-1-oxo-(5α)-witha-2,24-dienolide | [48] | ||
| Withacoagin, (20R,22R)-6α,7α-Epoxy-5α-20-hydroxy-1-oxowitha-2,24-dienolide | [19] |
| Explant Type | Sterilization Procedure | Reference |
|---|---|---|
| Nodes | Nodes → 5% Teepol →70% ethanol (30 s) → 0.1% HgCl2 (3 min) → DW | [54] |
| Leaf | Leaf → RTW → (15 min) → 20% Extran (5 min) → 0.1% (w/v) HgCl2 (3 min) → 4–5 DW. | [50] |
| Nodal | Nodes → RTW → 70% ethanol (30 s) → 2% NaOCl (10 min) → 4–5 × DW → Cefotaxime 100, 250, 500 and 750 mg/L (5 min). | [55] |
| Node and shoot tip | Nodes → 70% ethanol (6 s) → commercial bleach 7.5% →Tween 80 (15 min) → 3 × DW. | [56] |
| Nodes | Nodes → RTW → 0.1% bavistin (15–20 min) → streptomycin (15–20 min) → 3–5 × DW → 0.1% HgCl2 (2–5 min) → DW → antioxidant solution (2 min). | [57] |
| Seeds | seeds → 0.2% Tween-20 → RTW (10 min) → 90% ethyl alcohol (10–15 s) → 0.1% HgCl2 (2 min) → DW. | [57] |
| Nodes | Branches → 10% liquid detergent (10 min) → RTW (10 min) → 5 cm long segments of branches containing 3 to 5 nodes → 0.1% HgCl2 (3 min) → DW | [58] |
| Leaves | Leaf → 1.0% NaOCl (5 min) → SW. | [59] |
| Young shoot apical meristem | Young shoot apical meristem, nodes, tTCL → Tween-20 (10 min) → DW → 70% alcohol (30 s) → 0.1% HgCl2 (3 min) → 3–4 ×DW. | [60] |
| Nodes | Stems → 10% Teepol (5 min) → RTW (30 min) → Nodes containing segment → 0.1% HgCl2 (5 min) → 5 × DW. | [61] |
| Explant | Nutrient Medium | Culture Condition | Experimental Remark | Reference |
|---|---|---|---|---|
| Nodes | MS + 0.5 mg L−1 BA + 0.5 mg L−1 Kin + 0.5 PG (SIM, SMM) MS + 10 mg L−1 CC + 0.5 PG (PT) for 7 days MS + 0.25 mg L−1 IBA + 0.5 mg L−1 phenylacetic acid + 2 mg L−1 CC (RIM) 5.8 pH, 3% sucrose, 0.8% agar | Temp. 25 ± 2 °C, PP 16 h, 25 µmol m−2 s−1 PPFD provided by CWFT | SIM culture produced shoots in 83% of cases, with 23.4 buds per explant for in vitro recovered shoots and 24.6 buds per explant for shoot tips in SMM. A total of 80% of cultures had roots, and 75% of regenerated plants survived in the wild, as confirmed by RAPD markers. | [54] |
| Nodes | MS + 2 mg L−1 + BA + 0.5 mg L−1 IBA (SIM) MS + 2 mg L−1 + BA + 0.5 mg L−1 IBA (SMM), ½ MS + 2 mg L−1 IBA (RIM) 5.8 pH, 3% sucrose, 0.8% agar | Temp. 25 ± 2 °C, PP 16/8 h, 40 µmol m−2 s−1 PPFD provided by CWFT | SIM and SMM produced microshoots and shoot buds with average lengths of 1–7 and 7 cm, respectively. RIM had up to 35 roots per bud. Plants had a 75% survival rate after acclimatization. | [55] |
| Leaf | MS + 22.2 μM BA + 2.3 μM Kin (SIM). MS+ 0.5 mg L−1 BA+ 0.5 mg L−1 Kin + 0.5 PG (SMM) ½ MS + 71.6 μM CC + 3.9 μM PG (PT) for 7 days, ½ MS + 1.2 μM IBA + 3.6 μM phenylacetic acid + 14.3 μM CC (RIM) 5.8 pH, 3% sucrose, 0.9% agar | Temp. 26 ± 1 °C, PP 16/8 h, 25 µmol m−2 s−1 PPFD by CWFT | A total of 80% of cultures generated 17 shoots per leaf at SIM. Pulse-treated branches grew roots, and 75% of regenerated plants survived in natural environments. Genetic fidelity was confirmed with RAPD markers. | [50] |
| Shoot tip | MS (SIM), MS + IBA (RIM) 5.8 pH, 3% sucrose, 0.8% agar | Temp. 29 °C, PP 16/8 h, 24 µmol m−2 s−1 PPFD provided by CWFT | On SIM and RIM, 94% and 75% of the culture, respectively, develop shoots and roots. In every population, acclimatization and survival were observed. | [56] |
| Nodes | MS + 8.88 μM BAP + 0.57 μM IAA + 100 mg L−1 L-ascorbic acid + 25 mg L−1 (citric acid, adenine sulphate, L-arginine) (SIM) 1. 11 μM BAP + 0.57 μM IAA (SMM) ½ MS + 200 mg L−1 AC and 29.52 μM IBA (RIM) 5.8 pH, 3% sucrose, 0.8% agar | Temp. 27 ± 2 °C, PP 12/8 h, 35–40 µmol m−2 s−1 PPFD provided by CWFT | SIM produces 4.1 shoots per culture, while SMM produces 19.1 multiple shoots. Rooting was observed in 67.3% of microshoots after 15 days. A survival rate of 90% was observed after the acclimatization. | [51] |
| Nodes | MS + 2.50 mg L−1 mT + 0.1 mg L−1 NAA + 50 mg L−1 AdS (SIM) MS + BA 0.50 mg L−1 + 25, 50 or 75 mg L−1 AdS and YE (SMM) ½ MS + 2.00 mg L−1 NAA (RIM) 5.8 pH, 3% sucrose, 0.8% agar | Temp. 25 ± 2 °C, PP 16/8 h, 35 µmol m−2 s−1 PPFD provided by CWFT. | After two SMM culture passages, an average of 17.71 shoots with a mean length of 4.51 cm were produced. With an average shoot length of 2.43 cm and a length of 3.21 cm, RIM resulted in a response rate of 58.33%. A total of 85% of the acclimatized plant survived. | [61] |
| Leaf | MS + 4.44 μM BAP (SIM) MS + 1.11 μM BAP + 0.57 μM IAA (SEM) ½ MS + 0.2% AC (RIM), pulse treatment 2.46 mM IBA (5 min) pH/NS, sucrose/NS, 0.8% agar | Temp. 26 ± 2 °C, PP 12 h/d 35–40 μmol m−2 s−1 PPFD provided by CWFT | At SIM, 73.7% of the cultures responded, and each culture produced an average of 11.4 shoots with a mean shoot length per explant of 6.7 cm at SEM. A total of 90% of the shoots in vitro were rooted in 25–30 days. Acclimatized plantlets survived 90% when transplanted. | [59] |
| Leaves | MS + 4.93 μM IBA (ARIM) pH/NS, 3% sucrose, 0.8% agar | Temp. 25 ± 2 °C, PP 16 h 40 µmol m−2 s−1 PPFD provided by CWFT | After 30 days of root suspension culture at 7.48 g/dL, maximum root branching was observed in ARIM. A production of withanolide A reached 204.98 g/L in this root suspension culture. | [75] |
| Transverse thin cell layer (tTCL), | MS + 2.0 mg L−1 BAP + 0.5 mg L−1 NAA (SIM) ½ MS + 2 mg L−1 IBA (RIM) pH/NS, 3% sucrose, 0.8% agar | Temp. 25 ± 2 °C, PP 16 h 40 µmol m−2 s−1 PPFD provided by CWFT | A total of 31.94% of crops from SIM produced shoots with an average length of 1.44 cm and an average number of 1.70 cm. RIM produced an average of 97.22 roots. A total of 90.6% survived after acclimatization in the field. | [60] |
| Seeds | MS (SG), MS + 0.5 mg L−1 BAP (SIM) MS + 0.5 mg L−1 IBA (RIM) | Temp. 22 °C, PP 18 h PPFD by CWFT, NC | SIM was successful in propagation, but the study did not indicate how many shoots were produced per internode. A total of 90% of the shoots on RIM produced roots. The regenerated plants had 80% vitality when placed under field conditions. | [62] |
| Nodes | MS + 0.01 mg L−1 IBA + 0.5 mg L−1 BA + 0.5 mg L−1 PG + 0.5 mg L−1 GA (SIM) MS + NAA 0.1 mg L−1 (RIM) 5.8 pH, 3% sucrose, 0.8% agar | Temp. 25/18 °C, PP 14 h 60–80 μmol m−2 s−1 PPFD provided by CWFT | SIM produced 96.00% culture. Each node had an average of 6.75 shoots with a length of 2.830 cm. In RIM, rooting was observed in all cultures with a length of 3.075 cm and a number of 20.5 per shoot. The survival rate after acclimatization was 86%. | [75] |
| Explant | Nutrient Medium | Culture Condition | Experimental Remark | Reference |
|---|---|---|---|---|
| Seeds | MS (SG), MS + 2–4 mg L−1 2, 4-D (leaves) (CIM), MS + 2–4 mg L−1 2, 4-D or 0.25–0.5 mg−l BA (Internodes) (CIM) MS + 2 mg L−1 BA + 0.5 mg L−1 IBA (SIM) ½ MS + 2 mg L−1 IBA (RIM) 5.8 pH, 2% sucrose, 0.8% agar | 25 ± °C, PP 16 h, 40 µmol m−2 s−1 PPFD provided by CWFT | A total of 42% of internodes and 100% of leaves in CIM formed callus within 14–16 days. However, while the leaves callus failed to form shoots, the shoot formed only 33% of the internodal callus. 100% of the shoots on RIM formed roots. A total of 75% of plantlets survive in the field after acclimation. | [55] |
| Leaf | MS + 2.3 µM Kn + 13.3 µM BA (CIM) 2.3 µM Kn + 22.2 µM BA (SIM) 2.2 µM BA + 2.3 µM Kn, + 3.9 µM PG (SMM) 71.6 µM CC + 3.9 PG (PT) for 7 days MS + 2 µM IBA + 3.6 µM PAA + 14.3 µM CC (RIM) | 26 ± 1 °C, PP 16/8 h, 25 µmol m−2 s−1 PPFD provided by CWFT | In 80% of the cultures at SIM, the average number of shoots developed was 17.6, and the typical shoot length was 2–3 cm. Extensive roots formed on RIM within 3 weeks of planting. RAPD markers were used to verify genetic stability. | [50] |
| Leaf | MS + 2.5 mg L−1 2, 4-D + 0.5 mg L−1 BAP (CIM) | 25 ± 2 °C, PP 12 h, 2000 lx illumination provided by CWFT | After 28 days, 96.0% of the culture developed callus at CIM. The production of withnolide in the suspension culture was further increased by using the callus. Withaferin A (WFA) and withanolide A (WNA) were produced in the suspension culture at concentrations of 0.08 and 21 g/L, respectively. | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, Z.; Shaheen, A.; Wasi, A.; Rehman, S.u.; Tahseen, S.; Ramakrishnan, M.; Upadhyay, A.; Ganie, I.B.; Shahzad, A.; Ding, Y. Biotechnological Intervention and Withanolide Production in Withania coagulans. Agronomy 2023, 13, 1997. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy13081997
Ahmad Z, Shaheen A, Wasi A, Rehman Su, Tahseen S, Ramakrishnan M, Upadhyay A, Ganie IB, Shahzad A, Ding Y. Biotechnological Intervention and Withanolide Production in Withania coagulans. Agronomy. 2023; 13(8):1997. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy13081997
Chicago/Turabian StyleAhmad, Zishan, Arjumend Shaheen, Adla Wasi, Shams ur Rehman, Sabaha Tahseen, Muthusamy Ramakrishnan, Anamica Upadhyay, Irfan Bashir Ganie, Anwar Shahzad, and Yulong Ding. 2023. "Biotechnological Intervention and Withanolide Production in Withania coagulans" Agronomy 13, no. 8: 1997. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy13081997