Nutritional Enhancement of Polimaize Lines: Integrating Native Mexican Maize Alleles into High-Yield Varieties
Abstract
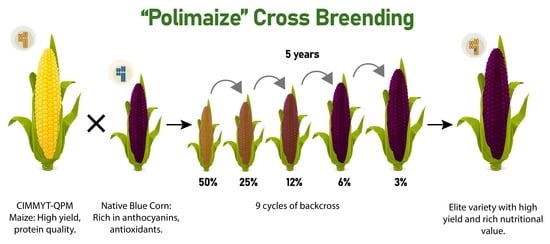
:1. Introduction
2. Materials and Methods
2.1. Metabolite Determination
2.2. Total Anthocyanin Determination
2.3. Antioxidant Capacity
2.4. Relative Abundance of Anthocyanins
2.5. Soluble Sugars (Glucose, Fructose, Sucrose)
2.6. Starch Analysis
2.7. Total Amino Acids
2.8. Tryptophan
2.9. Total Lipids
2.10. Statistical Analyses
3. Results
3.1. Anthocyanins
3.2. Antioxidant Activity
3.3. Relative Abundance of Anthocyanins
3.4. Soluble Sugars (Glucose, Fructose, Sucrose)
3.5. Starch
3.6. Amino Acids
3.7. Tryptophan
3.8. Lipids
3.9. Heritability and Correlation of Biochemical Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO). 2023. Available online: https://www.fao.org/faostat/en/#home (accessed on 29 January 2023).
- Kiran, A.; Wakeel, A.; Mahmood, K.; Mubaraka, R.; Hafsa; Haefele, S.M. Biofortification of staple crops to alleviate human malnutrition: Contributions and potential in developing countries. Agronomy 2022, 12, 452. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar]
- Curry, H.A. Endangered Maize: Industrial Agriculture and the Crisis of Extinction; University of California Press: Oakland, CA, USA, 2022. [Google Scholar]
- Sanchez, G.J.J.; Goodman, M.; Stuber, C.W. Isozymatic and morphological diversity in the races of maize of Mexico. Econ. Bot. 2000, 54, 3. [Google Scholar] [CrossRef]
- Patwardhan, A.; Ray, S.; Roy, A. Molecular markers in phylogenetic studies-a review. J. Phylogenetics Evol. Biol. 2014, 2, 131. [Google Scholar]
- Hossain, F.; Muthusamy, V.; Bhat, J.S.; Zunjare, R.U.; Kumar, S.; Prakash, N.R.; Mehta, B.K. Maize Breeding. In Fundamentals of Field Crop Breeding; Springer: Berlin/Heidelberg, Germany, 2022; pp. 221–258. [Google Scholar]
- Malenica, N.; Dunić, J.A.; Vukadinović, L.; Cesar, V.; Šimić, D. Genetic approaches to enhance multiple stress tolerance in maize. Genes 2021, 12, 1760. [Google Scholar] [CrossRef]
- Lago, C.; Landoni, M.; Cassani, E.; Doria, E.; Nielsen, E.; Pilu, R. Study and characterization of a novel functional food: Purple popcorn. Mol. Breed. 2013, 31, 575–585. [Google Scholar] [CrossRef]
- Casler, M. Breeding forage crops for increased nutritional value. Adv. Agron. 2001, 71, 51–107. [Google Scholar]
- Galili, G.; Galili, S.; Lewinsohn, E.; Tadmor, Y. Genetic, molecular, and genomic approaches to improve the value of plant foods and feeds. Crit. Rev. Plant Sci. 2002, 21, 167–204. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Beshir Issa, A.; Khokhar, E.S. Quality protein maize (QPM): Importance, genetics, timeline of different events, breeding strategies and varietal adoption. Plant Breed. 2021, 140, 375–399. [Google Scholar] [CrossRef]
- Ufaz, S.; Galili, G. Improving the content of essential amino acids in crop plants: Goals and opportunities. Plant Physiol. 2008, 147, 954–961. [Google Scholar] [CrossRef]
- Suriano, S.; Balconi, C.; Valoti, P.; Redaelli, R. Comparison of total polyphenols, profile anthocyanins, color analysis, carotenoids and tocols in pigmented maize. Lwt 2021, 144, 111257. [Google Scholar] [CrossRef]
- Chhoden, T.; Singh, A.; Aggarwal, P.; Sharma, S. Anthocyanins and its health benefits. In Functionality and Application of Colored Cereals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 161–184. [Google Scholar]
- Colombo, R.; Ferron, L.; Papetti, A. Colored corn: An up-date on metabolites extraction, health implication, and potential use. Molecules 2021, 26, 199. [Google Scholar] [CrossRef]
- Jin, H.-M.; Dang, B.; Zhang, W.-G.; Zheng, W.-C.; Yang, X.-J. Polyphenol and anthocyanin composition and activity of highland barley with different colors. Molecules 2022, 27, 3411. [Google Scholar] [CrossRef]
- Kumar, M.V.N.; Ramya, V.; Maheshwaramma, S.; Ganapathy, K.N.; Govindaraj, M.; Kavitha, K.; Vanisree, K. Exploiting Indian landraces to develop biofortified grain sorghum with high protein and minerals. Front. Nutr. 2023, 10, 1228422. [Google Scholar] [CrossRef]
- Martínez, O.; Ojeda, A.C.; Hayano-Kanashiro, C.; Reyes Valdés, M.H.; Hernández, J.L.P.; Simpson, J. Criteria for prioritizing selection of Mexican maize landrace accessions for conservation in situ or ex situ based on phylogenetic analysis. Front. Ecol. Evol. 2023, 11, 1139377. [Google Scholar] [CrossRef]
- Arellano-Vázquez, J.L.; Gutiérrez-Hernández, G.F.; Ceja-Torres, L.F.; Flores-Gómez, E.; García-Ramírez, E.; Quiroz-Figueroa, F.R.; Vázquez-Lozano, P. Combining Ability and Reciprocal Effects for the Yield of Elite Blue Corn Lines from the Central Highlands of Mexico. Plants 2023, 12, 3861. [Google Scholar] [CrossRef]
- Gupta, B.; Khandelwal, A.; Kumar, B.; Bhalothia, P. Genome Engineering for the Development of Climate-Resilient Crop Plants. In Applications of Genome Engineering in Plants; Upadhyay, S.K., Ed.; Wiley: Hoboken, NJ, USA, 2024; pp. 394–411. [Google Scholar]
- Panda, A.; Raju, M.; Rao, S.R.; Lavanya, G.; Reddy, E.P.K.; Sunder, G.S. Nutritional evaluation and utilisation of quality protein maize, Nityashree hybrid maize, and normal maize in broiler chickens. Br. Poult. Sci. 2011, 52, 632–638. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Chirinos, R.; Campos, D.; Costa, N.; Arbizu, C.; Pedreschi, R.; Larondelle, Y. Phenolic profiles of andean mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers: Identification by HPLC-DAD and evaluation of their antioxidant activity. Food Chem. 2008, 106, 1285–1298. [Google Scholar]
- Geigenberger, P.; Hajirezaei, M.; Geiger, M.; Deiting, U.; Sonnewald, U.; Stitt, M. Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 1998, 205, 428–437. [Google Scholar] [CrossRef]
- Angeles-Núñez, J.G.; Tiessen, A. Arabidopsis sucrose synthase 2 and 3 modulate metabolic homeostasis and direct carbon towards starch synthesis in developing seeds. Planta 2010, 232, 701–718. [Google Scholar] [CrossRef]
- Nurit, E.; Tiessen, A.; Pixley, K.V.; Palacios-Rojas, N. Reliable and inexpensive colorimetric method for determining protein-bound tryptophan in maize kernels. J. Agric. Food Chem. 2009, 57, 7233–7238. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F. Quantitative Genetics; Longman London: London, UK, 1983. [Google Scholar]
- Urias-Lugo, D.; Heredia, J.; Serna-Saldivar, S.; Muy-Rangel, M.; Valdez-Torres, J. Total phenolics, total anthocyanins and antioxidant capacity of native and elite blue maize hybrids (Zea mays L.). CyTA-J. Food 2015, 13, 336–339. [Google Scholar] [CrossRef]
- Urias-Peraldí, M.; Gutiérrez-Uribe, J.A.; Preciado-Ortiz, R.E.; Cruz-Morales, A.S.; Serna-Saldívar, S.O.; García-Lara, S. Nutraceutical profiles of improved blue maize (Zea mays) hybrids for subtropical regions. Field Crops Res. 2013, 141, 69–76. [Google Scholar] [CrossRef]
- Cuevas Montilla, E.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Soto-Gómez, D.; Pérez-Rodríguez, P. Sustainable agriculture through perennial grains: Wheat, rice, maize, and other species. A. review. Agric. Ecosyst. Environ. 2022, 325, 107747. [Google Scholar] [CrossRef]
- Mercer, K.L.; Perales, H.R. Evolutionary response of landraces to climate change in centers of crop diversity. Evol. Appl. 2010, 3, 480–493. [Google Scholar] [CrossRef]
- Menkir, A.; Rocheford, T.; Maziya-Dixon, B.; Tanumihardjo, S. Exploiting natural variation in exotic germplasm for increasing provitamin-A carotenoids in tropical maize. Euphytica 2015, 205, 203–217. [Google Scholar] [CrossRef]
- Menkir, A.; Gedil, M.; Tanumihardjo, S.; Adepoju, A.; Bossey, B. Carotenoid accumulation and agronomic performance of maize hybrids involving parental combinations from different marker-based groups. Food Chem. 2014, 148, 131–137. [Google Scholar] [CrossRef]
- Shahidi, F.; Danielski, R.; Ikeda, C. Phenolic compounds in cereal grains and effects of processing on their composition and bioactivities: A review. J. Food Bioact. 2021, 15, 39–50. [Google Scholar] [CrossRef]
- Liu, N.; Xue, Y.; Guo, Z.; Li, W.; Tang, J. Genome-wide association study identifies candidate genes for starch content regulation in maize kernels. Front. Plant Sci. 2016, 7, 1046. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, M.; Zhang, X.; Chen, W.; Song, X.; Fu, X.; Fang, H.; Xu, J.; Xiao, Y.; Li, Y. Genetic basis of kernel starch content decoded in a maize multi-parent population. Plant Biotechnol. J. 2021, 19, 2192–2205. [Google Scholar] [CrossRef]
- Meseka, S.; Fakorede, M.; Ajala, S.; Badu-Apraku, B.; Menkir, A. Introgression of alleles from maize landraces to improve drought tolerance in an adapted germplasm. J. Crop Improv. 2013, 27, 96–112. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, J.I.; Palacios-Rojas, N.; Meng, E.; Pixley, K.; Trethowan, R.; Pena, R. Enhancing the mineral and vitamin content of wheat and maize through plant breeding. J. Cereal Sci. 2007, 46, 293–307. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Palacios-Rojas, N.; Hossain, F.; Muthusamy, V.; Menkir, A.; Dhliwayo, T.; Ndhlela, T.; San Vicente, F.; Nair, S.K.; Vivek, B.S. Molecular breeding for nutritionally enriched maize: Status and prospects. Front. Genet. 2020, 10, 1392. [Google Scholar] [CrossRef]
- Pathirana, R.; Carimi, F. Management and utilization of plant genetic resources for a sustainable agriculture. Plants 2022, 11, 2038. [Google Scholar] [CrossRef]
- Altieri, M.A.; Merrick, L. In situ conservation of crop genetic resources through maintenance of traditional farming systems. Econ. Bot. 1987, 41, 86–96. [Google Scholar] [CrossRef]
- Louette, D.; Charrier, A.; Berthaud, J. In situ conservation of maize in Mexico: Genetic diversity and maize seed management in a traditional community. Econ. Bot. 1997, 51, 20–38. [Google Scholar] [CrossRef]









| ID Lines | Pedigree |
|---|---|
| IPN-007 | [(NBC-JIQ × LPSC7-F103-2-6-1-1-BB)//LPSC7-F103-2-6-1-1-BB//LPSC7-F103-2-6-1-1-BBB]//LPSC7-F103-2-6-1-1-BBB//LPSC7-F103-2-6-1-1-B*4]-B-B-B-B |
| IPN111-031 | [(CML254xNBC-JIQ)//CML254//CML254]//CML254//CML254//CML254//CML254//CML254]-B |
| IPN111-047 | [(NBC-JIQ × HLBP)-B//CML460]//CML460//CML460//CML460//CML460//CML460]-B |
| IPN111-055 | [(NBC-JIQ × CML343)//CML384//CML384]//CML384//CML384//CML384]//CML384//CML384]-B |
| IPN111-057 | [(NBC-JIQ × CML343)//CML321//CML321]//CML321//CML321//CML321]//CML321//CML321]-B |
| IPN111-059 | [(NBC-JIQ × CML343)//CML492//CML492//CML492//CML492//CML492]//CML492//CML492]-B |
| IPN111-061 | [(NBC-JIQ × CML343)//CML444//CML444//CML444//CML444//CML444]//CML444//CML444]-B |
| IPN111-065 | [(NBC-JIQ × LPSC7-F103-2-6-1-1-BB)//CML311//CML311//CML311//CML311//CML311]//CML311//CML311]-B |
| IPN111-067 | [(NBC-JIQ × LPSC7-F103-2-6-1-1-BB)//CML312//CML312//CML312//CML312//CML312//CML312//CML312]-B |
| IPN111-069 | [(NBC-JIQ × LPSC7-F103-2-6-1-1-BB)//CML491//CML491]//CML491//CML491//CML491//CML491//CML491]-B |
| IPN111-071 | [(NBC-JIQ × LPSC7-F103-2-6-1-1-BB)//CML494//CML494//CML494//CML494//CML494//CML494//CML494]-B |
| IPN111-077 | [[(NBC-JIQ × CML216)//CML216]//CML216//CML216//CML216]//CML216//CML216//CML216]-B |
| IPN111-079 | [(Pool17 × NBC-JIQ)//Pool17-f4//Pool17-f4//Pool17-f4//Pool17-f4//Pool17-f4//Pool17-f4//Pool17-f4]-B |
| Trait | Slope | Intercept | r2 | h2 | Significance |
|---|---|---|---|---|---|
| Antioxidant Activity | 1.72 | −0.43 | 0.74 | 1 | *** |
| Anthocyanins | −11.99 | 19.45 | 0.03 | 0 | NS |
| Sucrose | 0.70 | 10.47 | 0.27 | 1 | NS |
| Lipids | 0.53 | 1.58 | 0.10 | 1 | NS |
| Glucose | 0.14 | 46.64 | 0.01 | 0.27 | NS |
| Fructose | 0.76 | 5.65 | 0.25 | 1 | NS |
| Starch | 0.96 | 86.04 | 0.86 | 1 | *** |
| Amino acids | 0.65 | 0.38 | 0.70 | 1 | *** |
| Tryptophan | 0.85 | 0.41 | 0.70 | 1 | *** |
| Correlation Coefficient | Standard Error | p Value | Significance | |
|---|---|---|---|---|
| Intercept | 0.406 | 0.120 | 0.002 | ** |
| Antioxidant | 0.381 | 0.059 | 0.001 | *** |
| Glucose | −0.002 | 0.001 | 0.077 | NS |
| Fructose | −0.001 | 0.001 | 0.719 | NS |
| Sucrose | −0.008 | 0.002 | 0.001 | *** |
| Starch | −0.001 | 0.001 | 0.068 | NS |
| Lipids | 0.049 | 0.011 | 0.001 | *** |
| Amino Acids | 0.060 | 0.034 | 0.083 | NS |
| Tryptophan | −0.054 | 0.011 | 0.001 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyoque-Salcedo, G.; Arias-Martínez, S.; Gutiérrez-Cárdenas, O.G.; Montañez-Soto, J.L.; Oregel-Zamudio, E.; Torres-García, J.R. Nutritional Enhancement of Polimaize Lines: Integrating Native Mexican Maize Alleles into High-Yield Varieties. Agronomy 2024, 14, 403. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy14030403
Oyoque-Salcedo G, Arias-Martínez S, Gutiérrez-Cárdenas OG, Montañez-Soto JL, Oregel-Zamudio E, Torres-García JR. Nutritional Enhancement of Polimaize Lines: Integrating Native Mexican Maize Alleles into High-Yield Varieties. Agronomy. 2024; 14(3):403. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy14030403
Chicago/Turabian StyleOyoque-Salcedo, Guadalupe, Sergio Arias-Martínez, Oscar Giovanni Gutiérrez-Cárdenas, José Luis Montañez-Soto, Ernesto Oregel-Zamudio, and Jesús Rubén Torres-García. 2024. "Nutritional Enhancement of Polimaize Lines: Integrating Native Mexican Maize Alleles into High-Yield Varieties" Agronomy 14, no. 3: 403. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy14030403