Kir4.1 Dysfunction in the Pathophysiology of Depression: A Systematic Review
Abstract
:1. Introduction
2. Methods
3. Results
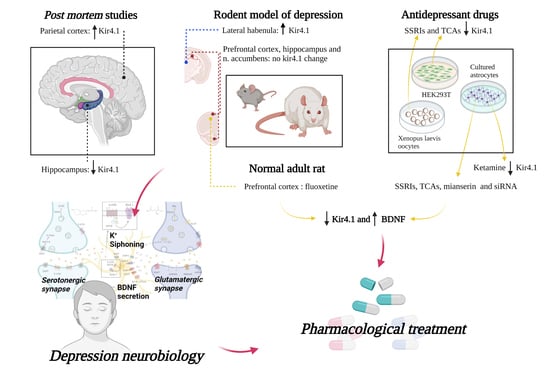
3.1. Kir4.1 Channel Expression and Depression
3.1.1. Down-Regulation of the Astrocytic Kir4.1 Channel
3.1.2. Up-Regulation of Astrocytic Kir4.1 Channel
3.1.3. No Change of Expression of Kir4.1 Astrocytic Channel
3.2. Kir4.1 Channels and Drugs with Antidepressant Action
3.2.1. Kir4.1 Channels, TCAs, and SSRIs
3.2.2. Kir4.1 Channels, Antidepressants and BDNF
3.2.3. Kir4.1 Channels and Ketamine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hasin, D.S.; Sarvet, A.L.; Meyers, J.L.; Saha, T.D.; Ruan, W.J.; Stohl, M.; Grant, B.F. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 2018, 75, 336–346. [Google Scholar]
- Thapar, A.; Collishaw, S.; Pine, D.S.; Thapar, A.K. Depression in adolescence. Lancet 2012, 379, 1056–1067. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Goldberg, D. The heterogeneity of “major depression”. World Psychiatry 2011, 10, 226–228. [Google Scholar]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61, 7–11. [Google Scholar]
- Hirschfeld, R.M. History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 2000, 61, 4–6. [Google Scholar]
- Cowen, P.J. Serotonin and depression: Pathophysiological mechanism or marketing myth? Trends Pharmacol. Sci. 2008, 29, 433–436. [Google Scholar]
- Coppen, A. The Biochemistry of Affective Disorders. Br. J. Psychiatry 1967, 113, 1237–1264. [Google Scholar]
- Cosci, F.; Chouinard, G. The monoamine hypothesis of depression revisited: Could it mechanistically novel antidepressant strategies? In Neurobiology of Depression: Road to Novel Therapeutics, 1st ed.; Quevedo, J., Carvalho, A.F., Zarate, C.A., Eds.; Elsevier Inc.: London, UK, 2019; pp. 63–73. [Google Scholar]
- Freis, E.D. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. N. Engl. J. Med. 1954, 251, 1006–1008. [Google Scholar]
- Feighner, J.P. Mechanism of Action of Antidepressant Medications. J. Clin. Psychiatry 1990, 60, 4–11. [Google Scholar]
- American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 2010. Available online: https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf (accessed on 7 August 2021).
- Fuller, R.W. Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci. 1994, 55, 163–167. [Google Scholar]
- American Academy of Pediatrics. Guidelines for Adolescent Depression in Primary Care (GLAD-PC): Part II. Treatment and Ongoing Management. 2018. Available online: https://pediatrics.aappublications.org/content/pediatrics/early/2018/02/22/peds.2017-4082.full.pdf (accessed on 7 August 2021).
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer Adherence 2012, 6, 369–388. [Google Scholar]
- Björkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar]
- Liu, B.; Liu, J.; Wang, M.; Zhang, Y.; Li, L. From Serotonin to Neuroplasticity: Evolvement of Theories for Major Depressive Disorder. Front. Cell Neurosci. 2017, 11, 305. [Google Scholar]
- Trullas, R.; Skolnick, P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur. J. Pharmacol. 1990, 185, 1–10. [Google Scholar]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar]
- Dwivedi, Y. Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsychiatr. Dis. Treat. 2009, 5, 433–449. [Google Scholar]
- Yu, H.; Chen, Z.Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011, 32, 3–11. [Google Scholar]
- Zhou, X.; Xiao, Q.; Xie, L.; Yang, F.; Wang, L.; Tu, J. Astrocyte, a Promising Target for Mood Disorder Interventions. Front. Mol. Neurosci. 2019, 12, 136. [Google Scholar]
- Rajkowska, G.; Stockmeier, C. Astrocyte Pathology in Major Depressive Disorder: Insights from Human Postmortem Brain Tissue. Curr. Drug Targets. 2013, 14, 1225–1236. [Google Scholar]
- Wang, Q.; Jie, W.; Yang, J.L.J. An astroglial basis of major depressive disorder? An overview. Glia 2017, 65, 1227–1250. [Google Scholar]
- Araque, A.; Sanzgiri, R.P.; Parpura, V.; Haydon, P.G. Astrocyte-induced modulation of synaptic transmission. Can. J. Physiol. Pharmacol. 1999, 77, 699–706. [Google Scholar]
- Bellot-Saez, A.; Kékesi, O.; Morley, J.W.; Buskila, Y. Astrocytic modulation of neuronal excitability through K+ spatial buffering. Neurosci. Biobehav. Rev. 2017, 77, 87–97. [Google Scholar]
- Molofsky, A.V.; Krenick, R.; Ullian, E.; Tsai, H.H.; Deneen, B.; Richardson, W.D.; Barres, B.A.; Rowitch, D.H. Astrocytes and disease: A neurodevelopmental perspective. Genes Dev. 2012, 26, 891–907. [Google Scholar]
- Pekny, M.; Pekna, M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta 2016, 1862, 483–491. [Google Scholar]
- Aida, T.; Yoshida, J.; Nomura, M.; Tanimura, A.; Iino, Y.; Soma, M.; Bai, N.; Ito, Y.; Cui, W.; Aizawa, H.; et al. Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology 2015, 40, 1569–1579. [Google Scholar]
- Altshuler, L.L.; Kupka, R.W.; Hellemann, G.; Frye, M.A.; Sugar, C.A.; McElroy, S.L.; Nolen, W.A.; Grunze, H.; Leverich, G.S.; Keck, P.E.; et al. Gender and depressive symptoms in 711 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Outcome Network. Am. J. Psychiatry 2010, 167, 708–715. [Google Scholar]
- Cobb, J.A.; O’Neill, K.; Milner, J.; Mahajan, G.J.; Lawrenca, T.J.; May, W.L.; Miguel-Hidalgo, J.; Rajkowska, G. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience 2016, 316, 209–220. [Google Scholar]
- Rial, D.; Lemos, C.; Pinheiro, H.; Duarte, J.M.; Gonçalves, F.Q.; Real, J.I.; Prediger, R.D.; Gonçalves, N.; Gomes, C.A.; Canas, P.M.; et al. Depression as a glial-based synaptic dysfunction. Front. Cell. Neurosci. 2016, 9, 521. [Google Scholar]
- Rubinow, M.J.; Mahajan, G.; May, W.; Overholser, J.C.; Jurjus, G.J.; Dieter, L.; Herbst, N.; Steffens, D.C.; Miguel-Hidalgo, J.J.; Rajkowska, G.; et al. Basolateral amygdala volume and cell numbers in major depressive disorder: A postmortem stereological study. Brain Struct. Funct. 2016, 221, 171–184. [Google Scholar]
- Sanacora, G. and Banasr, M. From pathophysiology to novel antidepressant drugs: Glial contributions to the pathology and treatment of mood disorders. Biol. Psychiatry 2013, 73, 1172–1179. [Google Scholar]
- Nwaobi, S.E.; Cuddapah, V.A.; Patterson, K.C.; Randolph, A.C.; Olsen, M.L. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. 2016, 132, 1–21. [Google Scholar]
- Cui, Y.; Yang, Y.; Ni, Z.; Dong, Y.; Cai, G.; Foncelle, A.; Ma, S.; Sang, K. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 2018, 554, 323–327. [Google Scholar]
- Medina, A.; Watson, S.J.; Bunney, W.; Myers, R.M.; Schatzberg, A.; Barchas, J.; Akil, H.; Thompson, R.C. Evidence for alterations of the glial syncytial function in major depressive disorder. J. Psychiatr. Res. 2016, 72, 15–21. [Google Scholar]
- Xiong, Z.; Zhang, K.; Ren, Q.; Chang, L.; Chen, J.; Hashimoto, K. Increased expression of inwardly rectifying Kir4.1 channel in the parietal cortex from patients with major depressive disorder. J. Affect. Disord. 2019, 245, 265–269. [Google Scholar]
- Ohno, Y.; Kinboshi, M.; Shimizu, S. Inwardly rectifying potassium channel Kir4.1 as a novel modulator of BDNF expression in astrocytes. Int. J. Mol. Sci. 2018, 19, 3313. [Google Scholar]
- Frizzo, M.E.; Ohno, Y. Perisynaptic astrocytes as a potential target for novel antidepressant drugs. J. Pharmacol. Sci. 2021, 145, 60–68. [Google Scholar]
- Larsen, B.R.; MacAulay, N. Kir4.1-mediated spatial buffering of K+: Experimental challenges in determination of its temporal and quantitative contribution to K+ clearance in the brain. Channels 2014, 8, 544–550. [Google Scholar]
- Olsen, M.L.; Khakh, B.S.; Skatchkov, S.N.; Zhou, M.; Lee, C.J.; Rouach, N. New insights on astrocyte ion channels: Critical for homeostasis and neuron-glia signaling. J. Neurosci. 2015, 35, 13827–13835. [Google Scholar]
- Su, S.; Ohno, Y.; Lossin, C.; Hibino, H.; Inanobe, A.; Kurachi, Y. Inhibition of astroglial inwardly rectifying Kir4.1 channels by a tricyclic antidepressant, nortriptyline. J. Pharmacol. Exp. Ther. 2007, 320, 573–580. [Google Scholar]
- Ohno, Y.; Hibino, H.; Lossin, C.; Inanobe, A.; Kurachi, Y. Inhibition of astroglial Kir4. 1 channels by selective serotonin reuptake inhibitors. Brain Res. 2007, 1178, 44–51. [Google Scholar]
- Stenovec, M.; Božić, M.; Pirnat, S.; Zorec, R. Astroglial Mechanisms of Ketamine Action Include Reduced Mobility of Kir4.1-Carrying Vesicles. Neurochem. Res. 2019, 45, 109–121. [Google Scholar]
- Zhang, Z.; Song, Z.; Shen, F.; Xie, P.; Wang, J.; Song Zhu, A.; Zhu, G. Ginsenoside Rg1 Prevents PTSD-Like Behaviors in Mice Through Promoting Synaptic Proteins, Reducing Kir4.1 and TNF-α in the Hippocampus. Mol. Neurobiol. 2021, 58, 1550–1563. [Google Scholar]
- McIntyre, R.S.; Rosenblat, J.D.; Nemeroff, C.B.; Sanacora, G.; Murrough, J.W.; Berk, M.; Brietzke, E.; Dodd, S.; Gorwood, P.; Ho, R.; et al. Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. Am. J. Psychiatry 2021, 178, 383–399. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar]
- Song, T.; Chen, W.; Chen, X.; Zhang, H.; Zou, Y.; Wu, H.; Lin, F.; Ren, L.; Kang, Y.; Lei, H. Repeated fluoxetine treatment induces transient and long-term astrocytic plasticity in the medial prefrontal cortex of normal adult rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 107, 110252. [Google Scholar]
- Song, T.; Wu, H.; Li, R.; Xu, H.; Rao, X.; Gao, L.; Zou, Y.; Lei, H. Repeated fluoxetine treatment induces long-lasting neurotrophic changes in the medial prefrontal cortex of adult rats. Behav. Brain Res. 2019, 365, 114–124. [Google Scholar]
- Furutani, K.; Ohno, Y.; Inanobe, A.; Hibino, H.; Kurachi, Y. Mutational and in silico analyses for antidepressant block of astroglial inward-rectifier Kir4.1 channel. Mol. Pharmacol. 2009, 75, 1287–1295. [Google Scholar]
- Kinboshi, M.; Mukai, T.; Nagao, Y.; Matsuba, Y.; Tsuji, Y.; Tanaka, S.; Tokudome, K.; Shimizu, S.; Ito, H.; Ikeda, A.; et al. Inhibition of inwardly rectifying potassium (Kir) 4.1 channels facilitates brain-derived neurotrophic factor (BDNF) expression in astrocytes. Front. Mol. Neurosci. 2017, 10, 408. [Google Scholar]
- Yang, Y.; Cui, Y.; Sang, K.; Dong, Y.; Ni, Z.; Ma, S.; Hu, H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018, 554, 317–322. [Google Scholar]
- Xiong, Z.; Zhang, K.; Ishima, T.; Ren, Q.; Ma, M.; Pu, Y.; Chang, L.; Chen, J.; Hashimoto, K. Lack of rapid antidepressant effects of Kir4.1 channel inhibitors in a chronic social defeat stress model: Comparison with (R)-ketamine. Pharmacol. Biochem. Behav. 2019, 176, 57–62. [Google Scholar]
- Toyoda, H.; Li, X.Y.; Wu, L.J.; Zhao, M.G.; Descalzi, G.; Chen, T.; Koga, K.; Zhuo, M. Interplay of amygdala and cingulate plasticity in emotional fear. Neural Plast. 2011, 2011, 813749. [Google Scholar]
- Anand, K.; Dhikav, V. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 2012, 15, 239–246. [Google Scholar]
- Campbell, S.; MacQueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 2004, 29, 417–426. [Google Scholar]
- Pandya, M.; Altinay, M.; Malone, D.A.; Anand, A. Where in the brain is depression? Curr. Psychiatry Rep. 2012, 14, 634–642. [Google Scholar]
- Strohschein, S.; Hüttmann, K.; Gabriel, S.; Binder, D.K.; Heinemann, U.; Steinhäuser, C. Impact of aquaporin-4 channels on K + buffering and gap junction coupling in the hippocampus. Glia 2011, 59, 973–980. [Google Scholar]
- Proulx, C.D.; Hikosaka, O.; Malinow, R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat. Neurosci. 2014, 17, 1146–1152. [Google Scholar]
- Yang, Y.; Wang, H.; Hu, J.; Hu, H. Lateral habenula in the pathophysiology of depression. Curr. Opin. Neurobiol. 2018, 48, 90–96. [Google Scholar]
- Browne, C.A.; Hammack, R.; Lucki, I. Dysregulation of the Lateral Habenula in Major Depressive Disorder. Front. Synaptic Neurosci. 2018, 10, 46. [Google Scholar]
- Hammen, C. Stress and depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar]
- George, M.S.; Ketter, T.A.; Post, R.M. Prefrontal cortex dysfunction in clinical depression. Depression 1994, 2, 59–72. [Google Scholar]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 201, 6871089. [Google Scholar]
- Heshmati, M.; Russo, S.J. Anhedonia and the Brain Reward Circuitry in Depression. Curr. Behav. Neurosci. Rep. 2015, 2, 146–153. [Google Scholar]
- Pizzagalli, D.A.; Holmes, A.J.; Dillon, D.G.; Goetz, E.L.; Birk, J.L.; Bogdan, R.; Dougherty, D.D.; Iosifescu, D.V.; Rauch, S.L.; Fava, M. Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Subjects with Major Depressive Disorder. Am. J. Psychiatry 2009, 166, 702–710. [Google Scholar]
- MacQueen, G.; Frodl, T. The hippocampus in major depression: Evidence for the convergence of the bench and bedside in psychiatric research. Mol. Psychiatry 2011, 16, 252–264. [Google Scholar]
- Groves, J.O. Is it time to reassess the BDNF hypothesis of depression? Mol. Psychiatry 2007, 12, 1079–1088. [Google Scholar]
- Binder, D.K.; Croll, S.D.; Gall, C.M.; Scharfman, H.E. BDNF and epilepsy: Too much of a good thing? Trends Neurosci. 2001, 24, 47–53. [Google Scholar]
- Iughetti, L.; Lucaccioni, L.; Fugetto, F.; Predieri, B.; Berardi, A.; Ferrari, F. Brain-derived neurotrophic factor and epilepsy: A systematic review. Neuropeptides 2018, 72, 23–29. [Google Scholar]
- Treadway, M.T.; Waskom, M.L.; Dillon, D.G.; Holmes, A.J.; Park, M.T.M.; Chakravarty, M.M.; Dutra, S.J.; Polli, F.E.; Iosifescu, D.V.; Fava, M.; et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol. Psychiatry 2015, 77, 285–294. [Google Scholar]
- Guo, F.; Zhang, Q.; Zhang, B.; Fu, Z.; Wu, B.; Huang, C.; Li, Y. Burst-firing patterns in the prefrontal cortex underlying the neuronal mechanisms of depression probed by antidepressants. Eur. J. Neurosci. 2014, 40, 3538–3547. [Google Scholar]
- Kucheryavykh, Y.V.; Kucheryavykh, L.Y.; Nichols, C.G.; Maldonado, H.M.; Baksi, K.; Reichenbach, A.; Skatchkov, S.N.; Eaton, M.J. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia 2007, 55, 274–281. [Google Scholar]
- Djukic, B.; Casper, K.B.; Philpot, B.D.; Chin, L.S.; McCarthy, K.D. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 2007, 27, 11354–11365. [Google Scholar]
- Sicca, F.; Ambrosini, E.; Marchese, M.; Sforna, L.; Servettini, I.; Valvo, G.; Brignone, M.S.; Lanciotti, A.; Moro, F.; Grottesi, A.; et al. Gain-of-function defects of astrocytic Kir4.1 channels in children with autism spectrum disorders and epilepsy. Sci. Rep. 2016, 6, 34325. [Google Scholar]
- Mathews, D.C.; Henter, I.D.; Zarate, C.A. Targeting the Glutamatergic System to Treat Major Depressive Disorder. Drugs 2012, 72, 1313–1333. [Google Scholar]
- Rodrigues, F.T.S.; de Souza, M.R.M.; Lima, C.N.C.; da Silva, F.E.R.; Costa, D.V.D.S.; Dos Santos, C.C.; Miyajima, F.; de Sousa, F.C.F.; Vasconcelos, S.M.M.; Barichello, T.; et al. Major depression model induced by repeated and intermittent lipopolysaccharide administration: Long-lasting behavioral, neuroimmune and neuroprogressive alterations. J. Psychiatr. Res. 2018, 107, 57–67. [Google Scholar]
- Jiang, B.; Xiong, Z.; Yang, J.; Wang, W.; Wang, Y.; Hu, Z.L.; Wang, F.; Chen, J.G. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br. J. Pharmacol. 2012, 166, 1872–1887. [Google Scholar]
- Kim, Y.; Cho, S.H. The effect of ginsenosides on depression in preclinical studies: A systematic review and meta-analysis. J. Ginseng Res. 2021, 45, 420–432. [Google Scholar]
- Wotton, C.A.; Cross, C.D.; Bekar, L.K. Serotonin, norepinephrine and acetylcholine differentially affect astrocytic potassium clearance to modulate somatosensory signaling in male mice. J. Neurosci Res. 2020, 98, 964–977. [Google Scholar]
- Puissant, M.M.; Mouradian, G.C., Jr.; Liu, P.; Hodges, M.R. Identifying Candidate Genes that Underlie Cellular pH Sensitivity in Serotonin Neurons Using Transcriptomics: A Potential Role for Kir5.1 Channels. Front. Cell. Neurosci. 2017, 11, 34. [Google Scholar]
- De Carvalho, D.; Patrone, L.G.; Taxini, C.L.; Biancardi, V.; Vicente, M.C.; Gargaglioni, L.H. Neurochemical and electrical modulation of the locus coeruleus: Contribution to CO2drive to breathe. Front. Physiol. 2014, 5, 288. [Google Scholar]
- Kim, M.A.; Lee, H.S.; Lee, B.Y.; Waterhouse, B.D. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004, 1026, 56–67. [Google Scholar]
- Benfenati, V.; Caprini, M.; Nobile, M.; Rapisarda, C.; Ferroni, S. Guanosine promotes the up-regulation of inward rectifier potassium current mediated by Kir4.1 in cultured rat cortical astrocytes. J. Neurochem. 2006, 98, 430–445. [Google Scholar]
- Hibino, H.; Higashi-Shingai, K.; Fujita, A.; Iwai, K.; Ishii, M.; Kurachi, Y. Expression of an inwardly rectifying K+ channel, Kir5.1, in specific types of fibrocytes in the cochlear lateral wall suggests its functional importance in the establishment of endocochlear potential. Eur. J. Neurosci. 2004, 19, 76–84. [Google Scholar]
- D’Adamo, M.C.; Shang, L.; Imbrici, P.; Brown, S.D.; Pessia, M.; Tucker, S.J. Genetic inactivation of Kcnj16 identifies Kir5.1 as an important determinant of neuronal PCO2/pH sensitivity. J. Biol. Chem. 2011, 286, 192–198. [Google Scholar]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar]
- Stenovec, M.; Li, B.; Verkhratsky, A.; Zorec, R. Astrocytes in rapid ketamine antidepressant action. Neuropharmacology 2020, 173, 108158. [Google Scholar]
- Tsankova, N.M.; Berton, O.; Renthal, W.; Kumar, A.; Neve, R.L.; Nestler, E.J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006, 9, 519–525. [Google Scholar]
- Salpekar, J.A.; Mula, M. Common psychiatric comorbidities in epilepsy: How big of a problem is it? Epilepsy Behav. 2019, 98, 293–297. [Google Scholar]
- Scholl, U.I.; Choi, M.; Liu, T.; Ramaekers, V.T.; Häusler, M.G.; Grimmer, J.; Tobe, S.W.; Farhi, A.; Nelson-Williams, C.; Lifton, R.P. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc. Natl. Acad. Sci. USA 2009, 106, 5842–5847. [Google Scholar]
- Bockenhauer, D.; Feather, S.; Stanescu, H.C.; Bandulik, S.; Zdebik, A.A.; Reichold, M.; Tobin, J.; Lieberer, E.; Sterner, C.; Landoure, G.; et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N. Engl. J. Med. 2009, 360, 1960–1970. [Google Scholar]
- Sicca, F.; Imbrici, P.; D’Adamo, M.C.; Moro, F.; Bonatti, F.; Brovedani, P.; Grottesi, A.; Guerrini, R.; Masi, G.; Santorelli, F.M.; et al. Autism with Seizures and Intellectual Disability: Possible Causative Role of Gain-of-function of the Inwardly-Rectifying K + Channel Kir4.1. Neurobiol. Dis. 2011, 43, 239–247. [Google Scholar]
- Harada, Y.; Nagao, Y.; Shimizu, S.; Serikawa, T.; Terada, R.; Fujimoto, M.; Okuda, A.; Mukai, T.; Sasa, M.; Kurachi, Y.; et al. Expressional analysis of inwardly rectifying Kir4.1 channels in Noda epileptic rat (NER). Brain Res. 2013, 1517, 141–149. [Google Scholar]
- Olsen, M.L.; Higashimori, H.; Campbell, S.L.; Hablitz, J.J.; Sontheimer, H. Functional expression of K(ir)4.1 channels in spinal cord astrocytes. Glia 2006, 53, 516–528. [Google Scholar]
- Connors, B.W.; Ransom, B.R.; Kunis, D.M.; Gutnick, M.J. Activity-dependent K+ accumulation in the developing rat optic nerve. Science 1982, 216, 1341–1343. [Google Scholar]
- Moroni, R.F.; Inverardi, F.; Regondi, M.C.; Pennacchio, P.; Frassoni, C. Developmental expression of Kir4.1 in astrocytes and oligodendrocytes of rat somatosensory cortex and hippocampus. Int. J. Dev. Neurosci. 2015, 47, 198–205. [Google Scholar]


| Reference | Model | Brain Areas | Kir4.1 Expression |
|---|---|---|---|
| Medina et al., 2016 [38] | Post-mortem study on brain samples from patients with depression | Hippocampus | ↓ Kir4.1 |
| Cui et al., 2018 [37] and Yang et al., 2018 [54] | Rodent models of depression (cLH rat and LPS-treated rat) | Lateral Habenula | ↑ Kir4.1 |
| Xiong et al., 2019 [39] | Post-mortem study on brain samples from patients with depression | Parietal cortex | ↑ Kir4.1 |
| Xiong et al., 2019 [55] | Rodent models of depression (CSDS model) | Prefrontal cortex, nucleus accumbens septi and hippocampus | No change |
| Reference | Drug | Model | Kir4.1 Expression/Function |
|---|---|---|---|
| Su et al., 2007 [44] | TCAs | HEK293T cells | ↓ Kir4.1 |
| Ohno et al., 2007 [45] | SSRIs | HEK293T cells | ↓ Kir4.1 |
| Furutani et al., 2009 [52] | TCAs and SSRIs | Chimeric and site directed mutants of Kir4.1 expressed in Xenopus Laevis oocytes and computational analyses of three-dimensional arrangements of the ligands. | ↓ Kir4.1 interacting with channel pore residues |
| Kinboshi et al., 2017 [53] | SSRIs, TCAs, mianserin, and siRNA | Primary mouse astrocytes | ↓ Kir4.1 and ↑ BDNF |
| Stenovec et al., 2020 [46] | Ketamine | Rat cortex astrocytes | ↓ Kir4.1 reducing mobility of Kir4.1-carrying vesicles. |
| Song et al., 2019 [51] and Song et al., 2021 [50] | Fluoxetine | Normal adult rats | ↓ Kir4.1 and ↑ BDNF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Vecchia, S.; Marchese, M.; Santorelli, F.M.; Sicca, F. Kir4.1 Dysfunction in the Pathophysiology of Depression: A Systematic Review. Cells 2021, 10, 2628. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10102628
Della Vecchia S, Marchese M, Santorelli FM, Sicca F. Kir4.1 Dysfunction in the Pathophysiology of Depression: A Systematic Review. Cells. 2021; 10(10):2628. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10102628
Chicago/Turabian StyleDella Vecchia, Stefania, Maria Marchese, Filippo Maria Santorelli, and Federico Sicca. 2021. "Kir4.1 Dysfunction in the Pathophysiology of Depression: A Systematic Review" Cells 10, no. 10: 2628. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10102628