Neonatal Porcine Germ Cells Dedifferentiate and Display Osteogenic and Pluripotency Properties
Abstract
:1. Introduction
2. Materials and Methods
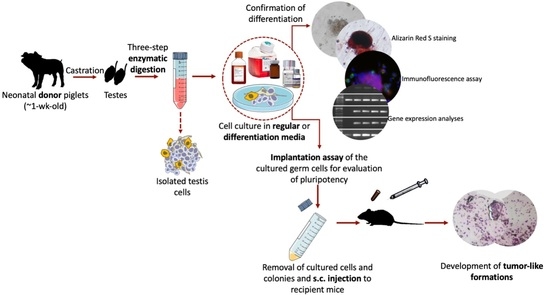
2.1. General Experimental Design
2.2. Castration of Donors, Testis Cell Isolation, and Culture in Regular Media
2.3. In Vitro Osteogenic Trans-Differentiation of Porcine Germ Cells
2.4. In Vitro Tri-Lineage Differentiation of Porcine Germ Cells
2.5. Imaging and Morphometrical Assessment
2.6. Staining and Immunocytochemistry for Evaluation of Dedifferentiation and Trans-Differentiation
2.6.1. Alizarin Red S Staining
2.6.2. Immunofluorescence Assay
2.7. Gene Expression Analyses for Confirmation of Dedifferentiation and Trans-Differentiation
2.8. Subcutaneous Implantation of Cultured Cells and Colonies
2.9. Retrieval of the Subcutaneous Implants and (Immuno)histochemistry
2.10. Statistical Analyses
3. Results
3.1. Viability and Morphometric Assessments
3.2. Alizarin Red S Staining and Immunofluorescence Assay
3.2.1. Osteogenic Differentiation
3.2.2. Pluripotency of Germ Cells and their Differentiation into Derivatives of Three Germinal Layers
3.3. Gene Expression Analyses
3.4. Characterization of Dedifferentiated Cell Implants
3.4.1. H&E Staining
3.4.2. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Rooji, D.G. Stem cells in the testis. Int. J. Exp. Pathol. 1998, 79, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Schmidt, J.A.; Avarbock, M.R.; Tobias, J.W.; Carlson, C.A.; Kolon, T.F.; Ginsberg, J.P.; Brinster, R.L. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc. Natl. Acad. Sci. USA 2009, 106, 21672–21677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roosen-Runge, E.C.; Giesel, L.O., Jr. Quantitative studies on spermatogenesis in the albino rat. Am. J. Anat. 1950, 87, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Ibtisham, F.; Awang-Junaidi, A.H.; Honaramooz, A. The study and manipulation of spermatogonial stem cells using animal models. Cell Tissue Res. 2020, 380, 393–414. [Google Scholar] [CrossRef]
- Lehmann, R. Germline stem cells: Origin and destiny. Cell Stem Cell 2012, 10, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, H.; Asgari, B.; Skutella, T. Pluripotency potential of embryonic stem cell-like cells derived from mouse testis. Cell J. 2019, 21, 281–289. [Google Scholar]
- Golestaneh, N.; Kokkinaki, M.; Pant, D.; Jiang, J.; DeStefano, D.; Fernandez-Bueno, C.; Rone, J.D.; Haddad, B.R.; Gallicano, G.I.; Dym, M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009, 18, 1115–1125. [Google Scholar] [CrossRef] [Green Version]
- Kanatsu-Shinohara, M.; Inoue, K.; Lee, J.; Yoshimoto, M.; Ogonuki, N.; Miki, H.; Baba, S.; Kato, T.; Kazuki, Y.; Toyokuni, S.; et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell 2004, 119, 1001–1012. [Google Scholar] [CrossRef] [Green Version]
- Guan, K.; Nayernia, K.; Maier, L.S.; Wagner, S.; Dressel, R.; Jae, H.L.; Nolte, J.; Wolf, F.; Li, M.; Engel, W.; et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 2006, 440, 1199–1203. [Google Scholar] [CrossRef]
- Seandel, M.; James, D.; Shmelkov, S.V.; Falciatori, I.; Kim, J.; Chavala, S.; Scherr, D.S.; Zhang, F.; Torres, R.; Gale, N.W.; et al. Generation of functional multipotent adult stem cells from GPR125 + germline progenitors. Nature 2007, 449, 346–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossack, N.; Meneses, J.; Shefi, S.; Nguyen, H.N.; Chavez, S.; Nicholas, C.; Gromoll, J.; Turek, P.J.; Reijo-Pera, R.A. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells 2009, 27, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hong, F.; Wang, Z.; Hao, D.; Yang, H. Spermatogonial stem cells are a promising and pluripotent cell source for regenerative medicine. Am. J. Transl. Res. 2020, 12, 7048–7059. [Google Scholar] [PubMed]
- Awang-Junaidi, A.H.; Fayaz, M.A.; Kawamura, E.; Sobchishin, L.; MacPhee, D.; Honaramooz, A. Live-cell imaging and ultrastructural analysis reveal remarkable features of cultured porcine gonocytes. Cell Tissue Res. 2020, 381, 361–377. [Google Scholar] [CrossRef]
- Ko, K.; Tapia, N.; Wu, G.; Kim, J.B.; Bravo, M.J.A.; Sasse, P.; Glaser, T.; Ruau, D.; Han, D.W.; Greber, B.; et al. Induction of Pluripotency in Adult Unipotent Germline Stem Cells. Cell Stem Cell 2009, 5, 87–96. [Google Scholar] [CrossRef]
- Holstein, A.F.; Maekawa, M.; Nagano, T.; Davidoff, M.S. Myofibroblasts in the lamina propria of human seminiferous tubules are dynamic structures of heterogeneous phenotype. Arch. Histol. Cytol. 1996, 59, 109–125. [Google Scholar] [CrossRef] [Green Version]
- Fayaz, M.A.; Honaramooz, A. Effects of different culture conditions on proliferation, colony-formation, and pluripotency state of porcine male germ cells. Reprod. Fertil. Dev. 2021, in press. [Google Scholar]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernig, M.; Meissner, A.; Foreman, R.; Brambrink, T.; Ku, M.; Hochedlinger, K.; Bernstein, B.E.; Jaenisch, R. In Vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 2007, 448, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoi, T.; Yae, K.; Nakagawa, M.; Ichisaka, T.; Okita, K.; Takahashi, K.; Chiba, T.; Yamanaka, S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 2008, 321, 699–702. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.B.; Zaehres, H.; Wu, G.; Gentile, L.; Ko, K.; Sebastiano, V.; Araúzo-Bravo, M.J.; Ruau, D.; Han, D.W.; Zenke, M. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 2008, 454, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hao, D.; Liu, C.; Huang, D.; Chen, B.; Fan, H.; Liu, C.; Zhang, L.; Zhang, Q.; An, J. Generation of functional dopaminergic neurons from human spermatogonial stem cells to rescue parkinsonian phenotypes. Stem Cell Res. Ther. 2019, 10, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Porath, I.; Thomson, M.W.; Carey, V.J.; Ge, R.; Bell, G.W.; Regev, A.; Weinberg, R.A. An embryonic stem cell–like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008, 40, 499–507. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and safety issues of stem cell-based therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awang-Junaidi, A.H.; Honaramooz, A. Optimization of culture conditions for short-term maintenance, proliferation, and colony formation of porcine gonocytes. J. Anim. Sci. Biotechnol. 2018, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yarahmadi, M.; Honaramooz, A. Development of novel strategies for the isolation of piglet testis cells with a high proportion of gonocytes. Reprod. Fertil. Dev. 2010, 22, 1057–1065. [Google Scholar] [CrossRef]
- Virtanen, P.; Isotupa, K. Staining properties of alizarin red S for growing bone in vitro. Cells Tissues Organs 1980, 108, 202–207. [Google Scholar] [CrossRef]
- Silva, M.J.; Brodt, M.D.; Ko, M.; Abu-Amer, Y. Impaired marrow osteogenesis is associated with reduced endocortical bone formation but does not impair periosteal bone formation in long bones of SAMP6 mice. J. Bone Miner. Res. 2005, 20, 419–427. [Google Scholar] [CrossRef]
- Zeineddine, D.; Abou Hammoud, A.; Mortada, M.; Boeuf, H. The Oct4 protein: More than a magic stemness marker. Am. J. Stem Cells 2014, 3, 74–82. [Google Scholar]
- Pan, G.J.; Chang, Z.Y.; Schöler, H.R.; Duanqing, P.E.I. Stem cell pluripotency and transcription factor Oct4. Cell Res. 2002, 12, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Schöler, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Goel, S.; Sugimoto, M.; Minami, N.; Yamada, M.; Kume, S.; Imai, H. Identification, isolation, and in vitro culture of porcine gonocytes. Biol. Reprod. 2007, 77, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, M.A.; Awang-Junaidi, A.H.; Singh, J.; Honaramooz, A. Validation of ultrasound biomicroscopy for the assessment of xenogeneic testis tissue grafts and cell implants in recipient mice. Andrology 2020, 8, 1332–1346. [Google Scholar] [CrossRef]
- Fayaz, M.A.; Awang-Junaidi, A.H.; Singh, J.; Honaramooz, A. Long-term monitoring of donor xenogeneic testis tissue grafts and cell implants in recipient mice using ultrasound biomicroscopy. Ultrasound Med. Biol. 2020, 46, 3088–3103. [Google Scholar] [CrossRef]
- Awang-Junaidi, A.H.; Singh, J.; Honaramooz, A. Regeneration of testis tissue after ectopic implantation of porcine testis cell aggregates in mice: Improved consistency of outcomes and in situ monitoring. Reprod. Fertil. Dev. 2020, 32, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried alive: How osteoblasts become osteocytes. Dev. Dyn. an Off. Publ. Am. Assoc. Anat. 2006, 235, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J. Osteoblasts: Novel roles in orchestration of skeletal architecture. Int. J. Biochem. Cell Biol. 2003, 35, 1301–1305. [Google Scholar] [CrossRef]
- Orriss, I.R.; Taylor, S.E.B.; Arnett, T.R. Rat osteoblast cultures. Bone Res. Protoc. 2012, 816, 31–41. [Google Scholar]
- Yin, X.; Chen, Z.; Liu, Z.; Dang, G. Icariine stimulates proliferation and differentiation of human osteoblasts by increasing production of bone morphogenetic protein 2. Chin. Med. J. 2007, 120, 204–210. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.-Y.; Tian, Z.; Xiao, X.-J.; Xu, Q.; Wei, C.-X.; Sun, H.-C.; Chen, G.-H. Directional differentiation of chicken spermatogonial stem cells in vitro. Cytotherapy 2010, 12, 326–331. [Google Scholar] [CrossRef]
- Qasemi-Panahi, B.; Tajik, P.; Movahedin, M.; Moghaddam, G.; Barzgar, Y.; Heidari-Vala, H. Differentiation of bovine spermatogonial stem cells into osteoblasts. Avicenna J. Med. Biotechnol. 2011, 3, 149–153. [Google Scholar]
- Parikka, V.; Väänänen, A.; Risteli, J.; Salo, T.; Sorsa, T.; Väänänen, H.K.; Lehenkari, P. Human mesenchymal stem cell derived osteoblasts degrade organic bone matrix in vitro by matrix metalloproteinases. Matrix Biol. 2005, 24, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Hanna, H.; Mir, L.M.; Andre, F.M. In Vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res. Ther. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.; Stoop, H.; Gillis, A.J.M.; Van Gurp, R.; van de Geijn, G.; de Boer, M.; Hersmus, R.; Saunders, P.T.K.; Anderson, R.A.; Oosterhuis, J.W. Differential expression of SOX17 and SOX2 in germ cells and stem cells has biological and clinical implications. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2008, 215, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Niwa, H.; Iwase, K.; Takiguchi, M.; Mori, M.; Abé, S.-I.; Abe, K.; Yamamura, K.-I. Endoderm-specific gene expression in embryonic stem cells differentiated to embryoid bodies. Exp. Cell Res. 1996, 229, 27–34. [Google Scholar] [CrossRef]
- Bernardo, A.S.; Faial, T.; Gardner, L.; Niakan, K.K.; Ortmann, D.; Senner, C.E.; Callery, E.M.; Trotter, M.W.; Hemberger, M.; Smith, J.C. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell 2011, 9, 144–155. [Google Scholar] [CrossRef] [Green Version]
- Babai, F.; Musevi-Aghdam, J.; Schurch, W.; Royal, A.; Gabbiani, G. Coexpression of α-sarcomeric actin, α-smooth muscle actin and desmin during myogenesis in rat and mouse embryos I. Skeletal muscle. Differentiation 1990, 44, 132–142. [Google Scholar] [CrossRef]
- Eng, L.F. Glial fibrillary acidic protein (GFAP): The major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 1985, 8, 203–214. [Google Scholar] [CrossRef]
- Ang, S.-L.; Conlon, R.A.; Jin, O.; Rossant, J. Positive and negative signals from mesoderm regulate the expression of mouse Otx2 in ectoderm explants. Development 1994, 120, 2979–2989. [Google Scholar] [CrossRef]
- Mortensen, A.H.; Schade, V.; Lamonerie, T.; Camper, S.A. Deletion of OTX2 in neural ectoderm delays anterior pituitary development. Hum. Mol. Genet. 2015, 24, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Mao, J.; Song, L.; Fan, A.; Zhang, S.; Wang, J.; Fan, N.; Liu, N.; Ye, X.; Fu, H. DNA repair and replication links to pluripotency and differentiation capacity of pig iPS cells. PLoS ONE 2017, 12, e0173047. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.; Yu, Y.; Zhang, H.; Wei, R.; Lv, J.; Cai, M.; Yang, X.; Zhang, Y.; Liu, Z. Derivation of porcine extraembryonic endoderm-like cells from blastocysts. Cell Prolif. 2020, 53, e12782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Kim, C.-H.; Moon, J.-I.; Chung, Y.-G.; Chang, M.-Y.; Han, B.-S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009, 4, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauritz, C.; Schwanke, K.; Reppel, M.; Neef, S.; Katsirntaki, K.; Maier, L.S.; Nguemo, F.; Menke, S.; Haustein, M.; Hescheler, J. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 2008, 118, 507. [Google Scholar] [CrossRef] [Green Version]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef] [Green Version]
- Acampora, D.; Di Giovannantonio, L.G.; Simeone, A. Otx2 is an intrinsic determinant of the embryonic stem cell state and is required for transition to a stable epiblast stem cell condition. Development 2013, 140, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, D.; Ohmura, T.; Sakurai, Y.; Inoue, K.; Suda, Y.; Aizawa, S. Otx2 expression in anterior neuroectoderm and forebrain/midbrain is directed by more than six enhancers. Dev. Biol. 2014, 387, 203–213. [Google Scholar] [CrossRef]
- Irie, N.; Weinberger, L.; Tang, W.W.C.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015, 160, 253–268. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Li, Z.; Zhao, Q.; Xiong, C.; Ni, K. Analysis of multi-lineage gene expression dynamics during primordial germ cell induction from human induced pluripotent stem cells. Stem Cell Res. Ther. 2020, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Viotti, M.; Niu, L.; Shi, S.-H.; Hadjantonakis, A.-K. Role of the gut endoderm in relaying left-right patterning in mice. PLoS Biol. 2012, 10, e1001276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostwick, D.G. Chapter 13—Immunohistology of the Prostate, Bladder, Testis and Kidney, 2nd ed.; Dabbs, D.J., Ed.; Churchill Livingstone: London, UK, 2006. [Google Scholar]
- Diekmann, U.; Wolling, H.; Dettmer, R.; Niwolik, I.; Naujok, O.; Buettner, F.F.R. Chemically defined and xenogeneic-free differentiation of human pluripotent stem cells into definitive endoderm in 3D culture. Sci. Rep. 2019, 9, 996. [Google Scholar] [CrossRef]
- Qu, S.; Yan, L.; Fang, B.; Ye, S.; Li, P.; Ge, S.; Wu, J.; Qu, D.; Song, H. Generation of enhanced definitive endoderm from human embryonic stem cells under an albumin/insulin-free and chemically defined condition. Life Sci. 2017, 175, 37–46. [Google Scholar] [CrossRef]
- Xu, X.; Browning, V.L.; Odorico, J.S. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech. Dev. 2011, 128, 412–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, S.; Chen, S.; Clark, J.; Hao, E.; Beattie, G.M.; Hayek, A.; Ding, S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc. Natl. Acad. Sci. USA 2006, 103, 6907–6912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barresi, V.; Ieni, A.; Branca, G.; Tuccari, G. Brachyury: A diagnostic marker for the differential diagnosis of chordoma and hemangioblastoma versus neoplastic histological mimickers. Dis. Markers 2014, 2014, 514753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Goodyear, S.M.; Tobias, J.W.; Avarbock, M.R.; Brinster, R.L. Spermatogonial stem cell self-renewal requires ETV5-mediated downstream activation of Brachyury in mice. Biol. Reprod. 2011, 85, 1114–1123. [Google Scholar] [CrossRef]
- Baba, S.; Heike, T.; Umeda, K.; Iwasa, T.; Kaichi, S.; Hiraumi, Y.; Doi, H.; Yoshimoto, M.; Kanatsu-Shinohara, M.; Shinohara, T. Generation of cardiac and endothelial cells from neonatal mouse testis-derived multipotent germline stem cells. Stem Cells 2007, 25, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Schlatt, S.; Van Pelt, A.; Neuhaus, N. Characterization and population dynamics of germ cells in adult macaque testicular cultures. PLoS ONE 2019, 14, e0218194. [Google Scholar] [CrossRef]
- Langenstroth, D.; Kossack, N.; Westernströer, B.; Wistuba, J.; Behr, R.; Gromoll, J.; Schlatt, S. Separation of somatic and germ cells is required to establish primate spermatogonial cultures. Hum. Reprod. 2014, 29, 2018–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zohar, R.; McCulloch, C.A. Multiple roles of α-smooth muscle actin in mechanotransduction. Exp. Cell Res. 2006, 312, 205–214. [Google Scholar] [CrossRef]
- Boyl, P.P.; Signore, M.; Acampora, D.; Martinez-Barbera, J.P.; Ilengo, C.; Annino, A.; Corte, G.; Simeone, A. Forebrain and midbrain development requires epiblast-restricted Otx2 translational control mediated by its 3′ UTR. Development 2001, 128, 2989–3000. [Google Scholar] [CrossRef]
- Beby, F.; Lamonerie, T. The homeobox gene Otx2 in development and disease. Exp. Eye Res. 2013, 111, 9–16. [Google Scholar] [CrossRef]
- Li, J.Y.H.; Joyner, A.L. Otx2 and Gbx2 are required for refinement and not induction of mid-hindbrain gene expression. Development 2001, 128, 4979–4991. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Y.; Xie, Y.; Wang, H. OTX2 impedes self–renewal of porcine iPS cells through downregulation of NANOG expression. Cell death Discov. 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, E.P.; Lanza, D.G.; Webster, N.J.; Benton, S.M.; Suetake, I.; Heaney, J.D. Delayed male germ cell sex-specification permits transition into embryonal carcinoma cells with features of primed pluripotency. Development 2018, 145, dev156612. [Google Scholar] [CrossRef] [Green Version]
- Davidoff, M.S.; Middendorff, R.; Köfüncü, E.; Müller, D.; Ježek, D.; Holstein, A.-F. Leydig cells of the human testis possess astrocyte and oligodendrocyte marker molecules. Acta Histochem. 2002, 104, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Haber, P.S.; Applegate, T.L.; Norton, I.D.; McCaughan, G.W.; Korsten, M.A.; Pirola, R.C.; Wilson, J.S. Periacinar stellate shaped cells in rat pancreas: Identification, isolation, and culture. Gut 1998, 43, 128–133. [Google Scholar] [CrossRef]
- Milose, J.C.; Filson, C.P.; Weizer, A.Z.; Hafez, K.S.; Montgomery, J.S. Role of biochemical markers in testicular cancer: Diagnosis, staging, and surveillance. Open Access J. Urol. 2012, 4, 1–8. [Google Scholar]
- Jacobsen, G.K. Alpha-fetoprotein (AFP) and human chorionic gonadotropin (HCG) in testicular germ cell tumours: A comparison of histologic and serologic occurrence of tumour markers. Acta Pathol. Microbiol. Scand. Ser. A Pathol. 1983, 91, 183–190. [Google Scholar] [CrossRef]
- Lempiäinen, A.; Sankila, A.; Hotakainen, K.; Haglund, C.; Blomqvist, C.; Stenman, U.-H. Expression of human chorionic gonadotropin in testicular germ cell tumors. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Mosbech, C.H.; Svingen, T.; Nielsen, J.E.; Toft, B.G.; Rechnitzer, C.; Petersen, B.L.; Rajpert-De Meyts, E.; Hoei-Hansen, C.E. Expression pattern of clinically relevant markers in paediatric germ cell-and sex-cord stromal tumours is similar to adult testicular tumours. Virchows Arch. 2014, 465, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Scandura, G.; Roe, A.; Beltran, L.; Shamash, J.; Alfrangis, C.; Daugaard, G.; Grantham, M.; Berney, D. Prospective molecular and morphological assessment of testicular prepubertal-type teratomas in postpubertal men. Mod. Pathol. 2020, 33, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wakao, S.; Kitada, M.; Kuroda, Y.; Ogura, F.; Murakami, T.; Niwa, A.; Dezawa, M. Morphologic and gene expression criteria for identifying human induced pluripotent stem cells. PLoS ONE 2012, 7, e48677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, Y.; Komiyama, M.; Miyata, H.; Yagoto, M.; Ashizawa, T.; Iizuka, A.; Oshita, C.; Kume, A.; Nogami, M.; Ito, I. Novel cancer-testis antigen expression on glioma cell lines derived from high-grade glioma patients. Oncol. Rep. 2014, 31, 1683–1690. [Google Scholar] [CrossRef]
- Gu, S.; Wu, Y.-M.; Hong, L.; Zhang, Z.-D.; Yin, M.-Z. Glial fibrillary acidic protein expression is an indicator of teratoma maturation in children. World J. Pediatr. 2011, 7, 262–265. [Google Scholar] [CrossRef]
- Sundström, J.; Pelliniemi, L.J.; Kuopio, T.; Veräjänkorva, E.; Fröjdman, K.; Harley, V.; Salminen, E.; Pöllänen, P. Characterization of the model for experimental testicular teratoma in 129/SvJ-mice. Br. J. Cancer 1999, 80, 149–160. [Google Scholar] [CrossRef]
- Singh, V.K.; Saini, A.; Kalsan, M.; Kumar, N.; Chandra, R. Describing the stem cell potency: The various methods of functional assessment and in silico diagnostics. Front. Cell Dev. Biol. 2016, 4, 134. [Google Scholar] [CrossRef] [Green Version]
- Goel, S.; Fujihara, M.; Tsuchiya, K.; Takagi, Y.; Minami, N.; Yamada, M.; Imai, H. Multipotential ability of primitive germ cells from neonatal pig testis cultured in vitro. Reprod. Fertil. Dev. 2009, 21, 696–708. [Google Scholar] [CrossRef] [Green Version]







| Antibody | Supplier | Catalogue No. | Dilution |
|---|---|---|---|
| Rabbit anti-POU5F1 | Abcam | AB18976 | 1:200 |
| Goat anti-OTX2 | R&D Systems | SC031B | 1:200 |
| Mouse anti-GFAP | Novus Biological | NBP1-05197SS | 1:200 |
| Goat anti-Brachyury | R&D Systems | SC030B | 1:200 |
| Mouse anti-ASM | Novus Biological | NBP1-33006 | 1:200 |
| Goat anti-SOX17 | R&D Systems | SC019B | 1:200 |
| Rabbit anti-AFP | Novus Biological | NBP1-76275 | 1:200 |
| Alexa Fluor 594 goat anti-rabbit | Abcam | AB150088 | 1:200 |
| Alexa Fluor 594 goat anti-mouse | Abcam | 150116 | 1:200 |
| Alexa Fluor 594 rabbit anti-goat | Abcam | 150148 | 1:200 |
| Target Name | Direction | Primer Sequence (5′-3′) | Annealing Temperature (°C) | Product Size (BP) |
|---|---|---|---|---|
| POU5F1 | Forward | AGAGAAAGCGGACAAGTA | 51.7 | 299 |
| Reverse | ATCCTCTCGTTGCGAATA | |||
| OTX2 | Forward | TTTATCTGGTCTCTCTCCCTCTC | 61 | 129 |
| Reverse | GTTAGTGGTGGAAAGTGGTAGG | |||
| GFAP | Forward | CAGAGCAGGACCGAGTTTATG | 61 | 117 |
| Reverse | CATAAAGAGAAGAGGGAAGGACAG | |||
| TBXT | Forward | GGGATTTGCTTCTGGGTCTAA | 63.7 | 124 |
| Reverse | GTTGAGAAGTCACTGGACAGAG | |||
| ACTA2 | Forward | CTGGGTCTGAGTCTTAGCTTTC | 63.7 | 114 |
| Reverse | GATAGGATGGCTGTGTGGATT | |||
| SOX17 | Forward | CATCTCAAGTGACCCTAGTCTTTAC | 61 | 136 |
| Reverse | GTTGAATCTTGAGGTCTGCCT | |||
| AFP | Forward | GCTCCATCTCCTTGCTTTCT | 66.1 | 100 |
| Reverse | AAGAGATGCCCATAAACCCTG | |||
| GAPDH | Forward | TCGGAGTGAACGGATTTG | 62 | 219 |
| Reverse | CCTGGAAGATGGTGATGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayaz, M.A.; Rosa, G.d.S.; Honaramooz, A. Neonatal Porcine Germ Cells Dedifferentiate and Display Osteogenic and Pluripotency Properties. Cells 2021, 10, 2816. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10112816
Fayaz MA, Rosa GdS, Honaramooz A. Neonatal Porcine Germ Cells Dedifferentiate and Display Osteogenic and Pluripotency Properties. Cells. 2021; 10(11):2816. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10112816
Chicago/Turabian StyleFayaz, Mohammad Amin, Gustavo dos Santos Rosa, and Ali Honaramooz. 2021. "Neonatal Porcine Germ Cells Dedifferentiate and Display Osteogenic and Pluripotency Properties" Cells 10, no. 11: 2816. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10112816