Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials
Abstract
:1. Introduction
2. Retinal Degenerative Diseases
3. Perspectives of Cell Therapy for Retinal Diseases
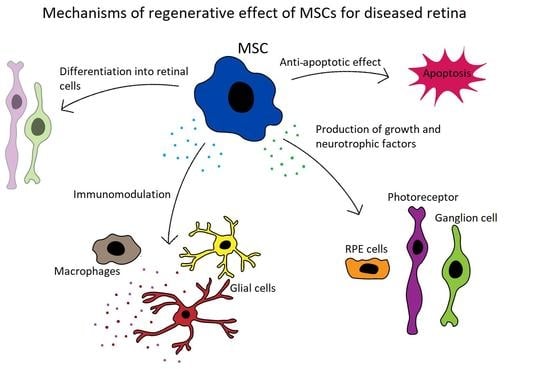
4. Mesenchymal Stem Cells
4.1. Characteristics of MSCs
4.2. Immunoregulatory and Anti-Inflammatory Properties of MSCs
4.3. Antiapoptotic Properties of MSCs
4.4. The Production of Growth Factors by MSCs
4.5. The Ability of MSCs to Differentiate into Cells with Retinal Cell Characteristics
4.6. Additional Mechanisms Contributing to the Therapeutic Action of MSCs
5. The Potential of MSCs for the Treatment of Retinal Diseases
6. Possible Problems and Limitations Associated with MSC-Based Therapy
7. The Use of MSCs for the Treatment of Retinal Diseases in Experimental Models
7.1. Experimental Models of AMD
7.2. Experimental Models of DR
7.3. Experimental Models for RP
7.4. Experimental Models for Glaucoma
8. Clinical Trials Using MSCs for Retinal Diseases
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol. Sci. 2016, 3, 196–221. [Google Scholar] [CrossRef]
- Semeraro, F.; Cancarini, A.; Dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Van Norren, D.; Vos, J.J. Light damage to the retina: An historical approach. Eye 2016, 30, 169–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiger, S.P.; Sullivan, L.S.; Bowne, S.J. Genes and mutations causing retinitis pigmentosa. Clin. Genet. 2013, 84, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.P.; Binkley, E.; Flamme-Wiese, M.J.; Zeng, S.; DeLuca, A.P.; Scheetz, T.E.; Tucker, B.A.; Mullins, R.F.; Stone, E.M. Single-Cell RNA Sequencing in Human Retinal Degeneration Reveals Distinct Glial Cell Populations. Cells 2020, 9, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, D.H.; Yun, J.-H.; Cho, C.S.; Kim, J.H.; Kim, J.H.; Cho, C.-H. Interaction between microglia and retinal pigment epithelial cells determines the integrity of outer blood-retinal barrier in diabetic retinopathy. Glia 2019, 67, 321–331. [Google Scholar] [CrossRef]
- Menon, M.; Mohammadi, S.; Davila-Velderrain, J.; Goods, B.A.; Cadwell, T.D.; Xing, Y.; Stemmer-Rachamimov, A.; Shalek, A.K.; Love, J.C.; Kellis, M.; et al. Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, J.; Liu, X.; Kokona, D.; Zinkernagel, M.S.; Ebneter, A. Inhibition of inflammatory cells delays retinal degeneration in experimental retinal vein occlusion in mice. Glia 2019, 68, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.K.; Lu, B.; Girman, S.; Wang, S. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog. Retin. Eye Res. 2017, 58, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.M.; Mendonça, L.; Brant, R.; Abud, M.; Regatieri, C.; Diniz, B. Stem cell therapy for retinal diseases. World J. Stem Cells 2015, 7, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alonso, M.L.; Srivastava, G.K. Current focus of stem cell application in retinal repair. World J. Stem Cells 2015, 7, 641–648. [Google Scholar] [CrossRef]
- Chen, M.; Xiang, Z.; Cai, J. The anti-apoptotic and neuro-protective effects of human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) on acute optic nerve injury is transient. Brain Res. 2013, 1532, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Mesentier-Louro, L.A.; Zaverucha-Do-Valle, C.; Da Silva-Junior, A.J.; Nascimento-Dos-Santos, G.; Gubert, F.; De Figueirêdo, A.B.P.; Torres, A.L.; Paredes, B.D.; Teixeira, C.; Tovar-Moll, F.; et al. Distribution of Mesenchymal Stem Cells and Effects on Neuronal Survival and Axon Regeneration after Optic Nerve Crush and Cell Therapy. PLoS ONE 2014, 9, e110722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, P.; Xu, H.; Zeng, Y.; Wang, Y.; Yin, Z.Q. Human Bone Marrow Stromal Cells can Differentiate to a Retinal Pigment Epithelial Phenotype when Co-Cultured with Pig Retinal Pigment Epithelium using a Transwell System. Cell. Physiol. Biochem. 2013, 31, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Rezanejad, H.; Soheili, Z.-S.; Haddad, F.; Matin, M.M.; Samiei, S.; Manafi, A.; Ahmadieh, H. In vitro differentiation of adipose-tissue-derived mesenchymal stem cells into neural retinal cells through expression of human PAX6 (5a) gene. Cell Tissue Res. 2014, 356, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Hermankova, B.; Kossl, J.; Javorkova, E.; Bohacova, P.; Hajkova, M.; Zajicova, A.; Krulova, M.; Holan, V. The Identification of Interferon-γ as a Key Supportive Factor for Retinal Differentiation of Murine Mesenchymal Stem Cells. Stem Cells Dev. 2017, 26, 1399–1408. [Google Scholar] [CrossRef]
- Le Blanc, K.; Ringdén, O. Immunomodulation by mesenchymal stem cells and clinical experience. J. Intern. Med. 2007, 262, 509–525. [Google Scholar] [CrossRef]
- Abumaree, M.; Al Jumah, M.; Pace, R.A.; Kalionis, B. Immunosuppressive Properties of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2011, 8, 375–392. [Google Scholar] [CrossRef]
- Reinshagen, H.; Sorg, R.V.; Boehringer, D.; Eberwein, P.; Sundmacher, R.; Reinhard, T.; Auw-Haedrich, C.; Schwartzkopff, J. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2009, 89, 741–748. [Google Scholar] [CrossRef]
- Holan, V.; Javorkova, E. Mesenchymal Stem Cells, Nanofiber Scaffolds and Ocular Surface Reconstruction. Stem Cell Rev. Rep. 2013, 9, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Čejková, J.; Trosan, P.; Čejka, Č.; Lencova, A.; Zajicova, A.; Javorkova, E.; Kubinová, Š.; Syková, E.; Holan, V. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp. Eye Res. 2013, 116, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Holan, V.; Trosan, P.; Cejka, C.; Javorkova, E.; Zajicova, A.; Hermankova, B.; Chudickova, M.; Cejkova, J. A Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells and Limbal Epithelial Stem Cells for Ocular Surface Reconstruction. Stem Cells Transl. Med. 2015, 4, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Foulsham, W.; Amouzegar, A.; Mittal, S.K.; Chauhan, S.K. The therapeutic application of mesenchymal stem cells at the ocular surface. Ocul. Surf. 2019, 17, 198–207. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.-B.; Ma, J.; Zuo, C.; Geng, J.; Gong, W.; Sun, Y.; Li, H.; Wang, B.; Zhang, L.; et al. A Toxicity Study of Multiple-Administration Human Umbilical Cord Mesenchymal Stem Cells in Cynomolgus Monkeys. Stem Cells Dev. 2012, 21, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Canadian Critical Care Trials Group. Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Phinney, D.G.; Prockop, D.J. Concise Review: Mesenchymal Stem/Multipotent Stromal Cells: The State of Transdifferentiation and Modes of Tissue Repair-Current Views. Stem Cells 2007, 25, 2896–2902. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Musina, R.A.; Bekchanova, E.S.; Sukhikh, G.T. Comparison of Mesenchymal Stem Cells Obtained from Different Human Tissues. Bull. Exp. Biol. Med. 2005, 139, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or Not the Same? Comparison of Adipose Tissue-Derived Versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Isobe, Y.; Koyama, N.; Nakao, K.; Osawa, K.; Ikeno, M.; Yamanaka, S.; Okubo, Y.; Fujimura, K.; Bessho, K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral Maxillofac. Surg. 2016, 45, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, I.; Ringdén, O.; Sundberg, B.; Le Blanc, K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp. Cell Res. 2005, 305, 33–41. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, L.; Ren, G.; Yuan, Z.; Zhang, Y.; Zhao, R.C.; Shi, Y. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007, 17, 240–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Jiao, C.; Zhao, S.; Li, X.; Ren, X.; Zhang, L.; Han, Z.C.; Zhang, X. Immunomodulatory effects of mesenchymal stem cells in a rat corneal allograft rejection model. Exp. Eye Res. 2012, 102, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Lee, R.H.; Yu, J.M.; Ko, J.H.; Lee, H.J.; Ko, A.Y.; Roddy, G.W.; Prockop, D.J. Intravenous Mesenchymal Stem Cells Prevented Rejection of Allogeneic Corneal Transplants by Aborting the Early Inflammatory Response. Mol. Ther. 2012, 20, 2143–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Lazarus, H.M.; Koc, O.N.; Devine, S.M.; Curtin, P.; Maziarz, R.T.; Holland, H.K.; Shpall, E.J.; McCarthy, P.; Atkinson, K.; Cooper, B.W.; et al. Cotransplantation of HLA-Identical Sibling Culture-Expanded Mesenchymal Stem Cells and Hematopoietic Stem Cells in Hematologic Malignancy Patients. Biol. Blood Marrow Transplant. 2005, 11, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.-J.; Zhang, F.-J.; Zhang, L.; Geng, Y.-Q.; Li, Q.-G.; Hong, Q.; Fu, B.; Zhu, F.; Cui, S.-Y.; Feng, Z.; et al. Mesenchymal Stem Cells Ameliorate Sepsis-associated Acute Kidney Injury in Mice. Shock 2014, 41, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Zappia, E.; Casazza, S.; Pedemonte, E.; Benvenuto, F.; Bonanni, I.; Gerdoni, E.; Giunti, D.; Ceravolo, A.; Cazzanti, F.; Frassoni, F.; et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005, 106, 1755–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augello, A.; Tasso, R.; Negrini, S.M.; Cancedda, R.; Pennesi, G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007, 56, 1175–1186. [Google Scholar] [CrossRef]
- English, K.; Ryan, J.M.; Tobin, L.M.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E2and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25Highforkhead box P3+regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase–mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-Stem-Cell-Induced Immunoregulation Involves FAS-Ligand-/FAS-Mediated T Cell Apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Ghannam, S.; Pène, J.; Torcy-Moquet, G.; Jorgensen, C.; Yssel, H. Mesenchymal Stem Cells Inhibit Human Th17 Cell Differentiation and Function and Induce a T Regulatory Cell Phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Svobodova, E.; Krulova, M.; Zajicova, A.; Pokorna, K.; Prochazkova, J.; Trosan, P.; Holan, V. The Role of Mouse Mesenchymal Stem Cells in Differentiation of Naive T-Cells into Anti-Inflammatory Regulatory T-Cell or Proinflammatory Helper T-Cell 17 Population. Stem Cells Dev. 2012, 21, 901–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holan, V.; Hermankova, B.; Bohacova, P.; Kossl, J.; Chudickova, M.; Hajkova, M.; Krulova, M.; Zajicova, A.; Javorkova, E. Distinct Immunoregulatory Mechanisms in Mesenchymal Stem Cells: Role of the Cytokine Environment. Stem Cell Rev. Rep. 2016, 12, 654–663. [Google Scholar] [CrossRef]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Kodati, S.; Lee, H.S.; Omoto, M.; Jin, Y.; Chauhan, S.K. Kinetics and Function of Mesenchymal Stem Cells in Corneal Injury. Investig. Opthalmol. Vis. Sci. 2012, 53, 3638–3644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assis, A.C.M.; Carvalho, J.L.; Jacoby, B.A.; Ferreira, R.L.B.; Castanheira, P.; Diniz, S.O.F.; Cardoso, V.N.; Goes, A.M.; Ferreira, A.J. Time-Dependent Migration of Systemically Delivered Bone Marrow Mesenchymal Stem Cells to the Infarcted Heart. Cell Transplant. 2010, 19, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Javorkova, E.; Trosan, P.; Zajicova, A.; Krulová, M.; Hajkova, M.; Holan, V. Modulation of the Early Inflammatory Microenvironment in the Alkali-Burned Eye by Systemically Administered Interferon-γ-Treated Mesenchymal Stromal Cells. Stem Cells Dev. 2014, 23, 2490–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holan, V.; Echalar, B.; Palacka, K.; Kossl, J.; Bohacova, P.; Krulova, M.; Brejchova, J.; Svoboda, P.; Zajicova, A. The Altered Migration and Distribution of Systemically Administered Mesenchymal Stem Cells in Morphine-Treated Recipients. Stem Cell Rev. Rep. 2021. [Google Scholar] [CrossRef]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Hérault, O.; Charbord, P.; Domenech, J. The In Vitro Migration Capacity of Human Bone Marrow Mesenchymal Stem Cells: Comparison of Chemokine and Growth Factor Chemotactic Activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J. Regulatory factors of mesenchymal stem cell migration into injured tissues and their signal transduction mechanisms. Front. Med. 2011, 5, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hermankova, B.; Kossl, J.; Bohacova, P.; Javorkova, E.; Hajkova, M.; Krulova, M.; Zajicova, A.; Holan, V. The Immunomodulatory Potential of Mesenchymal Stem Cells in a Retinal Inflammatory Environment. Stem Cell Rev. Rep. 2019, 15, 880–891. [Google Scholar] [CrossRef]
- Grunnet, L.G.; Aikin, R.; Tonnesen, M.F.; Paraskevas, S.; Blaabjerg, L.; Storling, J.; Rosenberg, L.; Billestrup, N.; Maysinger, D.; Mandrup-Poulsen, T. Proinflammatory Cytokines Activate the Intrinsic Apoptotic Pathway in -Cells. Diabetes 2009, 58, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhang, S.; Duan, H.; Dong, M.; Hu, X.; Zhang, Z.; Wang, Y.; Zhang, X.; Shi, W.; Zhou, Q. Different Effects of Pro-Inflammatory Factors and Hyperosmotic Stress on Corneal Epithelial Stem/Progenitor Cells and Wound Healing in Mice. Stem Cells Transl. Med. 2019, 8, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, A.M.; Di Zazzo, A.; Bonini, S.; Argüeso, P. Endoplasmic reticulum stress promotes inflammation-mediated proteolytic activity at the ocular surface. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khubutiya, M.S.; Vagabov, A.V.; Temnov, A.A.; Sklifas, A.N. Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy 2014, 16, 579–585. [Google Scholar] [CrossRef]
- Kossl, J.; Bohacova, P.; Hermankova, B.; Javorkova, E.; Zajicova, A.; Holan, V. Anti-Apoptotic Properties of Mesenchymal Stem Cells in a Mouse Model of Corneal Inflammation. Stem Cells Dev. 2021. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Aguiar, J.; Alberti, E.; De La Cuétara, K.; Pavón, N. Bone marrow stromal cells produce nerve growth factor and glial cell line-derived neurotrophic factors. Biochem. Biophys. Res. Commun. 2004, 316, 753–754. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W. Effects of Bone Marrow Mesenchymal Stem Cell Transplantation on Light-Damaged Retina. Investig. Opthalmol. Vis. Sci. 2010, 51, 3742–3748. [Google Scholar] [CrossRef] [PubMed]
- Zwart, I.; Hill, A.J.; Al-Allaf, F.; Shah, M.; Girdlestone, J.; Sanusi, A.B.; Mehmet, H.; Navarrete, R.; Navarrete, C.; Jen, L.-S. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp. Neurol. 2009, 216, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.D.S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Xu, G.; Guo, J. Light-induced retinal injury enhanced neurotrophins secretion and neurotrophic effect of mesenchymal stem cells in vitro. Arq. Bras. Oftalmol. 2013, 76, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, Y.; Iriyama, A.; Ueno, S.; Takahashi, H.; Kondo, M.; Tamaki, Y.; Araie, M.; Yanagi, Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp. Eye Res. 2007, 85, 234–241. [Google Scholar] [CrossRef]
- Notara, M.; Hernandez, D.; Mason, C.; Daniels, J.T. Characterization of the phenotype and functionality of corneal epithelial cells derived from mouse embryonic stem cells. Regen. Med. 2012, 7, 167–178. [Google Scholar] [CrossRef]
- Gu, S.; Xing, C.; Han, J.; Tso, M.O.; Hong, J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol. Vis. 2009, 15, 99–107. [Google Scholar]
- Jiang, T.-S.; Cai, L.; Ji, W.-Y.; Hui, Y.-N.; Wang, Y.-S.; Hu, D.; Zhu, J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol. Vis. 2010, 16, 1304–1316. [Google Scholar] [PubMed]
- Trosan, P.; Svobodova, E.; Chudickova, M.; Krulova, M.; Zajicova, A.; Holan, V. The Key Role of Insulin-Like Growth Factor I in Limbal Stem Cell Differentiation and the Corneal Wound-Healing Process. Stem Cells Dev. 2012, 21, 3341–3350. [Google Scholar] [CrossRef] [Green Version]
- Tropel, P.; Platet, N.; Platel, J.-C.; Noël, D.; Albrieux, M.; Benabid, A.-L.; Berger, F. Functional Neuronal Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells 2006, 24, 2868–2876. [Google Scholar] [CrossRef] [PubMed]
- Kicic, A.; Shen, W.-Y.; Wilson, A.S.; Constable, I.J.; Robertson, T.; Rakoczy, P.E. Differentiation of Marrow Stromal Cells into Photoreceptors in the Rat Eye. J. Neurosci. 2003, 23, 7742–7749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadri, S.; Kazemi, B.; Eeslaminejad, M.B.; Yazdani, S.; Soleimani, M.; Eslaminejad, M.B. High yield of cells committed to the photoreceptor-like cells from conjunctiva mesenchymal stem cells on nanofibrous scaffolds. Mol. Biol. Rep. 2013, 40, 3883–3890. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; Amirpour, N.; Razavi, S.; Esfandiari, E.; Zavar, R. Overview of retinal differentiation potential of mesenchymal stem cells: A promising approach for retinal cell therapy. Ann. Anat. Anat. Anz. 2017, 210, 52–63. [Google Scholar] [CrossRef]
- Castanheira, P.; Torquetti, L.; Nehemy, M.B.; Goes, A.M. Retinal incorporation and differentiation of mesenchymal stem cells intravitreally injected in the injured retina of rats. Arq. Bras. Oftalmol. 2008, 71, 644–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, D.-M.; Dong, F.-T.; Yu, W.-H.; Gao, F. Differentiation of mesenchymal stem cell in the microenviroment of retinitis pigmentosa. Int. J. Ophthalmol. 2010, 3, 216–219. [Google Scholar] [PubMed]
- Huang, C.; Zhang, J.; Ao, M.; Li, Y.; Zhang, C.; Xu, Y.; Li, X.; Wang, W. Combination of retinal pigment epithelium cell-conditioned medium and photoreceptor outer segments stimulate mesenchymal stem cell differentiation toward a functional retinal pigment epithelium cell phenotype. J. Cell. Biochem. 2011, 113, 590–598. [Google Scholar] [CrossRef]
- Mathivanan, I.; Trepp, C.M.; Brunold, C.; Baerlocher, G.M.; Enzmann, V. Retinal differentiation of human bone marrow-derived stem cells by co-culture with retinal pigment epithelium in vitro. Exp. Cell Res. 2015, 333, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Croitoru-Lamoury, J.; Lamoury, F.M.J.; Caristo, M.; Suzuki, K.; Walker, D.; Takikawa, O.; Taylor, R.; Brew, B.J. Interferon-γ Regulates the Proliferation and Differentiation of Mesenchymal Stem Cells via Activation of Indoleamine 2,3 Dioxygenase (IDO). PLoS ONE 2011, 6, e14698. [Google Scholar] [CrossRef] [Green Version]
- Wong, G.; Goldshmit, Y.; Turnley, A.M. Interferon-γ but not TNFα promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp. Neurol. 2004, 187, 171–177. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adak, S.; Magdalene, D.; Deshmukh, S.; Das, D.; Jaganathan, B.G. A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Rev. Rep. 2021, 1–20. [Google Scholar] [CrossRef]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, srep34562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, S.; Chaudhuri, R.; Agrawal, A.; Mohanty, S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 2018, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eells, J.T. Mitochondrial Dysfunction in the Aging Retina. Biology 2019, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Freeman, B.T.; Kouris, N.A.; Ogle, B.M. Tracking Fusion of Human Mesenchymal Stem Cells After Transplantation to the Heart. Stem Cells Transl. Med. 2015, 4, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Azizi, Z.; Lange, C.; Paroni, F.; Ardestani, A.; Meyer, A.; Wu, Y.; Zander, A.R.; Westenfelder, C.; Maedler, K. β-MSCs: Successful fusion of MSCs with β-cells results in a β-cell like phenotype. Oncotarget 2016, 7, 48963–48977. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Li, K.; Yan, X.; Dong, F.; Zhao, C. Amelioration of diabetic retinopathy by engrafted human adipose-derived mesenchymal stem cells in streptozotocin diabetic rats. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1415–1422. [Google Scholar] [CrossRef]
- Mendel, T.A.; Clabough, E.B.D.; Kao, D.S.; Demidova-Rice, T.N.; Durham, J.T.; Zotter, B.C.; Seaman, S.A.; Cronk, S.M.; Rakoczy, E.P.; Katz, A.J.; et al. Pericytes Derived from Adipose-Derived Stem Cells Protect against Retinal Vasculopathy. PLoS ONE 2013, 8, e65691. [Google Scholar] [CrossRef]
- Rajashekhar, G.; Ramadan, A.; Abburi, C.; Callaghan, B.; Traktuev, D.O.; Evans-Molina, C.; Maturi, R.; Harris, A.; Kern, T.S.; March, K.L. Regenerative Therapeutic Potential of Adipose Stromal Cells in Early Stage Diabetic Retinopathy. PLoS ONE 2014, 9, e84671. [Google Scholar] [CrossRef]
- Ezquer, F.; Ezquer, M.; Conget, P.; Arango-Rodriguez, M. Could donor multipotent mesenchymal stromal cells prevent or delay the onset of diabetic retinopathy? Acta Ophthalmol. 2013, 92, e86–e95. [Google Scholar] [CrossRef] [Green Version]
- Mead, B.; Berry, M.; Logan, A.; Scott, R.A.; Leadbeater, W.; Scheven, B.A. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015, 14, 243–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holan, V.; Hermankova, B.; Kossl, J. Perspectives of Stem Cell–Based Therapy for Age-Related Retinal Degenerative Diseases. Cell Transplant. 2017, 26, 1538–1541. [Google Scholar] [CrossRef] [Green Version]
- Holan, V.; Hermankova, B.; Krulova, M.; Zajicova, A. Cytokine interplay among the diseased retina, inflammatory cells and mesenchymal stem cell-a clue to stem cell-based therapy. World J. Stem Cells 2019, 11, 957–967. [Google Scholar] [CrossRef]
- Park, S.S.; Moisseiev, E.; Bauer, G.; Anderson, J.D.; Grant, M.B.; Zam, A.; Zawadzki, R.J.; Werner, J.S.; Nolta, J.A. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog. Retin. Eye Res. 2017, 56, 148–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, T.K.; Fortino, V.R.; Pelaez, D.; Cheung, H.S. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J. Stem Cells 2014, 6, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Alvarez-Sanchez, S.; González-Zamora, J.; Carretero-Barrio, I.; Pastor, J.C.; Fernandez-Bueno, I.; Srivastava, G.K. Mesenchymal stem cell therapy in retinal and optic nerve diseases: An update of clinical trials. World J. Stem Cells 2016, 8, 376–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-Y.; Ng, T.K.; Brelén, M.E.; Wu, D.; Wang, J.X.; Chan, K.P.; Yung, J.S.Y.; Cao, D.; Wang, Y.; Zhang, S.; et al. Continuous exposure to non-lethal doses of sodium iodate induces retinal pigment epithelial cell dysfunction. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowers, G.; Cohen, M.; Marks-Ohana, D.; Stika, S.; Eijzenberg, A.; Banin, E.; Obolensky, A. Course of Sodium Iodate–Induced Retinal Degeneration in Albino and Pigmented Mice. Investig. Opthalmol. Vis. Sci. 2017, 58, 2239–2249. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, T.; Fang, W.; Peng, G.; Wang, L.; Qin, L.; Liu, B.; Huang, Y.F. The temporal topography of the N-Methyl- N-nitrosourea induced photoreceptor degeneration in mouse retina. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Pirmardan, E.R.; Soheili, Z.-S.; Samiei, S.; Ahmadieh, H.; Mowla, S.J.; Naseri, M.; Daftarian, N. In Vivo Evaluation of PAX6 Overexpression and NMDA Cytotoxicity to Stimulate Proliferation in the Mouse Retina. Sci. Rep. 2018, 8, 17700. [Google Scholar] [CrossRef]
- Jin, Z.-B.; Gao, M.-L.; Deng, W.-L.; Wu, K.-C.; Sugita, S.; Mandai, M.; Takahashi, M. Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 2019, 69, 38–56. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Freeman, S.C.; Janot, A.C. Novel stem cell and gene therapy in diabetic retinopathy, age related macular degeneration, and retinitis pigmentosa. Int. J. Retin. Vitr. 2019, 5, 1–14. [Google Scholar] [CrossRef]
- Nuzzi, R.; Tridico, F. Perspectives of Autologous Mesenchymal Stem-Cell Transplantation in Macular Hole Surgery: A Review of Current Findings. J. Ophthalmol. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Wagner, W.; Ho, A.D.; Zenke, M. Different Facets of Aging in Human Mesenchymal Stem Cells. Tissue Eng. Part B Rev. 2010, 16, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, W.J.C.; Ploemacher, E.R. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 2003, 17, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggenhofer, E.; Benseler, V.; Kroemer, H.; Popp, F.; Geissler, E.; Schlitt, H.; Baan, C.; Dahlke, M.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Kolibabka, M.; Eshwaran, R.; Chatterjee, A.; Schlotterer, A.; Willer, H.; Bieback, K.; Hammes, H.-P.; Feng, Y. Intravitreal injection of mesenchymal stem cells evokes retinal vascular damage in rats. FASEB J. 2019, 33, 14668–14679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oner, A.; Gonen, Z.B.; Sinim, N.; Cetin, M.; Ozkul, Y. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: A phase I clinical safety study. Stem Cell Res. Ther. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahraman, N.S. Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: A 6-month follow-up results of a phase 3 trial. Int. J. Ophthalmol. 2020, 13, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Oumlzmert, E.; Arslan, U. Management of retinitis pigmentosa by Wharton’s jelly-derived mesenchymal stem cells: Prospective analysis of 1-year results. Stem Cell Res. Ther. 2020, 11, 353. [Google Scholar] [CrossRef]
- Niwa, M.; Aoki, H.; Hirata, A.; Tomita, H.; Green, P.G.; Hara, A. Retinal Cell Degeneration in Animal Models. Int. J. Mol. Sci. 2016, 17, 110. [Google Scholar] [CrossRef] [Green Version]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef]
- Mao, X.; Pan, T.; Shen, H.; Xi, H.; Yuan, S.; Liu, Q. The rescue effect of mesenchymal stem cell on sodium iodate-induced retinal pigment epithelial cell death through deactivation of NF-κB-mediated NLRP3 inflammasome. Biomed. Pharmacother. 2018, 103, 517–523. [Google Scholar] [CrossRef]
- Moriguchi, M.; Nakamura, S.; Inoue, Y.; Nishinaka, A.; Nakamura, M.; Shimazawa, M.; Hara, H. Irreversible Photoreceptors and RPE Cells Damage by Intravenous Sodium Iodate in Mice Is Related to Macrophage Accumulation. Investig. Opthalmol. Vis. Sci. 2018, 59, 3476–3487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, Y.; Wang, C.; Zhang, Y.; Su, G. Morphologic and histopathologic change of sodium iodate-induced retinal degeneration in adult rats. Int. J. Clin. Exp. Pathol. 2019, 12, 443–454. [Google Scholar]
- Bhutto, I.A.; Ogura, S.; Baldeosingh, R.; McLeod, D.S.; Lutty, G.A.; Edwards, M.M. An Acute Injury Model for the Phenotypic Characteristics of Geographic Atrophy. Investig. Opthalmol. Vis. Sci. 2018, 59, AMD143–AMD151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.M.; Ahn, J.; Cha, S.; Yun, C.; Park, T.K.; Kim, Y.-J.; Goo, Y.S.; Kim, S.-W. The effects of intravitreal sodium iodate injection on retinal degeneration following vitrectomy in rabbits. Sci. Rep. 2019, 9, 15696–15710. [Google Scholar] [CrossRef] [PubMed]
- Barzelay, A.; Algor, S.W.; Niztan, A.; Katz, S.; Benhamou, M.; Nakdimon, I.; Azmon, N.; Gozlan, S.; Mezad-Koursh, D.; Neudorfer, M.; et al. Adipose-Derived Mesenchymal Stem Cells Migrate and Rescue RPE in the Setting of Oxidative Stress. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Wu, Q.; Song, B.; Lu, B.; Zhang, Y. Differentiation of rat mesenchymal stem cells transplanted into the subretinal space of sodium iodate-injected rats. Clin. Exp. Ophthalmol. 2008, 36, 666–671. [Google Scholar] [CrossRef]
- Fiori, A.; Terlizzi, V.; Kremer, H.; Gebauer, J.; Hammes, H.-P.; Harmsen, M.C.; Bieback, K. Mesenchymal stromal/stem cells as potential therapy in diabetic retinopathy. Immunobiology 2018, 223, 729–743. [Google Scholar] [CrossRef]
- Ezquer, M.; Urzua, C.A.; Montecino, S.; Leal, K.; Conget, P.; Ezquer, F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res. Ther. 2016, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Wang, Y.; Kong, J.; Dong, M.; Duan, H.; Chen, S. Therapeutic efficacy of neural stem cells originating from umbilical cord-derived mesenchymal stem cells in diabetic retinopathy. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Kong, J.-H.; Zheng, D.; Chen, S.; Duan, H.-T.; Wang, Y.-X.; Dong, M.; Song, J. A comparative study on the transplantation of different concentrations of human umbilical mesenchymal cells into diabetic rats. Int. J. Ophthalmol. 2015, 8, 257–262. [Google Scholar] [PubMed]
- Cronk, S.M.; Kelly-Goss, M.R.; Ray, H.C.; Mendel, T.A.; Hoehn, K.L.; Bruce, A.C.; Dey, B.K.; Guendel, A.M.; Tavakol, D.N.; Herman, I.M.; et al. Adipose-Derived Stem Cells From Diabetic Mice Show Impaired Vascular Stabilization in a Murine Model of Diabetic Retinopathy. Stem Cells Transl. Med. 2015, 4, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Saijo, Y.; Tsuchida, H.; Ishioka, S.; Nishikawa, A.; Saito, T.; et al. Umbilical cord extracts improve diabetic abnormalities in bone marrow-derived mesenchymal stem cells and increase their therapeutic effects on diabetic nephropathy. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.; Barathi, V.A.; Chaurasia, S.S.; Wong, T.Y.; Kern, T.S. Update on animal models of diabetic retinopathy: From molecular approaches to mice and higher mammals. Dis. Model. Mech. 2012, 5, 444–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, A.K.W.; Lo, A.C.Y. Animal Models of Diabetic Retinopathy: Summary and Comparison. J. Diabetes Res. 2013, 2013, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.S.; Silva, M.S.; Santos, D.F.; Silva, G.A. Dysregulation of trophic factors contributes to diabetic retinopathy in the Ins2Akita mouse. Exp. Eye Res. 2020, 194. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, S.L.; Evans, W.; Pentecost, M.; Lenin, R.; Periasamy, R.; Jha, K.A.; Alli, S.; Gentry, J.; Thomas, S.M.; Sohl, N.; et al. Adipose stem cells and their paracrine factors are therapeutic for early retinal complications of diabetes in the Ins2Akita mouse. Stem Cell Res. Ther. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Van Hove, I.; De Groef, L.; Boeckx, B.; Modave, E.; Hu, T.-T.; Beets, K.; Etienne, I.; Van Bergen, T.; Lambrechts, D.; Moons, L.; et al. Single-cell transcriptome analysis of the Akimba mouse retina reveals cell-type-specific insights into the pathobiology of diabetic retinopathy. Diabetologia 2020, 63, 2235–2248. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Lim, R.R.; Parikh, B.H.; Wey, Y.S.; Tun, B.B.; Wong, T.Y.; Luu, C.D.; Agrawal, R.; Ghosh, A.; Mortellaro, A.; et al. The NLRP3 Inflammasome May Contribute to Pathologic Neovascularization in the Advanced Stages of Diabetic Retinopathy. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rivas, M.A.; Vecino, E. Animal models and different therapies for treatment of retinitis pigmentosa. Histol. Histopathol. 2009, 24, 1295–1322. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Liu, X.; Ghazaryan, E.; Li, Y.; Xie, J.; Su, G. Recent Advances of Stem Cell Therapy for Retinitis Pigmentosa. Int. J. Mol. Sci. 2014, 15, 14456–14474. [Google Scholar] [CrossRef] [Green Version]
- Tsubura, A.; Yoshizawa, K.; Kuwata, M.; Uehara, N. Animal models for retinitis pigmentosa induced by MNU, disease pro-gression, mechanisms and therapeutic trials. Histol. Histopathol. 2010, 25, 933–944. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, Z.; Sun, X.; Zhu, X.; Zhou, L.; Li, M.; Cheng, B.; Liu, X.; He, C. Microglia Polarization with M1/M2 Phenotype Changes in rd1 Mouse Model of Retinal Degeneration. Front. Neuroanat. 2017, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- Gargini, C.; Terzibasi, E.; Mazzoni, F.; Strettoi, E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: A morphological and ERG study. J. Comp. Neurol. 2006, 500, 222–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef]
- Kameya, S.; Hawes, N.L.; Chang, B.; Heckenlively, J.R.; Naggert, J.K.; Nishina, P.M. Mfrp, a gene encoding a frizzled related protein, is mutated in the mouse retinal degeneration 6. Hum. Mol. Genet. 2002, 11, 1879–1886. [Google Scholar] [CrossRef]
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; Lavail, M.M.; Vollrath, D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000, 9, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pierdomenico, J.; García-Ayuso, D.; Pinilla, I.; Cuenca, N.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas-Pérez, M.P. Early Events in Retinal Degeneration Caused by Rhodopsin Mutation or Pigment Epithelium Malfunction: Differences and Similarities. Front. Neuroanat. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otani, A.; Dorrell, M.I.; Kinder, K.; Moreno, S.K.; Nusinowitz, S.; Banin, E.; Heckenlively, J.; Friedlander, M. Rescue of retinal degen-eration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J. Clin. Investig. 2004, 114, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Lejkowska, R.; Kawa, M.P.; Pius-Sadowska, E.; Rogińska, D.; Łuczkowska, K.; Machaliński, B.; Machalińska, A. Preclinical Evaluation of Long-Term Neuroprotective Effects of BDNF-Engineered Mesenchymal Stromal Cells as Intravitreal Therapy for Chronic Retinal Degeneration in Rd6 Mutant Mice. Int. J. Mol. Sci. 2019, 20, 777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, L.; Gao, L.; Xu, H.; Duan, P.; Zeng, Y.; Liu, Y.; Yin, Z.Q. Combined transplantation of human mesenchymal stem cells and human retinal progenitor cells into the subretinal space of RCS rats. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.-L.; Hu, C.-B.; Wang, B.-Y.; Xiong, Y.-C.; Chen, T.; Zhao, N.; Bao, L.-H.; Quan, R.; Du, F.-Y.; Sui, B.-D.; et al. Bone progeria diminished the therapeutic effects of bone marrow mesenchymal stem cells on retinal degeneration. Biochem. Biophys. Res. Commun. 2020, 531, 180–186. [Google Scholar] [CrossRef]
- Johnson, T.V.; Tomarev, S.I. Rodent models of glaucoma. Brain Res. Bull. 2010, 81, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.; Kimura, A.; Guo, X.; Namekata, K.; Harada, T. Recent advances in genetically modified animal models of glaucoma and their roles in drug repositioning. Br. J. Ophthalmol. 2018, 103, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Overby, D.R.; Clark, A.F. Animal models of glucocorticoid-induced glaucoma. Exp. Eye Res. 2015, 141, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Wan, K.H. Review of rodent hypertensive glaucoma models. Acta Ophthalmol. 2019, 97, e331–e340. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhu, Y.; Chen, Q.; Xu, J.; Sarunic, M.V.; Saragovi, U.H.; Zhuo, Y. Validation of glaucoma-like features in the rat episcleral vein cauterization model. Chin. Med. J. 2014, 127, 359–364. [Google Scholar] [PubMed]
- Huang, W.; Hu, F.; Wang, M.; Gao, F.; Xu, P.; Xing, C.; Sun, X.; Zhang, S.; Wu, J. Comparative analysis of retinal ganglion cell damage in three glaucomatous rat models. Exp. Eye Res. 2018, 172, 112–122. [Google Scholar] [CrossRef]
- Mead, B.; Hill, L.J.; Blanch, R.J.; Ward, K.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Mesenchymal stromal cell–mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016, 18, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.V.; Bull, N.D.; Hunt, D.P.; Marina, N.; Tomarev, S.I.; Martin, K.R. Neuroprotective Effects of Intravitreal Mesenchymal Stem Cell Transplantation in Experimental Glaucoma. Investig. Opthalmol. Vis. Sci. 2010, 51, 2051–2059. [Google Scholar] [CrossRef]
- Manuguerra-Gagné, R.; Boulos, P.R.; Ammar, A.; Leblond, F.A.; Krosl, G.; Pichette, V.; Lesk, M.R.; Roy, D.-C. Transplantation of Mesenchymal Stem Cells Promotes Tissue Regeneration in a Glaucoma Model Through Laser-Induced Paracrine Factor Secretion and Progenitor Cell Recruitment. Stem Cells 2013, 31, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Emre, E.; Yüksel, N.; Duruksu, G.; Pirhan, D.; Subaşi, C.; Erman, G.; Karaöz, E. Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow–derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy 2015, 17, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Chang, X.; Xu, M.; Zhang, M.; Zhang, S.; Wang, Y.; Luo, X.; Xu, J.; Yang, X.; Sun, X. UMSC-derived exosomes promote retinal ganglion cels survival in a rat model of optic nerve crush. J. Chem. Neuroanat. 2019, 96, 134–139. [Google Scholar] [CrossRef]
- Çerman, E.; Akkoc, T.; Eraslan, M.; Şahin, O.; Ozkara, S.; Aker, F.V.; Subaşı, C.; Karaoz, E.; Akkoç, T. Retinal Electrophysiological Effects of Intravitreal Bone Marrow Derived Mesenchymal Stem Cells in Streptozotocin Induced Diabetic Rats. PLoS ONE 2016, 11, e0156495. [Google Scholar] [CrossRef]
- Roubeix, C.; Godefroy, D.; Mias, C.; Sapienza, A.; Riancho, L.; Degardin, J.; Fradot, V.; Ivkovic, I.; Picaud, S.; Sennlaub, F.; et al. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res. Ther. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Harper, M.M.; Grozdanic, S.D.; Blits, B.; Kuehn, M.H.; Zamzow, D.; Buss, J.E.; Kardon, R.H.; Sakaguchi, D.S. Transplantation of BDNF-Secreting Mesenchymal Stem Cells Provides Neuroprotection in Chronically Hypertensive Rat Eyes. Investig. Opthalmol. Vis. Sci. 2011, 52, 4506–4515. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.S.; Zawadzki, R.J.; Werner, J.S.; Nolta, A.J. Intravitreal Autologous Bone Marrow CD34+ Cell Therapy for Ischemic and Degenerative Retinal Disorders: Preliminary Phase 1 Clinical Trial Findings. Investig. Opthalmol. Vis. Sci. 2014, 56, 81–89. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Messias, A.; Voltarelli, J.C.; Scott, I.U.; Jorge, R. Intravitreal injection of autologous bone marrow–derived mononuclear cells for hereditary retinal dystrophy. Retina 2011, 31, 1207–1214. [Google Scholar] [CrossRef]
- Gu, X.; Yu, X.; Zhao, C.; Duan, P.; Zhao, T.; Liu, Y.; Li, S.; Yang, Z.; Li, Y.; Qian, C.; et al. Efficacy and Safety of Autologous Bone Marrow Mesenchymal Stem Cell Transplantation in Patients with Diabetic Retinopathy. Cell. Physiol. Biochem. 2018, 49, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study: Bone marrow derived stem cells in the treatment of Retinitis Pigmentosa. Stem Cell Investig. 2018, 5, 18. [Google Scholar] [CrossRef]
- Levy, S.; Weiss, J.N.; Malkin, A. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: A preliminary report. Neural Regen. Res. 2015, 10, 982–988. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S.; Benes, S.C. Stem Cell Ophthalmology Treatment Study: Bone marrow derived stem cells in the treatment of non-arteritic ischemic optic neuropathy (NAION). Stem Cell Investig. 2017, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Hoogduijn, M.J.; Dor, F.J.M.F. Mesenchymal Stem Cells: Are We Ready for Clinical Application in Transplantation and Tissue Regeneration? Front. Immunol. 2013, 4, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, S.; Gangaraju, R.; Chaum, E. Recent Advances in Retinal Stem Cell Therapy. Curr. Mol. Biol. Rep. 2017, 3, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Z.; Gu, P. Stem/progenitor cell-based transplantation for retinal degeneration: A review of clinical trials. Cell Death Dis. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Tzameret, A.; Sher, I.; Belkin, M.; Treves, A.J.; Meir, A.; Nagler, A.; Levkovitch-Verbin, H.; Rotenstreich, Y.; Solomon, A.S. Epiretinal transplantation of human bone marrow mesenchymal stem cells rescues retinal and vision function in a rat model of retinal degeneration. Stem Cell Res. 2015, 15, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.; Lin, S.; Chen, J.; Huang, X.; Wei, C.-C.; Li, Z.; Tang, S. Neuroprotection of Transplanting Human Umbilical Cord Mesenchymal Stem Cells in a Microbead Induced Ocular Hypertension Rat Model. Curr. Eye Res. 2018, 43, 810–820. [Google Scholar] [CrossRef]
- Velandia, S.L.; Di Lauro, S.; Alonso-Alonso, M.L.; Bartolomé, S.T.; Srivastava, G.K.; Pastor, J.C.; Fernandez-Bueno, I. Biocompatibility of intravitreal injection of human mesenchymal stem cells in immunocompetent rabbits. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 256, 125–134. [Google Scholar] [CrossRef]
- Lohan, P.; Treacy, O.; Morcos, M.; Donohoe, E.; O’Donoghue, Y.; Ryan, A.E.; Elliman, S.J.; Ritter, T.; Griffin, M.D. Interspecies Incompatibilities Limit the Immunomodulatory Effect of Human Mesenchymal Stromal Cells in the Rat. Stem Cells 2018, 36, 1210–1215. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.Y.; Kim, M.K.; Shin, M.S.; Wee, W.R.; Lee, J.H. Cytokine secretion by human mesenchymal stem cells cocultured with damaged corneal epithelial cells. Cytokine 2009, 46, 100–103. [Google Scholar] [CrossRef]
- Zhou, L.; Lopes, J.E.; Chong, M.M.W.; Ivanov, I.I.; Min, R.; Victora, G.D.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nat. Cell Biol. 2008, 453, 236–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Induction of Retinal Diseases | Species | Treatment | Result | Reference |
|---|---|---|---|---|
| NaIO3 | Mouse | Human A-MSCs | Protection of RPE cells, photoreceptors and outer nuclear layer | [123] |
| Rat | Rat BM-MSCs | Differentiation of transplanted MSCs into cells with retinal markers | [124] | |
| Streptozotocin | Mouse | Mouse A-MSCs | Enhanced levels of neurotrohpic factors, protection of retinal ganglion cells | [126] |
| Rat | Neural stem cells (derived from humal umbilical MSCs) | Enhanced levels of neurotrohpic factors, protection of retinal ganglion cells | [127] | |
| Human A-MSCs | Decreased apoptosis, decrease in expression of genes related to DR | [94] | ||
| Human umbilical MSCs | Increased expression of NGF | [128] | ||
| Rat BM-MSCs | Improvement in visual function | [161] | ||
| Insulin 2 gene mutation | Mouse | Human A-MSCs | Decreased vascular permeability | [134] |
| Conditioned medium from human A-MSCs | Decreased vascular permeability, improvement in visual function | [134] | ||
| Insulin 2/VEGFa gene mutation | Mouse | Mouse A-MSCs | Increased vascular density, incorporation of host MSCs into the retina | [129] |
| Cauterization of 3 episcleral veins | Rat | Rat BM-MSCs | Regulation of intraocular pressure, protection of retinal ganglion cells | [162] |
| Laser damage | Rat | Rat BM-MSCs | Protection of retinal ganglion cells | [157] |
| Rat BM-MSCs (engineered to express BDNF) | Improvement in ERG function, protection of retinal ganglion cells | [163] | ||
| Optic nerve crush injury | Rat | Exosomes from human BM-MSCs | Protection of retinal ganglion cells | [96] |
| PDE gene mutation (rd 10 mouse) | Mouse | Mouse BM-MSCs | Protection of photoreceptors | [146] |
| Mfrp mutation (rd 6 mouse) | Mouse | Mouse BM-MSCs (engineered to express BDNF) | Induction of antiapoptotic signaling, improvement in ERG | [147] |
| Mertk gene mutation (RCS rats) | Rat | Human BM-MSCs with human progentitor retinal cells | Inflammatory modulation, promoting differentiation of donors cells into photoreceptor | [148] |
| Retinal Disease | Cells For Treatment | Result | Reference |
|---|---|---|---|
| AMD, RP, retinal vascular occlusion | Autologous BM-MSC (intravitreal) | Phase 1, no severe safety issues associated with treatment | [164] |
| RP, cone-rod dystrophy | Autologous BM-MSC(intravitreal) | Phase 1, no severe safety issues associated with treatment | [165] |
| RP | Autologous BM-MSC (retrobulbar, subtenons, intravitreal, intravenous) | Improvement in visual function | [167] |
| Optic nerve diseases | Autologous BM-MSC (retrobulbar, subtenons, intravitreal, intravenous) | Improvement in visual function | [168] |
| Ischemic optic neuropathy | Autologous BM-MSC (retrobulbar, subtenons, intravitreal, intravenous) | Improvement in visual function | [169] |
| RP, inherited retinal dystrophy | Wharton’s jelly-derived MSC (subtenons) | Improvement in visual acuity and in outer retinal thickness | [115] |
| DR | Autologous MSCs (intravenous) | Improvements in macular thickness and in visual acuity | [166] |
| RP | Umbilical cord- derived MSC (suprachorodial) | Improvements in best corrected visual acuity, electroretinography and visual field | [114] |
| RP | A-MSC (subretinal) | Minor ocular complicaions, no severe safety issues associated with the treatment | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holan, V.; Palacka, K.; Hermankova, B. Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials. Cells 2021, 10, 588. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10030588
Holan V, Palacka K, Hermankova B. Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials. Cells. 2021; 10(3):588. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10030588
Chicago/Turabian StyleHolan, Vladimir, Katerina Palacka, and Barbora Hermankova. 2021. "Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials" Cells 10, no. 3: 588. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10030588