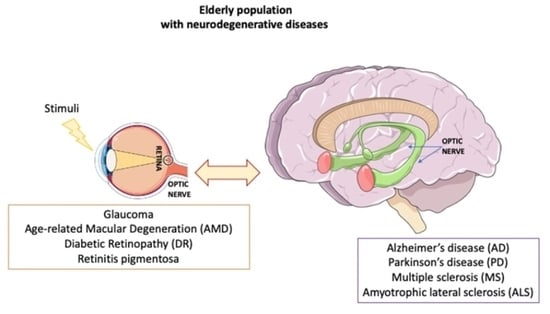
Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas
Abstract
:1. Introduction
| Diseases | Therapeutic Category | Drugs | Route of Drug Administration | Effect/Mechanism of Action | References |
|---|---|---|---|---|---|
| Glaucoma | Prostaglandin analogue | Latanoprost Bimatoprost Travoprost | Topical instillation (drops) | Reduction of intraocular pressure (IOP)/Increase uveoscleral outflow | [27,28] |
| Protaglandin/Rho-kinase transporter inhibitor association | Latanoprost/Netarsudil | Topical instillation (drops) | Increase of the trabecular outflow/Decreasing the aqueous production/Decrease the episcleral venous pressure | [28,29,30,31] | |
| Rho-kinase transporter inhibitor | Netarsudil | Topical instillation (drops) | Reduction of intraocular pressure (IOP)/Increase of the trabecular outflow | [32] | |
| β-receptor antagonists | Timolol Betaxolol Levobunolol Carteolol Metipranolol | Topical instillation (drops) | Reduction of intraocular pressure (IOP)/Decrease aqueous humor production | [28,33,34] | |
| α2-receptor agonists | Brimonidine Apraclonidine | Topical instillation (drops) | Reduction of intraocular pressure (IOP)/Decrease aqueous humor production | [28,33,34,35] | |
| Carbonic anhydrase inhibitors | Brinzolamide Dorzolamide | Topical instillation (drops) | Reduction of intraocular pressure (IOP)/Decrease aqueous humor production and increase uveoscleral outflow | [28,33,34,36] | |
| Cholinergic receptor agonists | Pilocarpine Carbachol | Topical instillation (drops) | Reduction of intraocular pressure (IOP)/Increase trabecular outflow | [28,34] | |
| AMD | Anti-VEGF | Ranibizumab Aflibercept Pegaptanib Conbercept Brolucizumab | Intravitreal injection | Reduction of new blood vessel growth/Inhibition of the biological activity of VEGF | [31,37,38,39] |
| Photodynamic therapy | Verteporfin | Intravenous | Elimination of the abnormal blood vessels in wet-form macular degeneration | [39,40] | |
| DR | Anti-VEGF | Ranibizumab Aflibercept Bevacizumab | Intravitreal injection | Reduction of new blood vessel growth/Inhibition of the biological activity of VEGF | [16,41] |
| RP | Supplements/Vitamin | Vitamin A Omega 3 (DHA) Lutein | Topical instillation/oral | Improve photoreceptor metabolism, slowing its death by apoptosis | [42,43,44,45] |
2. CNS and Eye Neurodegeneration
3. Ocular Neurodegenerative Diseases
3.1. Glaucoma
3.2. Age-Related Macular Degeneration
3.3. Diabetic Retinopathy
3.4. Retinitis Pigmentosa
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Moraes, C.G. Anatomy of the Visual Pathways. J. Glaucoma 2013, 22, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Guidoboni, G.; Sacco, R.; Szopos, M.; Sala, L.; Verticchio Vercellin, A.C.; Siesky, B.; Harris, A. Neurodegenerative Disorders of the Eye and of the Brain: A Perspective on Their Fluid-Dynamical Connections and the Potential of Mechanism-Driven Modeling. Front. Neurosci. 2020, 14, 566428. [Google Scholar] [CrossRef]
- Jindal, V. Interconnection Between Brain and Retinal Neurodegenerations. Mol. Neurobiol. 2015, 51, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Masland, R.H. The Neuronal Organization of the Retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmstaedter, M.; Briggman, K.L.; Turaga, S.C.; Jain, V.; Seung, H.S.; Denk, W. Connectomic Reconstruction of the Inner Plexiform Layer in the Mouse Retina. Nature 2013, 500, 168–174. [Google Scholar] [CrossRef]
- Takemura, S.; Bharioke, A.; Lu, Z.; Nern, A.; Vitaladevuni, S.; Rivlin, P.K.; Katz, W.T.; Olbris, D.J.; Plaza, S.M.; Winston, P.; et al. A Visual Motion Detection Circuit Suggested by Drosophila Connectomics. Nature 2013, 500, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Maisak, M.S.; Haag, J.; Ammer, G.; Serbe, E.; Meier, M.; Leonhardt, A.; Schilling, T.; Bahl, A.; Rubin, G.M.; Nern, A.; et al. A Directional Tuning Map of Drosophila Elementary Motion Detectors. Nature 2013, 500, 212–216. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The Inner Blood-Retinal Barrier: Cellular Basis and Development. Vis. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Soni, D.; Sagar, P.; Takkar, B. Diabetic Retinal Neurodegeneration as a Form of Diabetic Retinopathy. Int. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Ahmad, I.; Teotia, P.; Erickson, H.; Xia, X. Recapitulating Developmental Mechanisms for Retinal Regeneration. Prog. Retin. Eye Res. 2020, 76, 100824. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Chan, N.C.; Sadun, A.A. Glaucoma as Neurodegeneration in the Brain. Eye Brain 2021, 13, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults—Screening, Diagnosis, and Management: A Review. JAMA 2021, 325, 164. [Google Scholar] [CrossRef]
- Zhuang, J.; Madden, D.J.; Cunha, P.; Badea, A.; Davis, S.W.; Potter, G.G.; Lad, E.M.; Cousins, S.W.; Chen, N.-K.; Allen, K.; et al. Cerebral White Matter Connectivity, Cognition, and Age-Related Macular Degeneration. NeuroImage Clin. 2021, 30, 102594. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Singh, N.; Chaudhary, S.; Bellamkonda, V.; Kritikos, A.E.; Wise, A.S.; Rana, N.; McDonald, D.; Ayyagari, R. Retinal Degeneration and Alzheimer’s Disease: An Evolving Link. Int. J. Mol. Sci. 2020, 21, 7290. [Google Scholar] [CrossRef] [PubMed]
- Pillar, S.; Moisseiev, E.; Sokolovska, J.; Grzybowski, A. Recent Developments in Diabetic Retinal Neurodegeneration: A Literature Review. J. Diabetes Res. 2020, 2020, 5728674. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, F.; Megaw, R. Mechanisms of Photoreceptor Death in Retinitis Pigmentosa. Genes 2020, 11, 1120. [Google Scholar] [CrossRef]
- Milam, A.H.; Li, Z.Y.; Fariss, R.N. Histopathology of the Human Retina in Retinitis Pigmentosa. Prog. Retin. Eye Res. 1998, 17, 175–205. [Google Scholar] [CrossRef]
- Rita Machado, A.; Carvalho Pereira, A.; Ferreira, F.; Ferreira, S.; Quendera, B.; Silva, E.; Castelo-Branco, M. Structure-Function Correlations in Retinitis Pigmentosa Patients with Partially Preserved Vision: A Voxel-Based Morphometry Study. Sci. Rep. 2017, 7, 11411. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, S.; Tang, Y.; Guo, Y.; Gao, S. Intractable Ocular Diseases and Treatment Progress. AAPS PharmSciTech 2020, 21, 236. [Google Scholar] [CrossRef]
- Cheng, K.-J.; Hsieh, C.-M.; Nepali, K.; Liou, J.-P. Ocular Disease Therapeutics: Design and Delivery of Drugs for Diseases of the Eye. J. Med. Chem. 2020, 63, 10533–10593. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Tian, Y.; Funderburgh, J.; Pellegrini, G.; Zhang, K.; Goldberg, J.L.; Ali, R.R.; Young, M.; Xie, Y.; Temple, S. Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 2018, 22, 834–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharm. Exp. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Snyder, P.J.; Alber, J.; Alt, C.; Bain, L.J.; Bouma, B.E.; Bouwman, F.H.; DeBuc, D.C.; Campbell, M.C.W.; Carrillo, M.C.; Chew, E.Y.; et al. Retinal Imaging in Alzheimer’s and Neurodegenerative Diseases. Alzheimers Dement. 2021, 17, 103–111. [Google Scholar] [CrossRef]
- Czakó, C.; Kovács, T.; Ungvari, Z.; Csiszar, A.; Yabluchanskiy, A.; Conley, S.; Csipo, T.; Lipecz, A.; Horváth, H.; Sándor, G.L.; et al. Retinal Biomarkers for Alzheimer’s Disease and Vascular Cognitive Impairment and Dementia (VCID): Implication for Early Diagnosis and Prognosis. GeroScience 2020, 42, 1499–1525. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, V.B.; Chitranshi, N.; Gangoda, S.; Vander Wall, R.; Abbasi, M.; Golzan, M.; Dheer, Y.; Shah, T.; Avolio, A.; et al. One Protein, Multiple Pathologies: Multifaceted Involvement of Amyloid β in Neurodegenerative Disorders of the Brain and Retina. Cell. Mol. Life Sci. 2016, 73, 4279–4297. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Eaton, J.S. Medical Anti-glaucoma Therapy: Beyond the Drop. Vet. Ophthalmol. 2021, 24, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Marquis, R.E.; Whitson, J.T. Management of Glaucoma: Focus on Pharmacological Therapy. Drugs Aging 2005, 22, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mehran, N.A.; Sinha, S.; Razeghinejad, R. New Glaucoma Medications: Latanoprostene Bunod, Netarsudil, and Fixed Combination Netarsudil-Latanoprost. Eye 2020, 34, 72–88. [Google Scholar] [CrossRef]
- Ostler, E.; Rhee, D.; Burney, E.; Sozeri, Y. Advances in Medical Therapy for Glaucoma. Curr. Opin. Ophthalmol. 2021, 32, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. The Management of Glaucoma and Macular Degeneration. Expert Opin. Ther. Pat. 2019, 29, 745–747. [Google Scholar] [CrossRef] [Green Version]
- Kopczynski, C.C.; Heah, T. Netarsudil Ophthalmic Solution 0.02% for the Treatment of Patients with Open-Angle Glaucoma or Ocular Hypertension. Drugs Today 2018, 54, 467. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Supuran, C.T. Adrenergic Agonists and Antagonists as Antiglaucoma Agents: A Literature and Patent Review (2013–2019). Expert Opin. Ther. Pat. 2019, 29, 805–815. [Google Scholar] [CrossRef]
- Guglielmi, P.; Carradori, S.; Campestre, C.; Poce, G. Novel Therapies for Glaucoma: A Patent Review (2013-2019). Expert Opin. Ther. Pat. 2019, 29, 769–780. [Google Scholar] [CrossRef]
- Conti, F.; Romano, G.L.; Eandi, C.M.; Toro, M.D.; Rejdak, R.; Di Benedetto, G.; Lazzara, F.; Bernardini, R.; Drago, F.; Cantarella, G.; et al. Brimonidine Is Neuroprotective in Animal Paradigm of Retinal Ganglion Cell Damage. Front. Pharmacol. 2021, 12, 705405. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Glaucoma and the Applications of Carbonic Anhydrase Inhibitors. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Frost, S.C., McKenna, R., Eds.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2014; Volume 75, pp. 349–359. ISBN 978-94-007-7358-5. [Google Scholar]
- Al-Zamil, W.; Yassin, S. Recent Developments in Age-Related Macular Degeneration: A Review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, E.R.; Kaiser, P.K. Therapeutic Potential of the Ranibizumab Port Delivery System in the Treatment of AMD: Evidence to Date. Clin. Ophthalmol. 2020, 14, 1349–1355. [Google Scholar] [CrossRef]
- Supuran, C.T. Agents for the Prevention and Treatment of Age-Related Macular Degeneration and Macular Edema: A Literature and Patent Review. Expert. Opin. Ther. Pat. 2019, 29, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Goa, K.L. Verteporfin. Drugs Aging 2000, 16, 139–146. [Google Scholar] [CrossRef]
- Zhao, Y.; Singh, R.P. The Role of Anti-Vascular Endothelial Growth Factor (Anti-VEGF) in the Management of Proliferative Diabetic Retinopathy. Drugs Context 2018, 7, 212532. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, K.; Liu, R.; Pan, J.; Zhang, L.; Lu, X. Vitamins and Mineral Supplements for Retinitis Pigmentosa. J. Ophthalmol. 2019, 2019, 8524607. [Google Scholar] [CrossRef]
- Jia, Y.-P.; Sun, L.; Yu, H.-S.; Liang, L.-P.; Li, W.; Ding, H.; Song, X.-B.; Zhang, L.-J. The Pharmacological Effects of Lutein and Zeaxanthin on Visual Disorders and Cognition Diseases. Molecules 2017, 22, 610. [Google Scholar] [CrossRef]
- Brito-García, N.; del Pino-Sedeño, T.; Trujillo-Martín, M.M.; Coco, R.M.; Rodríguez de la Rúa, E.; del Cura-González, I.; Serrano-Aguilar, P. Effectiveness and Safety of Nutritional Supplements in the Treatment of Hereditary Retinal Dystrophies: A Systematic Review. Eye 2017, 31, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis Pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Madore, C.; Yin, Z.; Leibowitz, J.; Butovsky, O. Microglia, Lifestyle Stress, and Neurodegeneration. Immunity 2020, 52, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS Neurodegenerative Diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Mao, X. Role of Retinal Amyloid-β in Neurodegenerative Diseases: Overlapping Mechanisms and Emerging Clinical Applications. Int. J. Mol. Sci. 2021, 22, 2360. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589. [Google Scholar] [CrossRef]
- Mancino, R.; Cesareo, M.; Martucci, A.; Di Carlo, E.; Ciuffoletti, E.; Giannini, C.; Morrone, L.A.; Nucci, C.; Garaci, F. Neurodegenerative Process Linking the Eye and the Brain. Curr. Med. Chem. 2019, 26, 3754–3763. [Google Scholar] [CrossRef]
- Dinkin, M. Trans-Synaptic Retrograde Degeneration in the Human Visual System: Slow, Silent, and Real. Curr. Neurol. Neurosci. Rep. 2017, 17, 16. [Google Scholar] [CrossRef]
- Lawlor, M.; Danesh-Meyer, H.; Levin, L.A.; Davagnanam, I.; De Vita, E.; Plant, G.T. Glaucoma and the Brain: Trans-Synaptic Degeneration, Structural Change, and Implications for Neuroprotection. Surv. Ophthalmol. 2018, 63, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Y.; Gupta, N. Glaucoma of the brain: A disease model for the study of transsynaptic neural degeneration. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 173, pp. 465–478. ISBN 978-0-444-53256-5. [Google Scholar]
- You, M.; Rong, R.; Zeng, Z.; Xia, X.; Ji, D. Transneuronal Degeneration in the Brain During Glaucoma. Front. Aging Neurosci. 2021, 13, 643685. [Google Scholar] [CrossRef]
- Rocca, M.A.; Mesaros, S.; Preziosa, P.; Pagani, E.; Stosic-Opincal, T.; Dujmovic-Basuroski, I.; Drulovic, J.; Filippi, M. Wallerian and Trans-Synaptic Degeneration Contribute to Optic Radiation Damage in Multiple Sclerosis: A Diffusion Tensor MRI Study. Mult. Scler. 2013, 19, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Cutolo, C.A.; Rossi, T. Visual Defects and Ageing. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science; Harris, J.R., Korolchuk, V.I., Eds.; Subcellular Biochemistry; Springer: Singapore, 2019; Volume 91, pp. 393–434. ISBN 9789811336805. [Google Scholar]
- Gupta, S.; Zivadinov, R.; Ramanathan, M.; Weinstock-Guttman, B. Optical Coherence Tomography and Neurodegeneration: Are Eyes the Windows to the Brain? Expert Rev. Neurother. 2016, 16, 765–775. [Google Scholar] [CrossRef]
- Sen, S.; Saxena, R.; Tripathi, M.; Vibha, D.; Dhiman, R. Neurodegeneration in Alzheimer’s Disease and Glaucoma: Overlaps and Missing Links. Eye 2020, 34, 1546–1553. [Google Scholar] [CrossRef]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Dudvarski Stankovic, N.; Teodorczyk, M.; Ploen, R.; Zipp, F.; Schmidt, M.H.H. Microglia–Blood Vessel Interactions: A Double-Edged Sword in Brain Pathologies. Acta Neuropathol. 2016, 131, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal Microglia: Just Bystander or Target for Therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef] [PubMed]
- O’Bryhim, B.E.; Apte, R.S.; Kung, N.; Coble, D.; Van Stavern, G.P. Association of Preclinical Alzheimer Disease With Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018, 136, 1242. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, D.; Ji, J.; Wang, Y.; Zhang, R. Central Retina Changes in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neurol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.E.; Balendra, S.I.; Almonte, M.T.; Cordeiro, M.F. Retinal Correlates of Neurological Disorders. Ther. Adv. Chronic Dis. 2019, 10, 204062231988220. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Pediconi, N.; Oieni, F.; Pizzarelli, R.; Rosito, M.; Giubettini, M.; Santini, T.; Limatola, C.; Ruocco, G.; Ragozzino, D.; et al. Neuroinflammatory Processes, A1 Astrocyte Activation and Protein Aggregation in the Retina of Alzheimer’s Disease Patients, Possible Biomarkers for Early Diagnosis. Front. Neurosci. 2019, 13, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimaldi, A.; Brighi, C.; Peruzzi, G.; Ragozzino, D.; Bonanni, V.; Limatola, C.; Ruocco, G.; Di Angelantonio, S. Inflammation, Neurodegeneration and Protein Aggregation in the Retina as Ocular Biomarkers for Alzheimer’s Disease in the 3xTg-AD Mouse Model. Cell Death Dis. 2018, 9, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.K.H.; Li, Q.-X.; He, Z.; Vingrys, A.J.; Wong, V.H.Y.; Currier, N.; Mullen, J.; Bui, B.V.; Nguyen, C.T.O. The Eye As a Biomarker for Alzheimer’s Disease. Front. Neurosci. 2016, 10, 536. [Google Scholar] [CrossRef] [Green Version]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Indrieri, A.; Pizzarelli, R.; Franco, B.; De Leonibus, E. Dopamine, Alpha-Synuclein, and Mitochondrial Dysfunctions in Parkinsonian Eyes. Front. Neurosci. 2020, 14, 567129. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-J.; Xu, L.-L.; Mao, C.; Fu, Y.-T.; Ji, X.-Y.; Shen, Y.; Chen, J.; Yang, Y.; Liu, C.-F. Progressive Changes in the Retinal Structure of Patients with Parkinson’s Disease. J. Parkinsons Dis. 2018, 8, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Satue, M.; Garcia-Martin, E.; Fuertes, I.; Otin, S.; Alarcia, R.; Herrero, R.; Bambo, M.P.; Pablo, L.E.; Fernandez, F.J. Use of Fourier-Domain OCT to Detect Retinal Nerve Fiber Layer Degeneration in Parkinson’s Disease Patients. Eye 2013, 27, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological Mechanisms in Progressive Multiple Sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Faissner, S.; Gold, R. Progressive Multiple Sclerosis: Latest Therapeutic Developments and Future Directions. Adv. Neurol. Disord. 2019, 12, 175628641987832. [Google Scholar] [CrossRef] [Green Version]
- Spain, R.I.; Liu, L.; Zhang, X.; Jia, Y.; Tan, O.; Bourdette, D.; Huang, D. Optical Coherence Tomography Angiography Enhances the Detection of Optic Nerve Damage in Multiple Sclerosis. Br. J. Ophthalmol. 2018, 102, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Masrori, P.; Van Damme, P. Amyotrophic Lateral Sclerosis: A Clinical Review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.; Ramírez, A.I.; Fernández-Albarral, J.A.; López-Cuenca, I.; Salobrar-García, E.; Cadena, M.; Elvira-Hurtado, L.; Salazar, J.J.; de Hoz, R.; Ramírez, J.M. Amyotrophic Lateral Sclerosis: A Neurodegenerative Motor Neuron Disease With Ocular Involvement. Front. Neurosci. 2020, 14, 566858. [Google Scholar] [CrossRef]
- Soldatov, V.O.; Kukharsky, M.S.; Belykh, A.E.; Sobolev, A.M.; Deykin, A.V. Retinal Damage in Amyotrophic Lateral Sclerosis: Underlying Mechanisms. Eye Brain 2021, 13, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.A.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of Vision Loss Worldwide, 1990–2010: A Systematic Analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef] [Green Version]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The Molecular Basis of Retinal Ganglion Cell Death in Glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Saccà, S.C.; Paluan, F.; Gandolfi, S.; Manni, G.; Cutolo, C.A.; Izzotti, A. Common Aspects between Glaucoma and Brain Neurodegeneration. Mutat. Res./Rev. Mutat. Res. 2020, 786, 108323. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P.; De Groot, V. A New Glaucoma Hypothesis: A Role of Glymphatic System Dysfunction. Fluids Barriers CNS 2015, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Shimazawa, M.; Tsuruma, K.; Mayama, C.; Ishii, K.; Onoe, H.; Aihara, M.; Araie, M.; Hara, H. Induction of Amyloid-β(1-42) in the Retina and Optic Nerve Head of Chronic Ocular Hypertensive Monkeys. Mol. Vis. 2012, 18, 2647–2657. [Google Scholar]
- Gupta, N.; Yücel, Y.H. Glaucoma as a Neurodegenerative Disease. Curr. Opin. Ophthalmol. 2007, 18, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Kamel, K.; Farrell, M.; O’Brien, C. Mitochondrial Dysfunction in Ocular Disease: Focus on Glaucoma. Mitochondrion 2017, 35, 44–53. [Google Scholar] [CrossRef]
- Rolle, T.; Ponzetto, A.; Malinverni, L. The Role of Neuroinflammation in Glaucoma: An Update on Molecular Mechanisms and New Therapeutic Options. Front. Neurol. 2021, 11, 612422. [Google Scholar] [CrossRef]
- Bucolo, C.; Campana, G.; Di Toro, R.; Cacciaguerra, S.; Spampinato, S. Sigma1 Recognition Sites in Rabbit Iris-Ciliary Body: Topical Sigma1-Site Agonists Lower Intraocular Pressure. J. Pharm. Exp. 1999, 289, 1362–1369. [Google Scholar]
- Stahl, A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch. Aerztebl. Int. 2020, 117, 513–520. [Google Scholar] [CrossRef]
- Ricci, F.; Bandello, F.; Navarra, P.; Staurenghi, G.; Stumpp, M.; Zarbin, M. Neovascular Age-Related Macular Degeneration: Therapeutic Management and New-Upcoming Approaches. Int. J. Mol. Sci. 2020, 21, 8242. [Google Scholar] [CrossRef]
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial Oxidative Stress in the Retinal Pigment Epithelium (RPE) Led to Metabolic Dysfunction in Both the RPE and Retinal Photoreceptors. Redox Biol. 2019, 24, 101201. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Akhlaq, A. Sustained Suppression of VEGF for Treatment of Retinal/Choroidal Vascular Diseases. Prog. Retin. Eye Res. 2021, 83, 100921. [Google Scholar] [CrossRef]
- Kwak, N.; Okamoto, N.; Wood, J.M.; Campochiaro, P.A. VEGF Is Major Stimulator in Model of Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3158–3164. [Google Scholar]
- Intartaglia, D.; Giamundo, G.; Conte, I. The Impact of MiRNAs in Health and Disease of Retinal Pigment Epithelium. Front. Cell Dev. Biol. 2021, 8, 589985. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Gericke, A. Oxidative Stress and Vascular Dysfunction in the Retina: Therapeutic Strategies. Antioxidants 2020, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Whitson, H.E.; Ansah, D.; Whitaker, D.; Potter, G.; Cousins, S.W.; MacDonald, H.; Pieper, C.F.; Landerman, L.; Steffens, D.C.; Cohen, H.J. Prevalence and Patterns of Comorbid Cognitive Impairment in Low Vision Rehabilitation for Macular Disease. Arch. Gerontol. Geriatr. 2010, 50, 209–212. [Google Scholar] [CrossRef] [Green Version]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef] [PubMed]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 591. [Google Scholar] [CrossRef]
- Thagaard, M.S.; Vergmann, A.S.; Grauslund, J. Topical Treatment of Diabetic Retinopathy: A Systematic Review. Acta Ophthalmol. 2021, aos.14912. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Di Paola, L.; Leggio, G.M.; Romano, G.L.; Drago, F.; Salomone, S.; Bucolo, C. Molecular Features of Interaction between VEGFA and Anti-Angiogenic Drugs Used in Retinal Diseases: A Computational Approach. Front. Pharmacol. 2015, 6, 248. [Google Scholar] [CrossRef] [Green Version]
- Zafar, S.; Sachdeva, M.; Frankfort, B.J.; Channa, R. Retinal Neurodegeneration as an Early Manifestation of Diabetic Eye Disease and Potential Neuroprotective Therapies. Curr. Diabetes Rep. 2019, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Joltikov, K.A.; Sesi, C.A.; de Castro, V.M.; Davila, J.R.; Anand, R.; Khan, S.M.; Farbman, N.; Jackson, G.R.; Johnson, C.A.; Gardner, T.W. Disorganization of Retinal Inner Layers (DRIL) and Neuroretinal Dysfunction in Early Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5481. [Google Scholar] [CrossRef] [Green Version]
- Rudraraju, M.; Narayanan, S.P.; Somanath, P.R. Regulation of Blood-Retinal Barrier Cell-Junctions in Diabetic Retinopathy. Pharmacol. Res. 2020, 161, 105115. [Google Scholar] [CrossRef] [PubMed]
- Giurdanella, G.; Anfuso, C.D.; Olivieri, M.; Lupo, G.; Caporarello, N.; Eandi, C.M.; Drago, F.; Bucolo, C.; Salomone, S. Aflibercept, Bevacizumab and Ranibizumab Prevent Glucose-Induced Damage in Human Retinal Pericytes in Vitro, through a PLA2/COX-2/VEGF-A Pathway. Biochem. Pharmacol. 2015, 96, 278–287. [Google Scholar] [CrossRef]
- Barber, A.J. A New View of Diabetic Retinopathy: A Neurodegenerative Disease of the Eye. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 283–290. [Google Scholar] [CrossRef]
- Dogan, M.; Ozsoy, E.; Doganay, S.; Burulday, V.; Firat, P.G.; Ozer, A.; Alkan, A. Brain Diffusion-Weighted Imaging in Diabetic Patients with Retinopathy. Eur. Rev. Med. Pharm. Sci. 2012, 16, 126–131. [Google Scholar]
- Huang, X.; Tong, Y.; Qi, C.-X.; Xu, Y.-T.; Dan, H.-D.; Shen, Y. Disrupted Topological Organization of Human Brain Connectome in Diabetic Retinopathy Patients. Neuropsychiatr. Dis. Treat. 2019, 15, 2487–2502. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.F.; Chakarova, C.F.; Abd El-Aziz, M.M.; Bhattacharya, S.S. Photoreceptor Degeneration: Genetic and Mechanistic Dissection of a Complex Trait. Nat. Rev. Genet. 2010, 11, 273–284. [Google Scholar] [CrossRef]
- Sahel, J.-A.; Marazova, K.; Audo, I. Clinical Characteristics and Current Therapies for Inherited Retinal Degenerations. Col Sprin Harb. Perspect. Med. 2015, 5, a017111. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, T.B.; Luther, E.E. Retinitis Pigmentosa. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sahni, J.N.; Angi, M.; Irigoyen, C.; Angi, M.; Semeraro, F.; Romano, M.R.; Parmeggiani, F.; Parmeggiani, F. Therapeutic Challenges to Retinitis Pigmentosa: From Neuroprotection to Gene Therapy. Curr. Genom. 2011, 12, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.F.; Joo, K.; Kemp, J.A.; Fialho, S.L.; da Silva Cunha, A.; Woo, S.J.; Kwon, Y.J. Molecular Genetics and Emerging Therapies for Retinitis Pigmentosa: Basic Research and Clinical Perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, F.S.; Gallenga, C.E.; Bonifazzi, C.; Perri, P. A Challenge to the Striking Genotypic Heterogeneity of Retinitis Pigmentosa: A Better Understanding of the Pathophysiology Using the Newest Genetic Strategies. Eye 2016, 30, 1542–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobanova, E.S.; Finkelstein, S.; Li, J.; Travis, A.M.; Hao, Y.; Klingeborn, M.; Skiba, N.P.; Deshaies, R.J.; Arshavsky, V.Y. Increased Proteasomal Activity Supports Photoreceptor Survival in Inherited Retinal Degeneration. Nat. Commun. 2018, 9, 1738. [Google Scholar] [CrossRef]
- Campello, L.; Esteve-Rudd, J.; Cuenca, N.; Martín-Nieto, J. The Ubiquitin–Proteasome System in Retinal Health and Disease. Mol. Neurobiol. 2013, 47, 790–810. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Dopamine Outside the Brain: The Eye, Cardiovascular System and Endocrine Pancreas. Pharmacol. Ther. 2019, 203, 107392. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.L.; Platania, C.B.M.; Drago, F.; Salomone, S.; Ragusa, M.; Barbagallo, C.; Di Pietro, C.; Purrello, M.; Reibaldi, M.; Avitabile, T.; et al. Retinal and Circulating MiRNAs in Age-Related Macular Degeneration: An In Vivo Animal and Human Study. Front. Pharmacol. 2017, 8, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10, 2394. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10092394
Marchesi N, Fahmideh F, Boschi F, Pascale A, Barbieri A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells. 2021; 10(9):2394. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10092394
Chicago/Turabian StyleMarchesi, Nicoletta, Foroogh Fahmideh, Federica Boschi, Alessia Pascale, and Annalisa Barbieri. 2021. "Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas" Cells 10, no. 9: 2394. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10092394