Cholesterol Interaction Directly Enhances Intrinsic Activity of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)
Abstract
:1. Introduction
2. Results
2.1. CFTR: Lipid Complexes Are Purified Using Amphipol A8-35
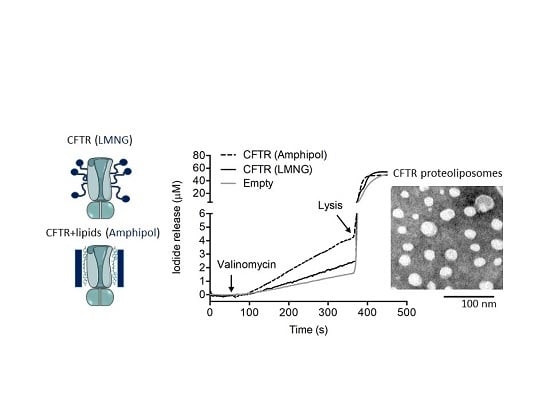
2.2. CFTR: Lipid: Amphipol Complexes Exhibit Higher Specific ATPase Activity and Higher Functional Reconstitution as Regulated Anion Channels than CFTR: Detergent Complexes
3. Discussion
4. Materials and Methods
4.1. Generation of FLAG-CFTR-His Construct
4.2. Expression of FLAG-CFTR-His Construct in HEK293F Cells and Generation of Crude Membranes
4.3. Purification of FLAG-CFTR-His in Amphipol or Detergent
4.4. CFTR Quantification
4.5. Phospholipid Assay
4.6. Lipid TLC
4.7. ATPase Assay–ATP Dose Response
4.8. ATPase Assay–Re-Introducing Lipids to Purified CFTR in LMNG Detergent Micelles
4.9. Iodide Efflux
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tsui, L.; Buchwald, M.; Barker, D.; Braman, J.; Knowlton, R.; Schumm, J.; Eiberg, H.; Mohr, J.; Kennedy, D.; Plavsic, N.; et al. Cystic fibrosis locus defined by a genetically linked polymorphic DNA marker. Science 1985, 230, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Kartner, N.; Hanrahan, J.W.; Jensen, T.J.; Naismith, A.; Sun, S.; Ackerley, C.A.; Reyes, E.F.; Tsui, L.-C.; Rommens, J.M.; Bear, C.E.; et al. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell 1991, 64, 681–691. [Google Scholar] [CrossRef]
- Kerem, B.; Rommens, J.; Buchanan, J.; Markiewicz, D.; Cox, T.; Chakravarti, A.; Buchwald, M.; Tsui, L. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989, 245, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.B.; Tabcharani, J.A.; Hou, Y.X.; Jensen, T.J.; Kartner, N.; Alon, N.; Hanrahan, J.W.; Riordan, J.R. Protein kinase a (pka) still activates cftr chloride channel after mutagenesis of all 10 pka consensus phosphorylation sites. J. Biol. Chem. 1993, 268, 11304–11311. [Google Scholar] [PubMed]
- Seibert, F.S.; Chang, X.B.; Aleksandrov, A.A.; Clarke, D.M.; Hanrahan, J.W.; Riordan, J.R. Influence of phosphorylation by protein kinase a on cftr at the cell surface and endoplasmic reticulum. Biochim. Biophys. Acta. 1999, 1461, 275–283. [Google Scholar] [CrossRef]
- Winter, M.C.; Welsh, M.J. Stimulation of cftr activity by its phosphorylated r domain. Nature 1997, 389, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.C.; Sheppard, D.N. Gating of the cftr cl- channel by atp-driven nucleotide-binding domain dimerisation. J. Physiol. 2009, 587, 2151–2161. [Google Scholar] [CrossRef]
- Ramjeesingh, M.; Ugwu, F.; Stratford, F.L.; Huan, L.J.; Li, C.; Bear, C.E. The intact cftr protein mediates atpase rather than adenylate kinase activity. Biochem. J. 2008, 412, 315–321. [Google Scholar] [CrossRef]
- Mihalyi, C.; Torocsik, B.; Csanady, L. Obligate coupling of cftr pore opening to tight nucleotide-binding domain dimerization. eLife 2016, 5, e18164. [Google Scholar] [CrossRef]
- Vergani, P.; Lockless, S.W.; Nairn, A.C.; Gadsby, D.C. Cftr channel opening by atp-driven tight dimerization of its nucleotide-binding domains. Nature 2005, 433, 876–880. [Google Scholar] [CrossRef]
- Aleksandrov, A.A.; Aleksandrov, L.A.; Riordan, J.R. Cftr (abcc7) is a hydrolyzable-ligand-gated channel. Pflugers Arch. Eur. J. Physiol. 2007, 453, 693–702. [Google Scholar] [CrossRef]
- Stratford, F.L.; Ramjeesingh, M.; Cheung, J.C.; Huan, L.J.; Bear, C.E. The walker b motif of the second nucleotide-binding domain (nbd2) of cftr plays a key role in atpase activity by the nbd1-nbd2 heterodimer. Biochem. J. 2007, 401, 581–586. [Google Scholar] [CrossRef]
- Kogan, I.; Ramjeesingh, M.; Li, C.; Bear, C.E. Studies of the molecular basis for cystic fibrosis using purified reconstituted cftr protein. Methods Mol. Med. 2002, 70, 143–157. [Google Scholar]
- Kogan, I.; Ramjeesingh, M.; Huan, L.J.; Wang, Y.; Bear, C.E. Perturbation of the pore of the cystic fibrosis transmembrane conductance regulator (cftr) inhibits its atpase activity. J. Biol. Chem. 2001, 276, 11575–11581. [Google Scholar] [CrossRef]
- Kidd, J.F.; Ramjeesingh, M.; Stratford, F.; Huan, L.J.; Bear, C.E. A heteromeric complex of the two nucleotide binding domains of cystic fibrosis transmembrane conductance regulator (cftr) mediates atpase activity. J. Biol. Chem. 2004, 279, 41664–41669. [Google Scholar] [CrossRef]
- Baker, J.M.R.; Hudson, R.P.; Kanelis, V.; Choy, W.-Y.; Thibodeau, P.H.; Thomas, P.J.; Forman-Kay, J.D. Cftr regulatory region interacts with nbd1 predominantly via multiple transient helices. Nat. Struct. Mol. Biol. 2007, 14, 738–745. [Google Scholar] [CrossRef]
- Kanelis, V.; Hudson, R.P.; Thibodeau, P.H.; Thomas, P.J.; Forman-Kay, J.D. Nmr evidence for differential phosphorylation-dependent interactions in wt and deltaf508 cftr. EMBO J. 2010, 29, 263–277. [Google Scholar] [CrossRef]
- Dawson, J.E.; Farber, P.J.; Forman-Kay, J.D. Allosteric coupling between the intracellular coupling helix 4 and regulatory sites of the first nucleotide-binding domain of cftr. PLoS ONE 2013, 8, e74347. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Csanady, L.; Gadsby, D.C.; Chen, J. Molecular structure of the human cftr ion channel. Cell 2017, 169, 85–95.e8. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, F.; Chen, J. Conformational changes of cftr upon phosphorylation and atp binding. Cell 2017, 170, 483–491.e8. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J. Atomic structure of the cystic fibrosis transmembrane conductance regulator. Cell 2016, 167, 1586–1597.e9. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, F.; Chen, J. Molecular structure of the atp-bound, phosphorylated human cftr. Proc. Natl. Acad. Sci. USA 2018, 115, 12757–12762. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Levit, A.; Levring, J.; Touhara, K.K.; Shoichet, B.K.; Chen, J. Structural identification of a hotspot on cftr for potentiation. Science 2019, 364, 1184–1188. [Google Scholar] [CrossRef]
- Jackson, S.M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N.M.I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B.; et al. Structural basis of small-molecule inhibition of human multidrug transporter abcg2. Nat. Struct. Mol. Biol. 2018, 25, 333–340. [Google Scholar] [CrossRef]
- Taylor, N.M.I.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the human multidrug transporter abcg2. Nature 2017, 546, 504–509. [Google Scholar] [CrossRef]
- Josts, I.; Nitsche, J.; Maric, S.; Mertens, H.D.; Moulin, M.; Haertlein, M.; Prevost, S.; Svergun, D.I.; Busch, S.; Forsyth, V.T.; et al. Conformational states of abc transporter msba in a lipid environment investigated by small-angle scattering using stealth carrier nanodiscs. Structure 2018, 26, 1072–1079.e4. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Lelong, I.H.; Zhang, J.; Cardarelli, C. Purification and reconstitution of human p-glycoprotein. Methods Enzymol. 1998, 292, 492–504. [Google Scholar]
- Ritchie, T.K.; Grinkova, Y.V.; Bayburt, T.H.; Denisov, I.G.; Zolnerciks, J.K.; Atkins, W.M.; Sligar, S.G. Chapter 11—reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 2009, 464, 211–231. [Google Scholar]
- Hirayama, H.; Kimura, Y.; Kioka, N.; Matsuo, M.; Ueda, K. Atpase activity of human abcg1 is stimulated by cholesterol and sphingomyelin. J. Lipid Res. 2013, 54, 496–502. [Google Scholar] [CrossRef]
- Infed, N.; Hanekop, N.; Driessen, A.J.; Smits, S.H.; Schmitt, L. Influence of detergents on the activity of the abc transporter lmra. Biochim. Biophys. Acta 2011, 1808, 2313–2321. [Google Scholar] [CrossRef]
- Kawai, T.; Caaveiro, J.M.; Abe, R.; Katagiri, T.; Tsumoto, K. Catalytic activity of msba reconstituted in nanodisc particles is modulated by remote interactions with the bilayer. FEBS Lett. 2011, 585, 3533–3537. [Google Scholar] [CrossRef]
- Doerrler, W.T.; Raetz, C.R. Atpase activity of the msba lipid flippase of escherichia coli. J. Biol. Chem. 2002, 277, 36697–36705. [Google Scholar] [CrossRef]
- Hildebrandt, E.; Khazanov, N.; Kappes, J.C.; Dai, Q.; Senderowitz, H.; Urbatsch, I.L. Specific stabilization of cftr by phosphatidylserine. Biochim. Biophys. Acta 2017, 1859, 289–293. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, C.; Zhou, Q.; An, J.; Hildebrandt, E.; Aleksandrov, L.A.; Kappes, J.C.; DeLucas, L.J.; Riordan, J.R.; Urbatsch, I.L.; et al. Membrane protein stability can be compromised by detergent interactions with the extramembranous soluble domains. Protein Sci. 2014, 23, 769–789. [Google Scholar] [CrossRef] [Green Version]
- Bechara, C.; Bolbach, G.; Bazzaco, P.; Sharma, K.S.; Durand, G.; Popot, J.-L.; Zito, F.; Sagan, S. Maldi-tof mass spectrometry analysis of amphipol-trapped membrane proteins. Anal Chem. 2012, 84, 6128–6135. [Google Scholar] [CrossRef]
- Gohon, Y.; Dahmane, T.; Ruigrok, R.W.H.; Schuck, P.; Charvolin, D.; Rappaport, F.; Timmins, P.; Engelman, D.M.; Tribet, C.; Popot, J.-L.; et al. Bacteriorhodopsin/amphipol complexes: Structural and functional properties. Biophys. J. 2008, 94, 3523–3537. [Google Scholar] [CrossRef]
- Martinez, K.L.; Gohon, Y.; Corringer, P.-J.; Tribet, C.; Merola, F.; Changeux, J.-P.; Popot, J.-L. Allosteric transitions of torpedo acetylcholine receptor in lipids, detergent and amphipols: Molecular interactions vs. physical constraints. FEBS Lett. 2002, 528, 251–256. [Google Scholar] [CrossRef]
- Ramjeesingh, M.; Li, C.; Wang, W.; Garami, E.; Hewryk, M.; Lee, D.; Rommens, J.M.; Galley, K.; Bear, C.E. Atpase activity of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 1996, 271, 28463–28468. [Google Scholar]
- Szellas, T.; Nagel, G. Apparent affinity of cftr for atp is increased by continuous kinase activity. FEBS Lett. 2003, 535, 141–146. [Google Scholar] [CrossRef]
- Eckford, P.D.; Li, C.; Ramjeesingh, M.; Bear, C.E. Cystic fibrosis transmembrane conductance regulator (cftr) potentiator vx-770 (ivacaftor) opens the defective channel gate of mutant cftr in a phosphorylation-dependent but atp-independent manner. J. Biol. Chem. 2012, 287, 36639–36649. [Google Scholar] [CrossRef]
- Eckford, P.D.; Li, C.; Bear, C.E. Functional reconstitution and channel activity measurements of purified wildtype and mutant cftr protein. J. Vis. Exp. 2015, 97, e52427. [Google Scholar] [CrossRef]
- Van Goor, F.; Burton, B.; Hadida, S.; Grootenhuis, P.; Olson, E.; Wine, J.; Frizzell, R.; Ashlock, M.; Negulescu, P. Rescue of cf airway epithelial cell function in vitro by a cftr potentiator, vx-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef]
- Van Goor, F.; Yu, H.; Burton, B.; Hoffman, B.J. Effect of ivacaftor on cftr forms with missense mutations associated with defects in protein processing or function. J. Cyst. Fibros. 2014, 13, 29–36. [Google Scholar] [CrossRef]
- Jih, K.Y.; Hwang, T.C. Vx-770 potentiates cftr function by promoting decoupling between the gating cycle and atp hydrolysis cycle. Proc. Natl. Acad. Sci. USA 2013, 110, 4404–4409. [Google Scholar] [CrossRef]
- Baroni, D.; Zegarra-Moran, O.; Svensson, A.; Moran, O. Direct interaction of a cftr potentiator and a cftr corrector with phospholipid bilayers. Eur. Biophys. J. 2014, 43, 341–346. [Google Scholar] [CrossRef]
- Hwang, T.C.; Yeh, J.T.; Zhang, J.; Yu, Y.C.; Yeh, H.I.; Destefano, S. Structural mechanisms of cftr function and dysfunction. J. Gen. Physiol. 2018, 150, 539–570. [Google Scholar] [CrossRef]
- Ramjeesingh, M.; Li, C.; Garami, E.; Huan, L.-J.; Hewryk, M.; Wang, Y.; Galley, K.; Bear, C.E. A novel procedure for the efficient purification of the cystic fibrosis transmembrane conductance regulator (cftr). Biochem. J. 1997, 327, 17–21. [Google Scholar] [CrossRef]
- Bear, C.E.; Li, C.; Kartner, N.; Bridges, R.J.; Jensen, T.J.; Ramjeesingh, M.; Riordan, J.R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (cftr). Cell 1992, 68, 809–818. [Google Scholar] [CrossRef]
- Gadsby, D.C.; Vergani, P.; Csanady, L. The abc protein turned chloride channel whose failure causes cystic fibrosis. Nature 2006, 440, 477–483. [Google Scholar] [CrossRef]
- Cheung, J.C.; Chiaw, P.K.; Pasyk, S.; Bear, C.E. Molecular basis for the atpase activity of cftr. Arch. Biochem. Biophys. 2008, 476, 95–100. [Google Scholar] [CrossRef]
- Li, C.; Ramjeesingh, M.; Bear, C.E. Purified cystic fibrosis transmembrane conductance regulator (cftr) does not function as an atp channel. J. Biol. Chem. 1996, 271, 11623–11626. [Google Scholar] [CrossRef]
- Byrnes, L.J.; Xu, Y.; Qiu, X.; Hall, J.D.; West, G.M. Sites associated with kalydeco binding on human cystic fibrosis transmembrane conductance regulator revealed by hydrogen/deuterium exchange. Sci. Rep. 2018, 8, 4664. [Google Scholar]
- Yeh, H.I.; Qiu, L.; Sohma, Y.; Conrath, K.; Zou, X.; Hwang, T.C. Identifying the molecular target sites for cftr potentiators glpg1837 and vx-770. J. Gen. Physiol. 2019, 151, 912–928. [Google Scholar] [CrossRef]
- Chin, S.; Hung, M.; Won, A.; Wu, Y.-S.; Ahmadi, S.; Yang, D.; Elmallah, S.; Toutah, K.; Hamilton, C.M.; Young, R.N.; et al. Lipophilicity of the cystic fibrosis drug, ivacaftor (vx-770), and its destabilizing effect on the major cf-causing mutation: F508del. Mol. Pharmacol. 2018, 94, 917–925. [Google Scholar] [CrossRef]
- Abu-Arish, A.; Pandzic, E.; Goepp, J.; Matthes, E.; Hanrahan, J.W.; Wiseman, P.W. Cholesterol modulates cftr confinement in the plasma membrane of primary epithelial cells. Biophys. J. 2015, 109, 85–94. [Google Scholar] [CrossRef]
- Cholon, D.M.; O’Neal, W.K.; Randell, S.H.; Riordan, J.R.; Gentzsch, M. Modulation of endocytic trafficking and apical stability of cftr in primary human airway epithelial cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L304–L314. [Google Scholar]
- Abu-Arish, A.; Pandzic, E.; Kim, D.; Tseng, H.W.; Wiseman, P.W.; Hanrahan, J.W. Agonists that stimulate secretion promote the recruitment of cftr into membrane lipid microdomains. J. Gen. Physiol. 2019, 151, 834–849. [Google Scholar] [CrossRef]
- Hildebrandt, E.; Zhang, Q.; Cant, N.; Ding, H.; Dai, Q.; Peng, L.; Fu, Y.; DeLucas, L.J.; Ford, R.; Kappes, J.C.; et al. A survey of detergents for the purification of stable, active human cystic fibrosis transmembrane conductance regulator (cftr). Biochim. Biophys. Acta 2014, 1838, 2825–2837. [Google Scholar] [CrossRef]
- Hajdu, P.; Varga, Z.; Pieri, C.; Panyi, G.; Gaspar, R. Cholesterol modifies the gating of kv1.3 in human t lymphocytes. Pflugers Arch. 2003, 445, 674–682. [Google Scholar] [CrossRef]
- Abi-Char, J.; Maguy, A.; Coulombe, A.; Balse, E.; Ratajczak, P.; Samuel, J.-L.; Nattel, S.; Hatem, S.N. Membrane cholesterol modulates kv1.5 potassium channel distribution and function in rat cardiomyocytes. J. Physiol. 2007, 582, 1205–1217. [Google Scholar] [CrossRef]
- Lundbæk, J.A.; Birn, P.; Hansen, A.J.; Søgaard, R.; Nielsen, C.; Girshman, J.; Bruno, M.J.; Tape, S.E.; Egebjerg, J.; Greathouse, D.V.; et al. Regulation of sodium channel function by bilayer elasticity: The importance of hydrophobic coupling. Effects of micelle-forming amphiphiles and cholesterol. J. Gen. Physiol. 2004, 123, 599–621. [Google Scholar]
- Tang, L.; El-Din, T.M.G.; Swanson, T.M.; Pryde, D.C.; Scheuer, T.; Zheng, N.; Catterall, W.A. Structural basis for inhibition of a voltage-gated ca(2+) channel by ca(2+) antagonist drugs. Nature 2016, 537, 117–121. [Google Scholar] [CrossRef]
- Ereño-Orbea, J.; Sicard, T.; Cui, H.; Mazhab-Jafari, M.T.; Benlekbir, S.; Guarné, A.; Rubinstein, J.L.; Julien, J.-P. Molecular basis of human cd22 function and therapeutic targeting. Nat. Commun. 2017, 8, 764. [Google Scholar] [CrossRef]
- Ereno-Orbea, J.; Sicard, T.; Cui, H.; Akula, I.; Julien, J.P. Characterization of glycoproteins with the immunoglobulin fold by x-ray crystallography and biophysical techniques. J. Vis. Exp. 2018, 137, e57750. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Jin, D.H.; Ogawa, T.; Muramoto, K. Acid hydrolysis of protein in a microcapillary tube for the recovery of tryptophan. Biosci. Biotechnol. Biochem. 2005, 69, 255–257. [Google Scholar] [CrossRef]
- Dörr, J.M.; Koorengevel, M.C.; Schäfer, M.; Prokofyev, A.V.; Scheidelaar, S.; van der Cruijsen, E.A.; Dafforn, T.R.; Baldus, M.; Killian, J.A. Detergent-free isolation, characterization, and functional reconstitution of a tetrameric k+ channel: The power of native nanodiscs. Proc. Natl. Acad. Sci. USA 2014, 111, 18607–18612. [Google Scholar] [CrossRef]
- Chifflet, S.; Torriglia, A.; Chiesa, R.; Tolosa, S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: Application to lens atpases. Anal. Biochem. 1988, 168, 1–4. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, S.; Ramjeesingh, M.; Hung, M.; Ereño-Oreba, J.; Cui, H.; Laselva, O.; Julien, J.-P.; Bear, C.E. Cholesterol Interaction Directly Enhances Intrinsic Activity of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). Cells 2019, 8, 804. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8080804
Chin S, Ramjeesingh M, Hung M, Ereño-Oreba J, Cui H, Laselva O, Julien J-P, Bear CE. Cholesterol Interaction Directly Enhances Intrinsic Activity of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). Cells. 2019; 8(8):804. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8080804
Chicago/Turabian StyleChin, Stephanie, Mohabir Ramjeesingh, Maurita Hung, June Ereño-Oreba, Hong Cui, Onofrio Laselva, Jean-Philippe Julien, and Christine E. Bear. 2019. "Cholesterol Interaction Directly Enhances Intrinsic Activity of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)" Cells 8, no. 8: 804. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8080804