Altered in Vitro Metabolomic Response of the Human Microbiota to Sweeteners
Abstract
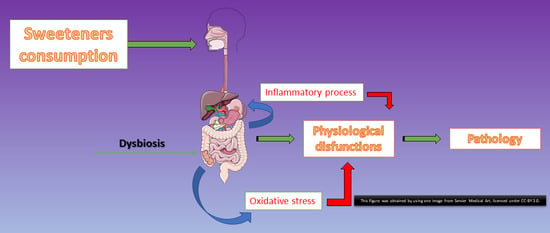
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Obtaining the Sweetener Samples
2.3. GIS1 In Vitro Model
2.4. Gut Microbiota Pattern Quantification by qPCR
2.5. Organic Acids and Ammonia Quantification by Capillary Electrophoresis (CE)
2.6. Statistical Analysis
3. Results
3.1. Alteration in the Metabolomic Pattern Post Sweetener In Vitro Treatment
3.2. Alterations in the Microbiota Pattern Post Sweetener In Vitro Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Sweeteners as food additives in the XXI century: A review of what is known, and what is to come. Food Chem. Toxicol. 2017, 107, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Kreuch, D.; Keating, D.J.; Wu, T.; Horowitz, M.; Rayner, C.K.; Young, R.L. Gut mechanisms linking intestinal sweet sensing to glycemic control. Front. Endocrinol. 2018, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016, 158, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of sweeteners on the gut microbiota: A review of experimental studies and clinical trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef] [PubMed]
- Aw, W.; Fukuda, S. Toward the comprehensive understanding of the gut ecosystem via metabolomics-based integrated omics approach. Semin. Immunopathol. 2015, 37, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucosesweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Invest. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Pelinescu, D.; Sarbu, I. Comparative fingerprinting of the human microbiota in diabetes and cardiovascular disease. J. Med. Food 2016, 19, 1188–1195. [Google Scholar] [CrossRef]
- Cárdenas-Castroa, A.P.; Bianchib, F.; Tallarico-Adornoc, M.A.; Montalvo-Gonzáleza, E.; Sáyago-Ayerdia, S.G.; Sivieri, K. In vitro colonic fermentation of Mexican “taco” from corn-tortilla and black beans in a simulator of human microbial ecosystem (SHIME®) system. Food Res. Int. 2019, 118, 81–88. [Google Scholar] [CrossRef]
- Umu, Ö.C.O.; Rudi, K.; Diep, D.B. Modulation of the gut microbiota by prebiotic fibers and bacteriocins. Microb. Ecol. Health Dis. 2017, 28, 1348886. [Google Scholar] [CrossRef] [PubMed]
- Kubica, P.; Namieśnik, J.; Wasik, A. Determination of eight artificial sweeteners and common Stevia rebaudiana glycosides in non-alcoholic and alcoholic beverages by reversed-phase liquid chromatography coupled with tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 407, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Pelinescu, D.; Avram, I. Antioxidative effects of phenolic compounds of mushrooms mycelia in simulated regions of the human colon, in vitro study. Pol. J. Food Nutr. Sci. 2018, 68, 83–90. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F.; Sârbu, I. In vitro ecological response of the human gut microbiome to bioactive extracts from edible wild mushrooms. Molecules 2018, 23, 2128. [Google Scholar] [CrossRef] [PubMed]
- Ketabi, A.; Dieleman, L.A.; Ganzle, M.G. Influence of isomalto-oligosaccharides on intestinalmicrobiota in rats. J. Appl. Micro. 2011, 110, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti De Gregoris, T.; Aldred, N.; Clare, A.S.; Burgess, J.G. Improvement of phylum-and class-specific primers for real-time PCR quantification of bacterial taxa. J. Microbiol. Methods 2011, 86, 351–356. [Google Scholar] [CrossRef]
- Gatea, F.; Teodor, E.D.; Paun, G.; Matei, A.O.; Radu, G.L. Capillary electrophoresis method validation for organic acids assessment in probiotics. Food Anal. Methods 2015, 8, 1335–1340. [Google Scholar] [CrossRef]
- Truică, G.I.; Teodor, E.D.; Radu, G.L. Organic acids assessments in medicinal plants by capillary electrophoresis. Rev. Roum. Chim. 2013, 58, 809–814. [Google Scholar]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 2019, 11, 51. [Google Scholar] [CrossRef]
- Ktsoyan, Z.A.; Mkrtchyan, M.S.; Zakharyan, M.K.; Mnatsakanyan, A.A.; Arakelova, K.A.; Gevorgyan, Z.U.; Sedrakyan, A.M.; Hovhannisyan, A.I.; Arakelyan, A.A.; Aminov, R.I. Systemic concentrations of short chain fatty acids are elevated in salmonellosis and exacerbation of familial mediterranean fever. Front. Microbiol. 2016, 7, 776. [Google Scholar] [CrossRef] [PubMed]
- Beloborodova, N.; Bairamov, I.; Olenin, A.; Shubina, V.; Teplova, V.; Fedotcheva, N. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J. Biomed. Sci. 2012, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Beloborodova, N.V.; Bairamov, I.T.; Olenin, A.Y.; Khabib, O.N.; Fedotcheva, N.I. Anaerobic microorganisms from human microbiota produce species-specific exometabolites important in heath and disease. Glob. J. Pathol. Microbiol. 2013, 1, 43–53. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Gokhale, D.V. Phenyllactic acid: A potential antimicrobial compound in lactic acid bacteria. J. Bacteriol. Mycol. 2016, 2, 121–125. [Google Scholar] [CrossRef]
- Yusuf, F.; Adewiah, S.; Syam, A.F.; Fatchiyah, F. Altered profile of gut microbiota and the level short chain fatty acids in colorectal cancer patients. J. Phys. Conf. Ser. 2019, 1146, 012037. [Google Scholar] [CrossRef]
- Kindt, A.; Liebisch, G.; Clavel, T.; Haller, D.; Hörmannsperger, G.; Yoon, H.; Kolmeder, D.; Sigruener, A.; Krautbauer, S.; Seeliger, C.; et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat. Commun. 2018, 14, 3760. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. Gut microbiome response to sucralose and its potential role in inducing liver inflammation in mice. Front. Physiol. 2017, 8, 487. [Google Scholar] [CrossRef]
- Sharma, A.; Amarnath, S.; Thulasimani, M.; Ramaswamy, S. Artificial sweeteners as a sugar substitute: Are they really safe? Indian J. Pharmacol. 2016, 48, 237–240. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Kunová, G.; Rada, V.; Vidaillac, A.; Lisova, I. Utilisation of steviol glycosides from Stevia rebaudiana (Bertoni) by lactobacilli and bifidobacteria in in vitro conditions. Folia Microbiol. 2014, 59, 251–255. [Google Scholar] [CrossRef]
- Farup, P.G.; Rudi, K.; Hestad, K. Faecal short-chain fatty acids—A diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol. 2016, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.P. Renwick AG biological fate of low-calorie sweeteners. Nutr. Rev. 2016, 74, 670–689. [Google Scholar] [CrossRef] [PubMed]
- Uebanso, T.; Ohnishi, A.; Kitayama, R.; Yoshimoto, A.; Nakahashi, M.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of low-dose non-caloric sweetener consumption on gut microbiota in mice. Nutrients 2017, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Deniņa, I.; Semjonovs, P.; Fomina, A.; Treimane, R.; Linde, R. The influence of stevia glycosides on the growth of Lactobacillus reuteri strains. Lett. Appl. Microbiol. 2014, 58, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Mansoorian, B.; Combet, E.; Alkhaldy, A.; Garcia, A.L.; Edwards, C.A. Impact of fermentable fibers on the colonic microbiota metabolism of dietary polyphenols rutin and quercetin. Int. J. Environ. Res. Pub. Health 2019, 16, 292. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, B.; Zambonin, L.; Angeloni, C.; Leoncini, E.; Dalla Sega, F.V.; Prata, C.; Fiorentini, D.; Hrelia, S. Steviol glycosides modulate glucose transport in different cell types. Oxid. Med. Cell. Longev. 2013, 2013, 348169. [Google Scholar] [CrossRef] [PubMed]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2018, 74, 799–809. [Google Scholar] [CrossRef]
- Ceunen, S.; Geuns, J.M.C. Steviol glycosides: Chemical diversity, metabolism, and function. J. Nat. Prod. 2013, 766, 1201–1228. [Google Scholar] [CrossRef]
- Murakami, S.; Goto, Y.; Ito, K.; Hayasaka, S.; Kurihara, S.; Soga, T.; Tomita, M.; Fukuda, S. The consumption of bicarbonate-rich mineral water improves glycemic control. Evid. Based Complement. Alt. Med. 2015, 2015, 10. [Google Scholar] [CrossRef]
- DuBois, G.E.; Prakash, I. Non-caloric sweeteners, sweetness modulators, and sweetener enhancers. Annu. Rev. Food Sci. Technol. 2012, 3, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, D.; Yeo, L.P.; Cecchini, F.; Koon, T.H.P.; Kushmaro, A.; Tok, A.I.Y.; Marks, R.S.; Eltzov, E. Measuring artificial sweeteners toxicity using a bioluminescent bacterial panel. Molecules 2018, 23, 2454. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.P.; Browman, D.; Herzog, H.; Neely, G.G. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS ONE 2018, 13, e0199080. [Google Scholar] [CrossRef]




| Samples Composition | Producer |
|---|---|
| Sodium cyclamate | KClassic Sweetener |
| Sucralose | Carrefour Quality BE |
| Sodium saccharin | Biscol LTD, Israel |
| Steviol powder | Kruger & Co., Germany |
| Steviol and brown sugar (1:1) | Tate & Lyle Sugars, Tesco, UK |
| Steviol capsule | Sly Nutricia SRL, Romania |
| White sugar | Sugar Factory Diamant, Romania |
| Oligofructose from chicory | Mărgăritar Sweet & Fit, AGRANA Zucker A.G. Vaslui, Romania |
| Groups | Sequence: 5′-3′ | Primer Conc. | Slope | Efficiency (%) | Reference | |
|---|---|---|---|---|---|---|
| Prokaryote | F | CGG YCC AGA CTC CTA CGG G | 0.2 µM | −3.22 | 104.16 | [15] |
| R | TTA CCG CGG CTG CTG GCA C | |||||
| Lactobacillus–Leuconostoc–Pediococcus Group | F | AGC AGT AGG GAA TCT TCC A | 0.5 µM | −3.51 | 92.70 | [16] |
| R | CAC CGC TAC ACA TGG AG | |||||
| Bifidobacterium sp. | F | TCG CGT CYG GTG TGA AAG | 0.3 µM | −3.49 | 93.38 | [16] |
| R | CCA CAT CCA GCR TCC AC | |||||
| Enterobacteriaceae Family | F | CAT TGA CGT TAC CCG CAG AAG AAG C | 0.3 µM | −3.39 | 97.17 | [15] |
| R | CTC TAC GAG ACT CAA GCT TGC | |||||
| Bacteroides–Prevotella–Porphyromonas Group | F | GGTGTCGGCTTAAGTGCCAT | 0.3 µM | −3.32 | 99.68 | [16] |
| R | CGGAYGTAAGGGCCGTGC | |||||
| Firmicutes Phylum | F | GGAGYATGTGGTTTAATTCGAAGCA | 0.5 µM | −3.28 | 101.52 | [17] |
| Samples | Formic Acid | Lactic Acid | Benzoic Acid | Phenyllactic Acid | HO-Phenyllactic Acid |
|---|---|---|---|---|---|
| Control | 34.21 ± 5.35 | nd | 1.71 ± 0.10 | 17.60 ± 0.36 | 44.58 ± 0.76 |
| Sodium cyclamate | 49.63 ± 3.70 c | nd | 1.71 ± 0.05 a | 24.19 ± 0.18 b | 48.81 ± 0.89 a |
| Sucralose | 41.29 ± 3.40 b | nd | 1.60 ± 0.07 a | 38.50 ± 1.99 c | 56.48 ± 1.57 b |
| Sodium saccharin | 32.13 ± 1.18 a | nd | 1.34 ± 0.1 c | 28.86 ± 1.02 a | 53.49 ± 1.36 b |
| Steviol powder | 12.68 ± 0.98 a | nd | 7.87 ± 0.15 b | 179.22 ± 4.46 c | 72.96 ± 1.36 b |
| Steviol and brown sugar | 46.85 ± 1.96 c | 314.43 ± 5.89 b | 12.06 ± 0.12c | 120.35 ± 4.15 a | 41.83 ± 1.07 c |
| Steviol capsule | nd | nd | 2.74 ± 0.09 a | 42.52 ± 0.52 b | 118.15 ± 1.40 a |
| White sugar | 313.51 ± 8.56 b | 291.52 ± 5.60 a | 23.66 ± 0.23 a | 8.68 ± 1.29 a | 141.00 ± 0.24 c |
| Oligofructose from chicory | 435.74 ± 3.93 c | nd | 3.90 ± 0.13 c | 55.96 ± 0.75 c | 42.38 ± 0.17 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamanu, E.; Pelinescu, D.; Gatea, F.; Sârbu, I. Altered in Vitro Metabolomic Response of the Human Microbiota to Sweeteners. Genes 2019, 10, 535. https://0-doi-org.brum.beds.ac.uk/10.3390/genes10070535
Vamanu E, Pelinescu D, Gatea F, Sârbu I. Altered in Vitro Metabolomic Response of the Human Microbiota to Sweeteners. Genes. 2019; 10(7):535. https://0-doi-org.brum.beds.ac.uk/10.3390/genes10070535
Chicago/Turabian StyleVamanu, Emanuel, Diana Pelinescu, Florentina Gatea, and Ionela Sârbu. 2019. "Altered in Vitro Metabolomic Response of the Human Microbiota to Sweeteners" Genes 10, no. 7: 535. https://0-doi-org.brum.beds.ac.uk/10.3390/genes10070535