Are Cyanobacteria an Ancestor of Chloroplasts or Just One of the Gene Donors for Plants and Algae?
Abstract
:1. Introduction
2. Historical and Theoretical Problems on the Origin of Chloroplasts
2.1. Historical Demarcation
2.2. Genocentrism and the Changes in Thinking at the Beginning of the 21st Century
“All of these data are consistent with the hypothesis that the chlDNA genes diverged from the blue-green algae well after the divergence of the eubacterial and eucaryotic nuclear lines. Therefore, chlDNAs must have originated by a symbiotic event between members of the eubacteria and eucaryotic nuclear lineages. Similarly, sequence data have indicated that the mtDNA rRNA genes are more homologous to bacterial rRNA genes than to rRNA genes of eukaryotic nuclei. They too probably also had an endosymbiotic origin.” ([35] p. 227)
2.3. Spread of Endosymbiotic Gene Transfers (EGT)
2.4. Currently Accepted Evidence for the Endosymbiotic, Cyanobacterial Origin of Chloroplasts
2.5. Archaeplastida and the Cyanobacterial Origin of Chloroplasts
2.6. Recent Discussions on the Mitochondrial Origin
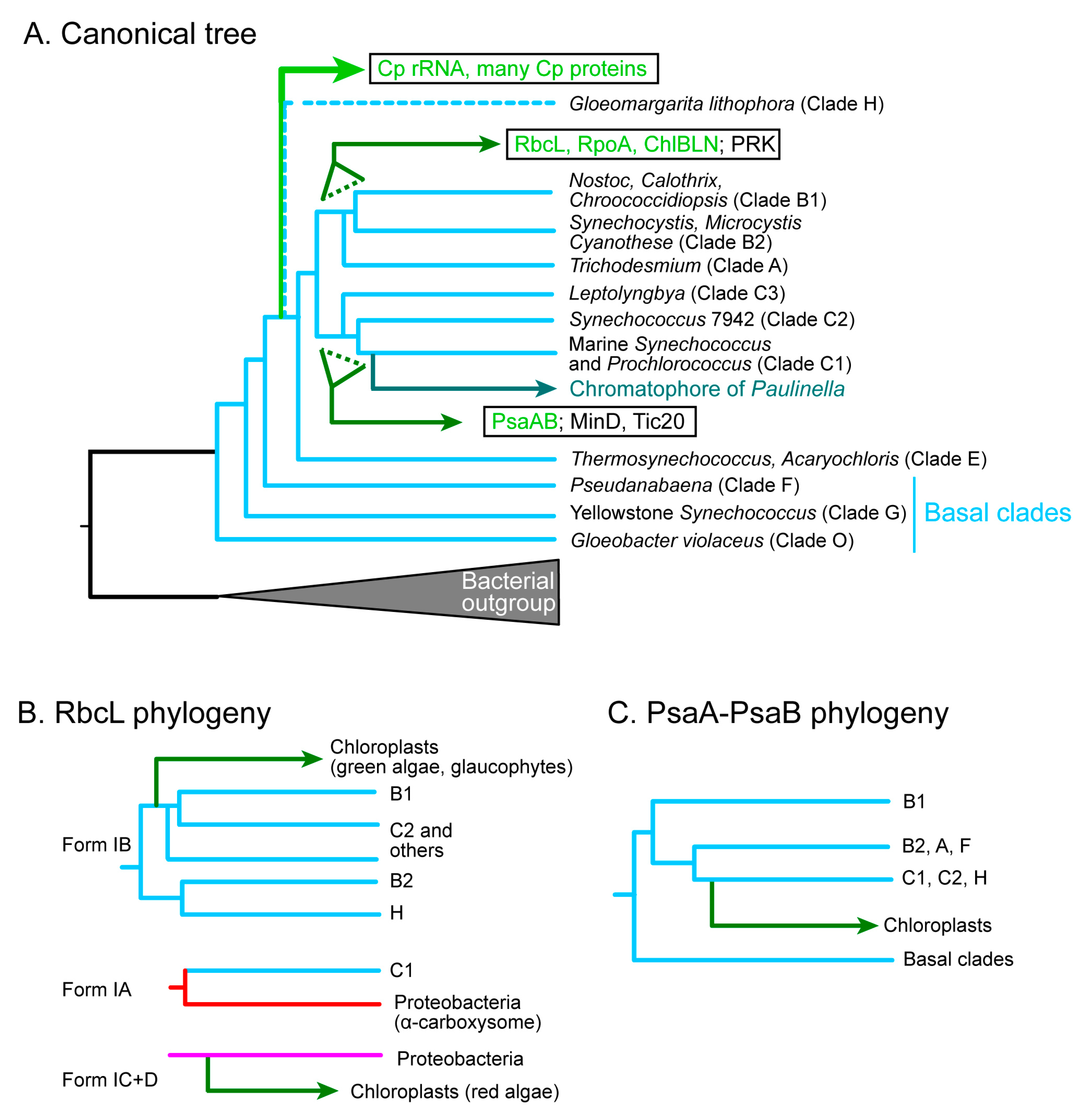
2.7. Phylogenetic Analysis of Chloroplast Enzymes
3. Origin of Structural Elements of Chloroplasts
3.1. Peptidoglycan Synthesis Enzymes
3.2. Lipid Biosynthesis Enzymes
3.2.1. Fatty Acid Synthesis
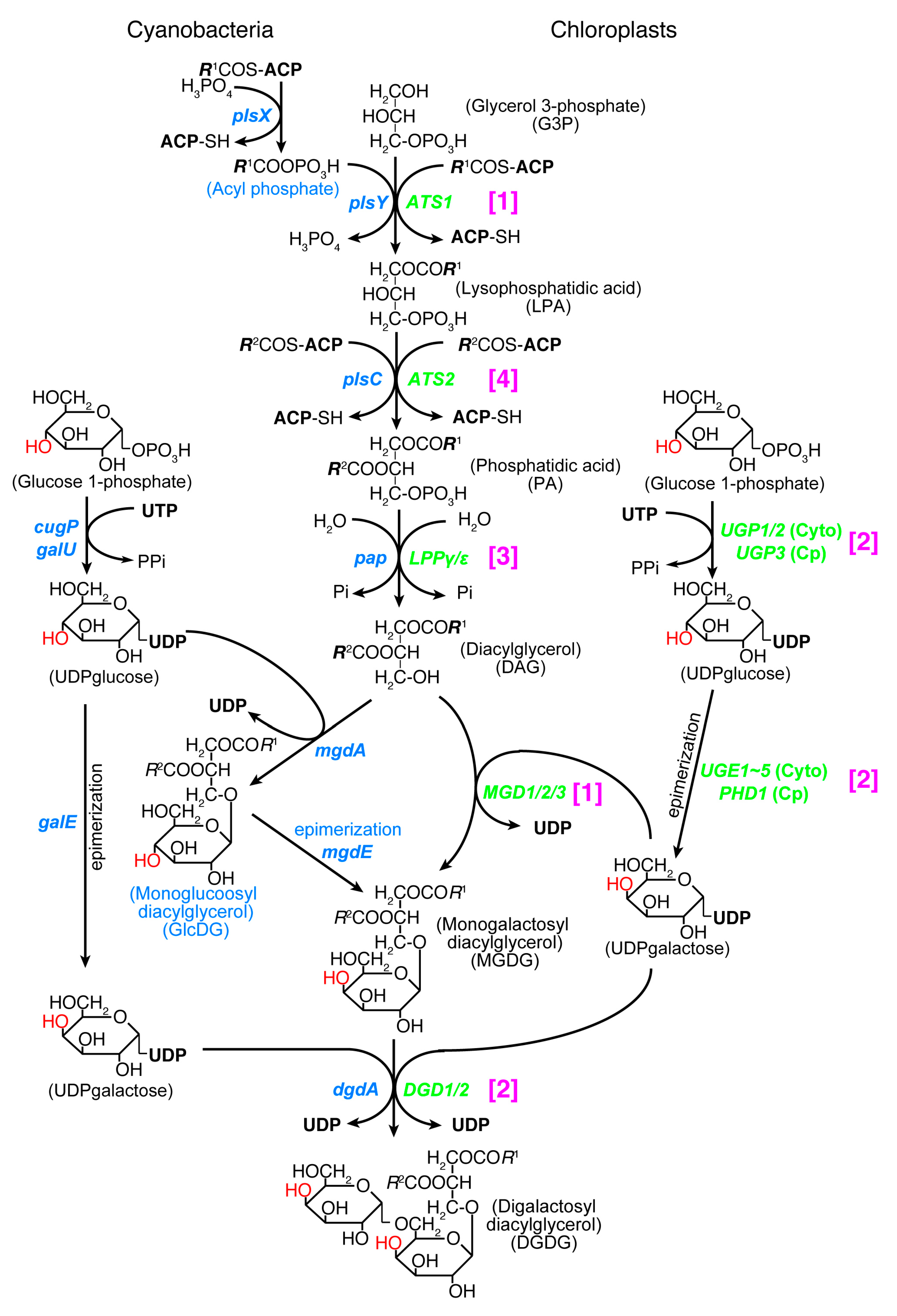
3.2.2. Chloroplast Lipid Biosynthesis
4. Diverse Origins of Chloroplast-Encoded Genes
5. Other Chloroplast Enzymes
5.1. Enzymes Related to DNA Replication
5.2. Division Machinery
5.3. Carbon Fixation and Amino Acid Synthesis
5.4. Translocon Components
6. Essential Differences in Chloroplasts from Paulinella Chromatophores
6.1. Chloroplasts and Chromatophores
6.2. Possibly Different Histories of Chloroplasts and Chromatophores
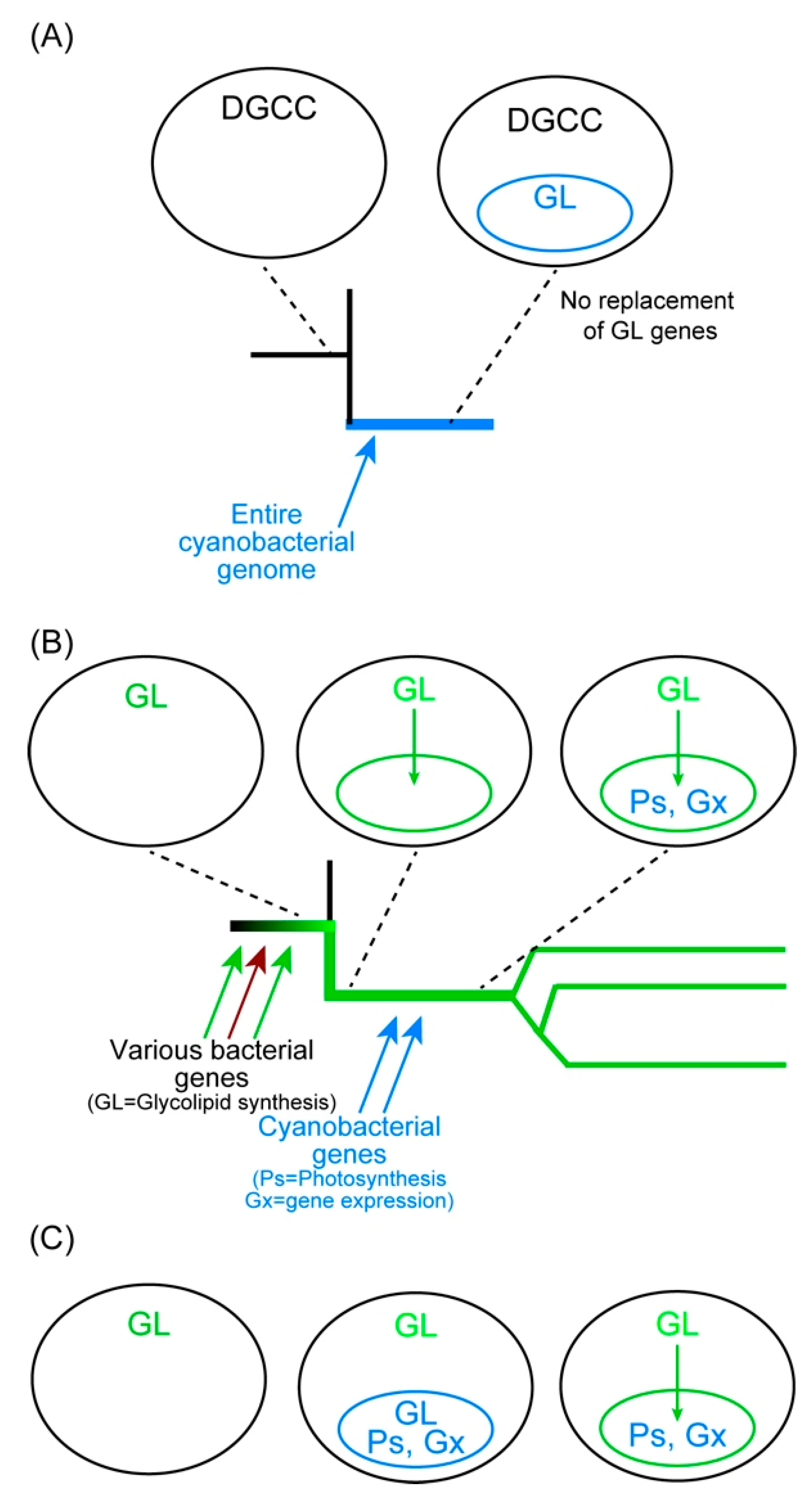
6.3. Extrachloroplast Glycolipids Are Traces of Their Eukaryotic Origin
6.4. Unified Model of Chromatophore and Chloroplast Formation
6.5. Additional Notes on the Models
6.5.1. DGDG Synthesis in Red Algae
6.5.2. Multiple Gene Transfers from Cyanobacteria and Other Bacteria
6.5.3. Eukaryotic Nature of Chloroplast Envelope
7. Conclusions: Flexible Views on the Origin
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mereschkowsky, C. Über Natur und Ursprung der Chromatophoren im Pflanzenreiche. Biol. Centralblatt 1905, 25, 593–604. [Google Scholar]
- Schimper, A.F.W. Über die Entwicklung der Chlorophyllkörper und Farbkörper. Bot. Z. 1883, 41, 105–162. [Google Scholar]
- Martin, W.; Kowallik, K. Annotated English translation of Mereschkowsky’s 1905 paper ‘Über Natur und Ursprung der Chromatophoren im Pflanzenreiche’. Eur. J. Phycol. 1999, 34, 287–295. [Google Scholar] [CrossRef] [Green Version]
- McFadden, G.I. Origin and evolution of plastids and photosynthesis in eukaryotes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archibald, J. One Plus One Equals One. Symbiosis and the Evolution of Complex Life; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Archibald, J.M. Endosymbiosis and eukaryotic cell evolution. Curr. Biol. 2015, 25, R911–R921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, W.F. Physiology, anaerobes, and the origin of mitosing cells 50 years on. J. Theor. Biol. 2017, 434, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Sato, N. Revisiting the theoretical basis of the endosymbiotic origin of plastids in the original context of Lynn Margulis on the origin of mitosing, eukaryotic cells. J. Theor. Biol. 2017, 434, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Sato, N. Endosymbiotic Theories of Organelles Revisited. In Retrospects and Prospects; Springer: Singapore, 2020. [Google Scholar]
- Kowallik, K.V.; Martin, W.F. The origin of symbiogenesis: An annotated English translation of Mereschkowsky’s 1910 paper on the theory of two plasma lineages. Biosystems 2021, 199, 104281. [Google Scholar] [CrossRef]
- Stiller, J.W. Toward an empirical framework for interpreting plastid evolution. J. Phycol. 2014, 50, 462–471. [Google Scholar] [CrossRef]
- Sagan, L. On the origin of mitosing cells. J. Theor. Biol. 1967, 14, 225–274. [Google Scholar] [CrossRef]
- Margulis, L. Origin of Eukaryotic Cells. Evidence and Research Implications for a Theory of the Origin and Evolution of Microbial, Plant, and Animal Cells on the Precambrian Earth; Yale University Press: New Haven, CT, USA, 1970. [Google Scholar]
- Gray, M.W. The pre-endosymbiont hypothesis: A new perspective on the origin and evolution of mitochondria. Cold Spring Harb. Perspect. Biol. 2014, 6, a016097. [Google Scholar] [CrossRef] [Green Version]
- Gray, M.W. Mosaic nature of the mitochondrial proteome: Implications for the origin and evolution of mitochondria. Proc. Natl. Acad. Sci. USA 2015, 112, 10133–10138. [Google Scholar] [CrossRef] [Green Version]
- Keeling, P.J. The impact of history on our perception of evolutionary events: Endosymbiosis and the origin of eukaryotic complexity. Cold Spring Harb. Perspect. Biol. 2014, 6, a016196. [Google Scholar] [CrossRef] [Green Version]
- Sapp, J. Evolution by Association; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Pascher, A. Studien über Symbiosen. Über einige Endosymbiosen von Blaualgen in Einzellern. Jahrb. Wiss. Bot. 1929, 71, 386–462. [Google Scholar]
- Buchner, P. Endosymbiose der Tiere mit Pflanzlichen Mikroorganismen; Birkhäuser: Basel, Switzerland, 1953. [Google Scholar]
- Lederberg, J. Cell genetics and hereditary symbiosis. Physiol. Rev. 1952, 32, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Kozo-Polyanski, B.M. Symbiogenesis: A New Principle of Evolution; Translated from Russian to English by Fet, V.; Harvard University Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Myer-Abich, A. Beiträge zur Theorie der Evolution der Organismen: II. Typensynthese durch Holobiose. Bibl. Biotheor. 1950, 5, 1–206. [Google Scholar]
- Echlin, P. The cyanophytic origin of higher plant chloroplasts. Br. Phycol. Bull. 1966, 3, 150–151. [Google Scholar]
- Goksøyr, J. Evolution of Eucaryotic cells. Nature 1967, 214, 1161. [Google Scholar] [CrossRef]
- Taylor, F.J.R. Implications and extensions of the serial endosymbiosis theory of the origin of eukaryotes. Taxon 1974, 23, 229–258. [Google Scholar] [CrossRef]
- Uzzell, T.; Spolsky, C. Mitochondria and plastids as endosymbionts: A rivival of special creation? Am. Sci. 1974, 62, 334–343. [Google Scholar]
- Cavalier-Smith, T. The origin of nuclei and eukaryotic cells. Nature 1975, 256, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, L. Evolution of organelles and eukaryotic genomes. Science 1975, 188, 891–898. [Google Scholar] [CrossRef]
- Stanier, R.Y. Some aspects of the biology of cells and their possible evolutionary significance. Symp. Soc. Gen. Microbiol. 1970, 20, 1–38. [Google Scholar]
- Cohen, S.S. Are/Were mitochondria and chloroplasts microorganisms? Am. Sci. 1970, 58, 281–289. [Google Scholar] [PubMed]
- Cohen, S.S. Mitochondria and chloroplasts revisited. Am. Sci. 1973, 61, 437–445. [Google Scholar] [PubMed]
- Stanier, R.Y. The origins of photosynthesis in eukaryotes. Symp. Soc. Gen. Microbiol. 1974, 24, 219–242. [Google Scholar]
- Schwartz, R.M.; Dayhoff, M.O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. A perspective is derived from protein and nucleic acid sequence data. Science 1978, 199, 395–403. [Google Scholar] [CrossRef]
- Gray, M.W.; Doolittle, W.F. Has the endosymbiont hypothesis been proven? Microbiol. Rev. 1982, 46, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Structure and evolution of organelle genomes. Microbiol. Rev. 1982, 46, 208–240. [Google Scholar] [CrossRef]
- Delwiche, C.F.; Kuhsel, M.; Palmer, J.D. Phylogenetic analysis of tufA sequences indicates a cyanobacterial origin of all plastids. Mol. Phylogenet. Evol. 1995, 4, 110–128. [Google Scholar] [CrossRef]
- Moreira, D.; Philippe, H. Sure facts and open questions about the origin and evolution of photosynthetic plastids. Res. Microbiol. 2001, 152, 771–780. [Google Scholar] [CrossRef]
- Delwiche, C.F. Tracing the thread of plastid diversity through the tapestry of life. Amer. Nat. 1999, 154, S164–S177. [Google Scholar] [CrossRef]
- Palmer, J.D. The symbiotic birth and spread of plastids: How many times and whodunit? J. Phycol. 2003, 39, 4–11. [Google Scholar] [CrossRef]
- Stiller, J.W.; Reel, D.C.; Johnson, J.C. A single origin of plastids revisited: Convergent evolution in organellar genome content. J. Phycol. 2003, 39, 95–105. [Google Scholar] [CrossRef]
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109–136. [Google Scholar] [CrossRef] [Green Version]
- McFadden, G.J. Primary and secondary endosymbiosis and the origin of plastids. J. Phycol. 2001, 37, 951–959. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. The origin of plastids. Biol. J. Linn. Soc. 1982, 17, 289–306. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. Membrane heredity and early chloroplast evolution. Trends Plant Sci. 2000, 5, 174–182. [Google Scholar] [CrossRef]
- Martin, W.F.; Garg, S.; Zimorski, V. Endosymbiotic theories for eukaryote origin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, N.A.; Reece, J.B.; Urry, L.A.; Cain, M.L.; Wasserman, S.A.; Minorsky, P.V.; Jackson, R.B. Biology, 10th ed.; Pearson Education: San Francisco, CA, USA, 2016. [Google Scholar]
- Martin, W.; Stoebe, B.; Goremykin, V.; Hansmann, S.; Hasegawa, M.; Kowallik, K.V. Gene transfer to the nucleus and the evolution of chloroplasts. Nature 1998, 393, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Ayliffe, M.A.; Timmis, J.N. Direct measurement of the transfer rate of chloroplast DNA into the nucleus. Nature 2003, 422, 72–76. [Google Scholar] [CrossRef]
- Timmis, J.N.; Ayliffe, M.A.; Huang, C.Y.; Martin, W. Endosymbiotic gene transfer: Organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 2004, 5, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Sato, N. Complex origins of chloroplast membranes with photosynthetic machineries: Multiple transfers of genes from divergent organisms at different times or a single endosymbiotic event? J. Plant Res. 2020, 133, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Ohta, N.; Sato, N.; Nozaki, H.; Kuroiwa, T. Analysis of the cluster of ribosomal protein genes in the plastid genome of a unicellular red alga Cyanidioschyzon merolae: Translocation of the str cluster as an early event in the Rhodophyte–Chromophyte lineage of plastid evolution. J. Mol. Evol. 1997, 45, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Stoebe, B.; Kowallik, K.V. Gene-cluster analysis in chloroplast genomics. Trends Genet. 1999, 15, 344–347. [Google Scholar] [CrossRef]
- Shih, P.M.; Wu, D.; Latifi, A.; Axen, S.; Fewer, D.P.; Talla, E.; Calteau, A.; Cai, F.; de Marsac, N.T.; Rippka, R.; et al. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 1053–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponce-Toledo, R.I.; Deschamps, P.; López-García, P.; Zivanovic, Y.; Benzerara, K.; Moreira, D. An early-branching freshwater cyanobacterium at the origin of plastids. Curr. Biol. 2017, 27, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Ezpeleta, N.; Brinkmann, H.; Burey, S.C.; Roure, B.; Burger, G.; Löffelhardt, W.; Bohnert, H.J.; Philippe, H.; Lang, B.F. Monophyly of primary photosynthetic eukaryotes: Green plants, red algae, and glaucophytes. Curr. Biol. 2005, 15, 1325–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, M.; Misumi, O.; Shin-I, T.; Maruyama, S.; Takahara, M.; Miyagishima, S.-Y.; Mori, T.; Nishida, K.; Yagisawa, F.; Nishida, K.; et al. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 2004, 428, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Prieto, A.; Bhattacharya, D. Phylogeny of Calvin cycle enzymes supports Plantae monophyly. Mol. Phyl. Evol. 2007, 45, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Stiller, J.W. Plastid endosymbiosis, genome evolution and the origin of green plants. Trends Plant Sci. 2007, 12, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, H.; Iseki, M.; Hasegawa, M.; Misawa, K.; Nakada, T.; Sasaki, N.; Watanabe, M. Phylogeny of primary photosynthetic eukaryotes as deduced from slowly evolving nuclear genes. Mol. Biol. Evol. 2007, 24, 1592–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawryluk, R.M.R.; Tikhonenkov, D.V.; Hehenberger, E.; Husnik, F.; Mylnikov, A.P.; Keeling, P.J. Non-photosynthetic predators are sister to red algae. Nature 2019, 572, 240–243. [Google Scholar] [CrossRef]
- Adl, S.M.; Simpson, A.G.B.; Farmer, M.A.; Andersen, R.A.; Anderson, O.R.; Barta, J.; Bowser, S.S.; Brugerolle, G.; Fensome, R.A.; Fredericq, S.; et al. The new higher level classification of Eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005, 52, 399–451. [Google Scholar] [CrossRef] [PubMed]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittis, A.A.; Gabaldón, T. Late acquisition of mitochondria by a host with chimaeric prokaryotic ancestry. Nature 2016, 531, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Gabaldón, T. Relative timing of mithochondrial endosymbiosis and the “pre-mitochondrial symbioses” hypothesis. IUBMB Life 2018, 70, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Harish, A.; Kurland, C.G. Mitochondria are not captive bacteria. J. Theor. Biol. 2017, 434, 88–98. [Google Scholar] [CrossRef]
- Pfanzagl, B.; Zenker, A.; Pittenauer, E.; Allmaier, G.; Martinez-Torrecuadrada, J.; Schmid, E.R.; De Pedro, M.A.; Löffelhardt, W. Primary structure of cyanelle peptidoglycan of Cyanophora paradoxa: A prokaryotic cell wall as part of an organelle envelope. J. Bacteriol. 1996, 178, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanier, R.Y.; Adelberg, E.A.; Ingraham, J.L. The Microbial World, 4th ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1976. [Google Scholar]
- Takano, H.; Takechi, K. Plastid peptidoglycan. Biochim. Biophys. Acta 2010, 1800, 144–151. [Google Scholar] [CrossRef]
- Lin, X.; Li, N.; Kudo, H.; Zhang, Z.; Li, J.; Wang, L.; Zhang, W.; Takechi, K.; Takano, H. Genes sufficient for synthesizing peptidoglycan are retained in gymnosperm genomes, and MurE from Larix gmelinii can rescue the albino phenotype of Arabidopsis MurE mutation. Plant Cell Physiol. 2017, 58, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Myouga, F.; Takechi, K.; Sato, H.; Nabeshima, K.; Nagata, N.; Takio, S.; Shinozaki, K.; Takano, H. An Arabidopsis homolog of the bacterial peptidoglycan synthesis enzyme MurE has an essential role in chloroplast development. Plant J. 2008, 53, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Nowack, E.C.; Melkonian, M.; Glöckner, G. Chromatophore genome sequence of Paulinella sheds light on acquisition of photosynthesis by eukaryotes. Curr. Biol. 2008, 18, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Nowack, E.C.; Price, D.C.; Bhattacharya, D.; Singer, A.; Melkonian, M.; Grossman, A.R. Gene transfers from diverse bacteria compensate for reductive genome evolution in the chromatophore of Paulinella chromatophora. Proc. Natl. Acad. Sci. USA 2016, 113, 12214–12219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lhee, D.; Yang, E.C.; Kim, J.I.; Nakayama, T.; Zuccarello, G.; Andersen, R.A.; Yoon, H.S. Diversity of the photosynthetic Paulinella species, with the description of Paulinella micropora sp. nov. and the chromatophore genome sequence for strain KR01. Protist 2017, 168, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Yoshitomi, T.; Mori-Moriyama, N. Characterization and biosynthesis of lipids in Paulinella micropora MYN1. Evidence for efficient integration of chromatophores into cellular lipid metabolism. Plant Cell Physiol. 2020, 61, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Tanidokoro, K.; Shimizu, Y.; Kawarabayasi, Y.; Ohshima, T.; Sato, M.; Tadano, S.; Ishikawa, H.; Takio, S.; Takechi, K.; et al. Moss chloroplasts are surrounded by a peptidoglycan wall containing D-amino acids. Plant Cell 2016, 28, 1521–1532. [Google Scholar] [CrossRef] [Green Version]
- Sato, N.; Toyoshima, M.; Tajima, N.; Takechi, K.; Takano, H. Single-pixel densitometry revealed the presence of peptidoglycan in the intermembrane space of moss chloroplast envelope in conventional electron micrographs. Plant Cell Physiol. 2017, 58, 1743–1751. [Google Scholar] [CrossRef]
- Sato, N.; Takano, H. Diverse origins of enzymes involved in the biosynthesis of chloroplast peptidoglycan. J. Plant Res. 2017, 130, 635–645. [Google Scholar] [CrossRef]
- Price, D.C.; Goodenough, U.W.; Roth, R.; Lee, J.-H.; Kariyawasam, T.; Mutwil, M.; Ferrari, C.; Facchinelli, F.; Ball, S.G.; Cenci, U.; et al. Analysis of an improved Cyanophora paradoxa genome assembly. DNA Res. 2019, 26, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Zepke, H.D.; Heinz, E.; Radunz, A.; Linscheid, M.; Pesch, R. Combination and positional distribution of fatty acids in lipid from blue-green algae. Arch. Microbiol. 1978, 119, 157–162. [Google Scholar] [CrossRef]
- Sato, N.; Murata, N. Lipid biosynthesis in the blue-green alga, Anabaena variabilis I. Lipid classes. Biochim. Biophys. Acta 1982, 710, 271–278. [Google Scholar] [CrossRef]
- Sato, N.; Murata, N. Lipid biosynthesis in the blue-green alga (cyanobacterium), Anabaena variabilis III. UDP-glucose:diacylglycerol glucosyltransferase activity in vitro. Plant Cell Physiol. 1982, 23, 1115–1120. [Google Scholar] [CrossRef]
- Shimojima, M.; Ohta, H.; Iwamatsu, A.; Masuda, T.; Shioi, Y.; Takamiya, K. Cloning of the gene for monogalactosyldiacylglycerol synthase and its evolutionary origin. Proc. Natl. Acad. Sci. USA 1997, 94, 333–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dörmann, P.; Balbo, I.; Benning, C. Arabidopsis galactolipid biosynthesis and lipid trafficking mediated by DGD1. Science 1999, 284, 2181–2184. [Google Scholar] [CrossRef]
- Awai, K.; Kakimoto, T.; Awai, C.; Kaneko, T.; Nakamura, Y.; Takamiya, K.-I.; Wada, H.; Ohta, H. Comparative genomic analysis revealed a gene for monoglucosyldiacylglycerol synthase, an enzyme for photosynthetic membrane lipid synthesis. Plant Physiol. 2006, 141, 1120–1127. [Google Scholar] [CrossRef] [Green Version]
- Awai, K.; Watanabe, H.; Benning, C.; Nishida, I. Digalactosyldiacylglycerol is required for better photosynthetic growth of Synechocystis sp. PCC6803 under phosphate limitation. Plant Cell Physiol. 2007, 48, 1517–1523. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, I.; Mizusawa, N.; Wada, H.; Sato, N. Digalactosyldiacylglycerol is required for stabilization of the oxygen-evolving complex in photosystem II. Plant Physiol. 2007, 145, 1361–1370. [Google Scholar] [CrossRef] [Green Version]
- Awai, K.; Ohta, H.; Sato, N. Oxygenic photosynthesis without galactolipids. Proc. Natl. Acad. Sci. USA 2014, 111, 13571–13575. [Google Scholar] [CrossRef] [Green Version]
- Sato, N. Is monoglucosyl diacylglycerol a precursor to monogalactosyl diacylglycerol in all cyanobacteria? Plant Cell Physiol. 2015, 56, 1890–1899. [Google Scholar] [CrossRef] [Green Version]
- Sato, N.; Okazaki, Y.; Saito, K. Isotopic combinatomer analysis provides in vivo evidence of the direct epimerization of monoglucosyl diacylglycerol in cyanobacteria. Biochemistry 2016, 55, 5689–5701. [Google Scholar] [CrossRef]
- Maeda, K.; Narikawa, R.; Ikeuchi, M. CugP is a novel ubiquitous non-GalU-type bacterial UDP-glucose pyrophosphorylase found in cyanobacteria. J. Bacteriol. 2014, 196, 2348–2354. [Google Scholar] [CrossRef] [Green Version]
- Mori, N.; Moriyama, T.; Sato, N. Uncommon properties of lipid biosynthesis of isolated plastids in the unicellular red alga Cyanidioschyzon merolae. FEBS Open Bio 2019, 9, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.J.; Zhang, Y.M.; Grimes, K.D.; Qi, J.; Lee, R.E.; Rock, C.O. Acyl-phosphates initiate membrane phospholipid synthesis in Gram-positive pathogens. Mol. Cell. 2006, 23, 765–772. [Google Scholar] [CrossRef]
- Sato, N.; Awai, K. “Prokaryotic Pathway” is not prokaryotic: Noncyanobacterial origin of the chloroplast lipid biosynthetic pathway revealed by comprehensive phylogenomic analysis. Genome Biol. Evol. 2017, 9, 3162–3178. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Awai, K. Adaptations in chloroplast membrane lipid synthesis from synthesis in ancestral cyanobacterial endosymbionts. Biochem. Biophys. Res. Commun. 2020, 528, 473–477. [Google Scholar] [CrossRef]
- Sato, N.; Awai, K. Diversity in biosynthetic pathways of galactolipids in the light of endosymbiotic origin of chloroplasts. Front. Plant Sci. 2016, 7, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Moriyama, T. Genomic and biochemical analysis of lipid biosynthesis in the unicellular rhodophyte Cyanidioschyzon merolae: Lack of plastidic desaturation pathway results in mixed pathway of galactolipid synthesis. Eukaryot. Cell 2007, 6, 1006–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essigmann, B.; Güler, S.; Narang, R.A.; Linke, D.; Benning, C. Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1998, 95, 1950–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Xu, C.; Benning, C. Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth. Proc. Natl. Acad. Sci. USA 2002, 99, 5732–5737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabita, F.R. Microbial ribulose 1,5-bisphosphate carboxylase/oxygenase: A different perspective. Photosynth. Res. 1999, 60, 1–28. [Google Scholar] [CrossRef]
- Delwiche, C.F.; Palmer, J.D. Rampant horizontal transfer and duplication of rubisco genes in eubacteria and plastids. Mol. Biol. Evol. 1996, 13, 873–882. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.; Meurer, J.; Bhattacharya, D. Evidence of a chimeric genome in the cyanobacterial ancestor of plastids. BMC Evol. Biol. 2008, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Badger, M.R.; Bek, E.J. Multiple Rubisco forms in proteobacteria: Their functional significance in relation to CO2 acquisition by the CBB cycle. J. Exp. Bot. 2008, 59, 1525–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, G.C.; Heinhorst, S.; Kerfeld, C.A. Carboxysomal carbonic anhydrase: Structure and role in microbial CO2 fixation. Biochim. Biophys. Acta 2010, 1804, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, A.; Archibald, J.M. Evolutionary dynamics of light-independent protochlorophyllide oxidoreductase genes in the secondary plastids of cryptophyte algae. Eukaryot. Cell 2008, 7, 550–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Staay, G.W.M.; Moon-van der Staay, S.Y.; Garczarek, L.; Partensky, F. Rapid evolutionary divergence of Photosystem I core subunits PsaA and PsaB in the marine prokaryote Prochlorococcus. Photosynth. Res. 2000, 65, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Yang, E.C.; Bhattacharya, D.; Yoon, H.S. Ancient gene paralogy may mislead inference of plastid phylogeny. Mol. Biol. Evol. 2012, 29, 3333–3343. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, T.; Terasawa, K.; Sato, N. Conservation of POPs, the plant organellar DNA polymerases, in eukaryotes. Protist 2011, 162, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Sato, N. Enzymes involved in organellar DNA replication in photosynthetic eukaryotes. Front. Plant Sci. 2014, 5, 480. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, T.; Tajima, N.; Sekine, K.; Sato, N. Localization and phylogenetic analysis of enzymes related to organellar genome replication in the unicellular rhodophyte Cyanidioschyzon merolae. Genome Biol. Evol. 2014, 6, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Sato, N. Was the evolution of plastid genetic machinery discontinuous? Trends Plant Sci. 2001, 6, 151–156. [Google Scholar] [CrossRef]
- Miyagishima, S.Y.; Nakanishi, H.; Kabeya, Y. Structure, regulation, and evolution of the plastid division machinery. Int. Rev. Cell Mol. Biol. 2011, 291, 115–153. [Google Scholar] [CrossRef]
- Yoshida, Y. Insights into the Mechanisms of Chloroplast Division. Int. J. Mol. Sci. 2018, 19, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, M.T.; Sato, N. Plastid replication in Arabidopsis: Complexity of the molecular components for the control of division. Recent Res. Devel. Plant Sci. 2004, 2, 219–248. [Google Scholar]
- Martin, W.; Schnarrenberger, C. The evolution of the Calvin cycle from prokaryotic to eukaryotic chromosomes: A case study of functional redundancy in ancient pathways through endosymbiosis. Curr. Genet. 1997, 32, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Prieto, A.; Moustafa, A. Plastid-localized amino acid biosynthetic pathways of Plantae are predominantly composed of non-cyanobacterial enzymes. Sci. Rep. 2012, 2, 955. [Google Scholar] [CrossRef]
- Richardson, L.G.L.; Schnell, D.J. Origins, function, and regulation of the TOC–TIC general protein import machinery of plastids. J. Exp. Bot. 2020, 71, 1226–1238. [Google Scholar] [CrossRef]
- Gagat, P.; Bodył, A.; Mackiewicz, P. How protein targeting to primary plastids via the endomembrane system could have evolved? A new hypothesis based on phylogenetic studies. Biol. Direct. 2013, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.; Bhattacharya, D. Revaluating the evolution of the Toc and Tic protein translocons. Trends Plant Sci. 2009, 14, 13–20. [Google Scholar] [CrossRef]
- Chotewutmontri, P.; Holbrook, K.; Bruce, B.D. Plastid protein targeting: Preprotein recognition and translocation. Int. Rev. Cell Mol. Biol. 2017, 330, 227–294. [Google Scholar] [CrossRef] [PubMed]
- Lauterborn, R. Protozoenstudien. II. Paulinella chromatophora nov. gen. nov. spec., ein beschalter Rhizopode des Sü.wassers mit blaugrünen chromatophorenartigen Einschlüssen. Z. Wiss. Zool. 1895, 59, 537–544. [Google Scholar]
- Pascher, A. Über die Natur der blaugrünen Chromatophoren des Rhizopoden Paulinella chromatophora. Zool. Anz. 1929, 81, 189–194. [Google Scholar]
- Marin, B.; Nowack, E.C.; Melkonian, M. A plastid in the making: Evidence for a second primary endosymbiosis. Protist 2005, 156, 425–432. [Google Scholar] [CrossRef]
- Maréchal, E. Primary Endosymbiosis: Emergence of the Primary Chloroplast and the Chromatophore, Two Independent Events. Chapter 1. In Plastids: Methods and Protocols; Methods in Molecular, Biology; Maréchal, E., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1829, pp. 3–16. [Google Scholar]
- Delaye, L.; Valadez-Cano, C.; Pérez-Zamorano, B. How really ancient is Paulinella chromatophora? PLoS Curr. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Parfrey, L.W.; Lahr, D.J.; Knoll, A.H.; Katz, L.A. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc. Natl. Acad. Sci. USA 2011, 108, 13624–13629. [Google Scholar] [CrossRef] [Green Version]
- Eme, L.; Sharpe, S.C.; Brown, M.W.; Roger, A.J. On the age of eukaryotes: Evaluating evidence from fossils and molecular clocks. Cold Spring Harb. Perspect. Biol. 2014, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Härtel, H.; Benning, C. Can digalactosyldiacylglycerol substitute for phosphatidylcholine upon phosphate deprivation in leaves and roots of Arabidopsis? Biochem. Soc. Trans. 2000, 28, 729–732. [Google Scholar] [CrossRef]
- Andersson, M.X.; Stridh, M.H.; Larsson, K.E.; Liljenberg, C.; Sandelius, A.S. Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett. 2003, 537, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Jouhet, J.; Maréchal, E.; Baldan, B.; Bligny, R.; Joyard, J.; Block, M.A. Phosphate deprivation induces transfer of DGDG galactolipid from chloroplast to mitochondria. J. Cell Biol. 2004, 167, 863–874. [Google Scholar] [CrossRef]
- Russo, M.A.; Quartacci, M.F.; Izzo, R.; Belligno, A.; Navari-Izzo, F. Long- and short-term phosphate deprivation in bean roots: Plasma membrane lipid alterations and transient stimulation of phospholipases. Phytochemistry 2007, 68, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Botté, C.Y.; Deligny, M.; Roccia, A.; Bonneau, A.-L.; Saïdani, N.; Hardré, H.; Aci, S.; Yamaryo-Botté, Y.; Jouhet, J.; Dubots, E.; et al. Chemical inhibitors of monogalactosyldiacylglycerol synthases in Arabidopsis thaliana. Nat. Chem. Biol. 2011, 7, 834–842. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fujii, S.; Sato, M.; Toyooka, K.; Wada, H. Specific role of phosphatidylglycerol and functional overlaps with other thylakoid lipids in Arabidopsis chloroplast biogenesis. Plant Cell Rep. 2015, 34, 631–642. [Google Scholar] [CrossRef]
- Semeniuk, A.; Sohlenkamp, C.; Duda, K.; Hölzl, G.A. bifunctional glycosyltransferase from Agrobacterium tumefaciens synthesizes monoglucosyl and glucuronosyl diacylglycerol under phosphate deprivation. J. Biol. Chem. 2014, 289, 10104–10114. [Google Scholar] [CrossRef] [Green Version]
- Geiger, O.; Röhrs, V.; Weissenmayer, B.; Finan, T.M.; Thomas-Oates, J.E. The regulator gene phoB mediates phosphate stress-controlled synthesis of the membrane lipid diacylglyceryl-N,N,N-trimethylhomoserine in Rhizobium (Sinorhizobium) meliloti. Mol. Microbiol. 1999, 32, 63–73. [Google Scholar] [CrossRef]
- Riekhof, W.R.; Naik, S.; Bertrand, H.; Benning, C.; Voelker, D.R. Phosphate starvation in fungi induces the replacement of phosphatidylcholine with the phosphorus-free betaine lipid diacylglyceryl-N,N,N-trimethylhomoserine. Eukaryot. Cell 2014, 13, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Sato, N. Betaine lipids. Bot. Mag. Tokyo 1992, 105, 185–197. [Google Scholar] [CrossRef]
- Tajima, N.; Sato, S.; Maruyama, F.; Kurokawa, K.; Ohta, H.; Tabata, S.; Sekine, K.; Moriyama, T.; Sato, N. Analysis of the complete plastid genome of the unicellular red alga Porphyridium purpureum. J. Plant Res. 2014, 127, 389–397. [Google Scholar] [CrossRef]
- Ku, C.; Nelson-Sathi, S.; Roettger, M.; Garg, S.; Hazkani-Covo, E.; Martin, W.F. Endosymbiotic gene transfer from prokaryotic pangenomes: Inherited chimerism in eukaryotes. Proc. Natl. Acad. Sci. USA 2015, 112, 10139–10146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schötz, F. Dreidimensionale, maßstabgetreue Rekonstruktion einer grünen Flagellatenzelle nach Elektronenmikroskopie von Serienschnitten. Planta 1972, 102, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Toyoshima, M.; Saito, M.; Wada, H.; Sato, N. Revisiting the algal “chloroplast lipid droplet”: The absence of an entity that is unlikely to exist. Plant Physiol. 2018, 176, 1519–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oi, T.; Enomoto, S.; Nakao, T.; Arai, S.; Yamane, K.; Taniguchi, M. Three-dimensional intracellular structure of a whole rice mesophyll cell observed with FIB-SEM. Ann. Bot. 2017, 120, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, K.; Oi, T.; Enomoto, S.; Nakao, T.; Arai, S.; Miyake, H.; Taniguchi, M. Three-dimensional ultrastructure of chloroplast pockets formed under salinity stress. Plant Cell Environ. 2018, 41, 563–575. [Google Scholar] [CrossRef]
- Kobayashi, K.; Masuda, T.; Tajima, N.; Wada, H.; Sato, N. Molecular phylogeny and intricate evolutionary history of the three isofunctional enzymes involved in the oxidation of Protoporphyrinogen IX. Genome. Biol. Evol. 2014, 6, 2141–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Moriyama, T. Photosynthesis. Chapter 17. In Cyanidioschyzon merolae. A New Model Eukaryote for Cell and Organelle Biology; Kuroiwa, T., Miyagishima, S., Matsunaga, S., Sato, N., Nozaki, H., Tanaka, K., Misumi, O., Eds.; Springer: Singapore, 2018; pp. 263–281. [Google Scholar]


| Item | Evidence | Explanation |
|---|---|---|
| 1 | Similarity of photosynthesis | (a) Photosynthesis with oxygen evolution (b) Presence of chlorophyll a and carotenoids (c) Similarity in the structure consisting of two limiting membranes and internal thylakoid membranes containing galactolipids |
| 2 | Similarity of the genetic system | Prokaryotic RNA polymerase and ribosome |
| 3 | Similarity in reproduction | Binary fission |
| 4 | Presence of peptidoglycan | Glaucophytes, some green algae, charophytes, bryophytes, pteridophytes, some gymnosperms |
| 5 | Phylogenetic relationship | (a) rRNA genes (b) Ribosomal proteins and house-keeping enzymes (c) Photosynthetic components (orthologs found only in cyanobacteria and chloroplasts) |
| 6 | Synteny of gene clusters | (a) Ribosomal proteins (b) ATPase subunits (c) RNA polymerase subunits |
| Apparent Similarity | Revealed Difference | Cyanobacteria | Chloroplasts | Reference |
|---|---|---|---|---|
| Chlorophylls and tetrapyrroles | Partially different biosynthesis pathway (PPO) 1 | HemJ (or HemY) | HemY | [143] |
| Galactolipids (MGDG and DGDG) 2 | Different biosynthesis pathway | MgdA- > MgdE- > DgdA | MGD1- > DGD1 | [50] |
| Peptidoglycan 3 | Different origins of biosynthesis enzymes | Cyanobacterial enzymes | Most enzymes originated from bacteria other than cyanobacteria | [77,78] |
| DNA replication | Different enzyme | PolIII | POP 4 | [108] |
| Transcription | Additional enzyme in chloroplast | Prokaryotic RNAP | Prokaryotic RNAP + NEP 5 | [110] |
| Division by binary fission | Additional components of division machinery in chloroplast | MinC, MinD, MinE, FtsZ, etc. | MinD, MinE, FtsZ, dynamin, etc (no MinC) | [111,112,113] |
| Oxygen producing photosynthesis | Partially different components of oxygen-evolving complex | PsbO, PsbU, PsbV | PsbO, PsbP, PsbQ (Red algae retain cyanobacteria-like system) | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, N. Are Cyanobacteria an Ancestor of Chloroplasts or Just One of the Gene Donors for Plants and Algae? Genes 2021, 12, 823. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12060823
Sato N. Are Cyanobacteria an Ancestor of Chloroplasts or Just One of the Gene Donors for Plants and Algae? Genes. 2021; 12(6):823. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12060823
Chicago/Turabian StyleSato, Naoki. 2021. "Are Cyanobacteria an Ancestor of Chloroplasts or Just One of the Gene Donors for Plants and Algae?" Genes 12, no. 6: 823. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12060823






