Exploration of the Moderating Effects of Physical Activity and Early Life Stress on the Relation between Brain-Derived Neurotrophic Factor (BDNF) rs6265 Variants and Depressive Symptoms among Adolescents
Abstract
:1. Introduction
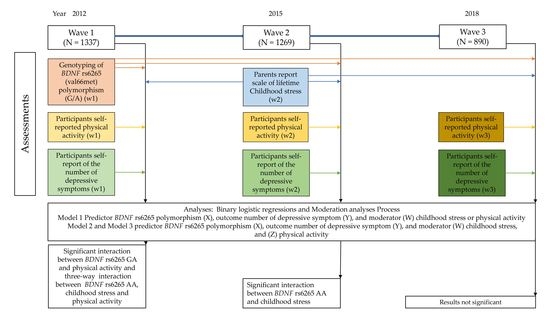
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Statistical Analyses
3. Results
3.1. Crude Analyses
3.2. Interactions
3.2.1. One-Way Interactions
3.2.2. Two-Way Interactions
3.2.3. Three-Way Interactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.L.; Lopez, A.D. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020; The World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Costello, E.J.; Angold, A.; Keeler, G.P. Adolescent outcomes of childhood disorders: The consequences of severity and impairment. J. Am. Acad. Child Adolesc. Psychiatry 1999, 38, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Feehan, M.; McGee, R.; Raja, S.N.; Williams, S.M. DSM-III-R disorders in New Zealand 18-year-olds. Aust. N. Z. J. Psychiatry 1994, 28, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Rohde, P.; Lewinsohn, P.M.; Klein, D.N.; Seeley, J.R.; Gau, J.M. Key characteristics of major depressive disorder occurring in childhood, adolescence, emerging adulthood, and adulthood. Clin. Psychol. Sci. 2013, 1, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003, 289, 3095–3105. [Google Scholar] [CrossRef]
- Hankin, B.L.; Abramson, L.Y.; Moffitt, T.E.; Silva, P.A.; McGee, R.; Angell, K.E. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. J. Abnorm. Psychol. 1998, 107, 128–140. [Google Scholar] [CrossRef]
- Fergusson, D.M.; Boden, J.M.; Horwood, L.J. Recurrence of major depression in adolescence and early adulthood, and later mental health, educational and economic outcomes. Br. J. Psychiatry 2007, 191, 335–342. [Google Scholar] [CrossRef]
- Glied, S.; Pine, D.S. Consequences and correlates of adolescent depression. Arch. Pediatr. Adolesc. Med. 2002, 156, 1009–1014. [Google Scholar] [CrossRef] [Green Version]
- Lewinsohn, P.M.; Rohde, P.; Seeley, J.R. Psychosocial characteristics of adolescents with a history of suicide attempt. J. Am. Acad. Child Adolesc. Psychiatry 1993, 32, 60–68. [Google Scholar] [CrossRef]
- Green, K.M.; Zebrak, K.A.; Fothergill, K.E.; Robertson, J.A.; Ensminger, M.E. Childhood and adolescent risk factors for comorbid depression and substance use disorders in adulthood. Addict. Behav. 2012, 37, 1240–1247. [Google Scholar] [CrossRef] [Green Version]
- Harrington, R.; Bredenkamp, D.; Groothues, C.; Rutter, M.; Fudge, H.; Pickles, A.J.J.o.C.P.; Psychiatry. Adult outcomes of childhood and adolescent depression. III Links with suicidal behaviours. J. Am. Acad. Child Adolesc. Psychiatry 1994, 35, 1309–1319. [Google Scholar] [CrossRef]
- McLaughlin, K.A. The public health impact of major depression: A call for interdisciplinary prevention efforts. Prev. Sci. 2011, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Cairns, K.E.; Yap, M.B.H.; Pilkington, P.D.; Jorm, A.F. Risk and protective factors for depression that adolescents can modify: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2014, 169, 61–75. [Google Scholar] [CrossRef]
- Liu, R.T. Childhood adversities and depression in adulthood: Current findings and future directions. Clin. Psychol. 2017, 24, 140. [Google Scholar] [CrossRef]
- Kudinova, A.Y.; McGeary, J.E.; Knopik, V.S.; Gibb, B.E. Brain derived neurotrophic factor (BDNF) polymorphism moderates the interactive effect of 5-HTTLPR polymorphism and childhood abuse on diagnoses of major depression in women. Psychiatry Res. 2015, 225, 746–747. [Google Scholar] [CrossRef] [Green Version]
- Frodl, T.; Reinhold, E.; Koutsouleris, N.; Reiser, M.; Meisenzahl, E.M. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J. Psychiatr. Res. 2010, 44, 799–807. [Google Scholar] [CrossRef]
- Rose, D.T.; Abramson, L. IX Developmental predictors of depressive cognitive style: Research and theory. In Developmental Perspectives on Depression, 1st ed.; Cicchetti, D., Toth, S., Eds.; University of Rochester Press: New York, NY, USA, 1992; pp. 323–350. [Google Scholar]
- Beck, A.T. Cognitive Therapy and the Emotional Disorders, 1st ed.; Penguin: New York, NY, USA, 1976. [Google Scholar]
- Massing-Schaffer, M.; Liu, R.T.; Kraines, M.A.; Choi, J.Y.; Alloy, L.B. Elucidating the relation between childhood emotional abuse and depressive symptoms in adulthood: The mediating role of maladaptive interpersonal processes. Personal. Individ. Differ. 2015, 74, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Colman, I.; Kingsbury, M.; Garad, Y.; Zeng, Y.; Naicker, K.; Patten, S.; Jones, P.B.; Wild, T.C.; Thompson, A.H. Consistency in adult reporting of adverse childhood experiences. Psychol. Med. 2016, 46, 543–549. [Google Scholar] [CrossRef]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J. Neurosci. 1997, 17, 2295–2313. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Rosenfeld, R.; Matheson, C.; Hawkins, N.; Lopez, O.; Bennett, L.; Welcher, A. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience 1997, 78, 431–448. [Google Scholar] [CrossRef]
- Casey, B.J.; Glatt, C.E.; Tottenham, N.; Soliman, F.; Bath, K.; Amso, D.; Altemus, M.; Pattwell, S.; Jones, R.; Levita, L.; et al. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience 2009, 164, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Maisonpierre, P.C.; Le Beau, M.M.; Espinosa, R.; Ip, N.Y.; Belluscio, L.; de la Monte, S.M.; Squinto, S.; Furth, M.E.; Yancopoulos, G.D. Human and rat brain-derived neurotrophic factor and neurotrophin-3: Gene structures, distributions, and chromosomal localizations. Genomics 1991, 10, 558–568. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Gatt, J.M.; Nemeroff, C.B.; Dobson-Stone, C.; Paul, R.H.; Bryant, R.A.; Schofield, P.R.; Gordon, E.; Kemp, A.H.; Williams, L.M. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol. Psychiatry 2009, 14, 681–695. [Google Scholar] [CrossRef] [Green Version]
- SNPedia. rs6265. Available online: https://www.snpedia.com/index.php/Rs6265 (accessed on 8 March 2022).
- Chen, Z.-Y.; Ieraci, A.; Teng, H.; Dall, H.; Meng, C.-X.; Herrera, D.G.; Nykjaer, A.; Hempstead, B.L.; Lee, F.S. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. Neuroscience 2005, 25, 6156–6166. [Google Scholar] [CrossRef]
- Karege, F.; Perret, G.; Bondolfi, G.; Schwald, M.; Bertschy, G.; Aubry, J.-M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002, 109, 143–148. [Google Scholar] [CrossRef]
- Duman, R. Synaptic plasticity and mood disorders. Mol. Psychiatry 2002, 7, S29–S34. [Google Scholar] [CrossRef] [Green Version]
- Gujral, S.; Manuck, S.B.; Ferrell, R.E.; Flory, J.D.; Erickson, K.I. The BDNF Val66Met polymorphism does not moderate the effect of self-reported physical activity on depressive symptoms in midlife. Psychiatry Res. 2014, 218, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, M.; Arias, B.; Wichers, M.; Barrantes-Vidal, N.; Moya, J.; Villa, H.; Van Os, J.; Ibáñez, M.I.; Ruipérez, M.A.; Ortet, G. Early adversity and 5-HTT/BDNF genes: New evidence of gene–environment interactions on depressive symptoms in a general population. Psychol. Med. 2009, 39, 1425–1432. [Google Scholar] [CrossRef] [Green Version]
- Nanni, V.; Uher, R.; Danese, A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am. J. Psychiatry 2012, 169, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Gilman, S.E.; Trinh, N.H.; Smoller, J.W.; Fava, M.; Murphy, J.M.; Breslau, J. Psychosocial stressors and the prognosis of major depression: A test of Axis IV. Psychol. Med. 2013, 43, 303–316. [Google Scholar] [CrossRef] [Green Version]
- Rhebergen, D.; Lamers, F.; Spijker, J.; De Graaf, R.; Beekman, A.; Penninx, B. Course trajectories of unipolar depressive disorders identified by latent class growth analysis. Psychol. Med. 2012, 42, 1383–1396. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.C.; Birnbaum, H.; Bromet, E.; Hwang, I.; Sampson, N.; Shahly, V. Age differences in major depression: Results from the National Comorbidity Survey Replication (NCS-R). Psychol. Med. 2010, 40, 225–237. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, K.A. Future Directions in Childhood Adversity and Youth Psychopathology. J. Clin. Child Adolesc. Psychol. 2016, 45, 361–382. [Google Scholar] [CrossRef]
- Polanczyk, G.; Caspi, A.; Williams, B.; Price, T.S.; Danese, A.; Sugden, K.; Uher, R.; Poulton, R.; Moffitt, T.E. Protective Effect of CRHR1 Gene Variants on the Development of Adult Depression Following Childhood Maltreatment: Replication and Extension. Arch. Gen. Psychiatry 2009, 66, 978–985. [Google Scholar] [CrossRef]
- Bradley, R.G.; Binder, E.B.; Epstein, M.P.; Tang, Y.; Nair, H.P.; Liu, W.; Gillespie, C.F.; Berg, T.; Evces, M.; Newport, D.J. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Arch. Gen. Psychiatry 2008, 65, 190–200. [Google Scholar] [CrossRef]
- Zimmermann, P.; Brückl, T.; Nocon, A.; Pfister, H.; Binder, E.B.; Uhr, M.; Lieb, R.; Moffitt, T.E.; Caspi, A.; Holsboer, F. Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: Results from a 10-year prospective community study. Am. J. Psychiatry 2011, 168, 1107–1116. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-M.; Stewart, R.; Kim, S.-W.; Yang, S.-J.; Shin, I.-S.; Kim, Y.-H.; Yoon, J.-S. Interactions between Life Stressors and Susceptibility Genes (5-HTTLPR and BDNF) on Depression in Korean Elders. Biol. Psychiatry 2007, 62, 423–428. [Google Scholar] [CrossRef]
- Grassi-Oliveira, R.; Stein, L.M.; Lopes, R.P.; Teixeira, A.L.; Bauer, M.E. Low Plasma Brain-Derived Neurotrophic Factor and Childhood Physical Neglect Are Associated with Verbal Memory Impairment in Major Depression—A Preliminary Report. Biol. Psychiatry 2008, 64, 281–285. [Google Scholar] [CrossRef]
- Vinberg, M.; Trajkovska, V.; Bennike, B.; Knorr, U.; Knudsen, G.M.; Kessing, L.V. The BDNF Val66Met polymorphism: Relation to familiar risk of affective disorder, BDNF levels and salivary cortisol. Psychoneuroendocrinology 2009, 34, 1380–1389. [Google Scholar] [CrossRef]
- Brown, G.W.; Craig, T.K.; Harris, T.O.; Herbert, J.; Hodgson, K.; Tansey, K.E.; Uher, R. Functional Polymorphism in the Brain-Derived Neurotrophic Factor Gene Interacts with Stressful Life Events but Not Childhood Maltreatment in The Etiology of Depression. Depress. Anxiety 2014, 31, 326–334. [Google Scholar] [CrossRef]
- Frodl, T.; Skokauskas, N.; Frey, E.-M.; Morris, D.; Gill, M.; Carballedo, A. BDNF Val66Met Genotype Interacts with Childhood Adversity and Influences the Formation of Hippocampal Subfields. Hum. Brain Mapp. 2014, 35, 5776–5783. [Google Scholar] [CrossRef]
- Bland, H.W.; Melton, B.F.; Bigham, L.E.; Welle, P.D. Quantifying the impact of physical activity on stress tolerance in college students. Coll. Stud. J. 2014, 48, 559–568. [Google Scholar]
- McPhie, M.L.; Rawana, J.S. The effect of physical activity on depression in adolescence and emerging adulthood: A growth-curve analysis. J. Adolesc. 2015, 40, 83–92. [Google Scholar] [CrossRef]
- Norris, R.; Carroll, D.; Cochrane, R. The effects of physical activity and exercise training on psychological stress and well-being in an adolescent population. J. Psychosom. Res. 1992, 36, 55–65. [Google Scholar] [CrossRef]
- Ferris, L.T.; Williams, J.S.; Shen, C.-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 2007, 39, 728. [Google Scholar] [CrossRef]
- Tang, S.W.; Chu, E.; Hui, T.; Helmeste, D.; Law, C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci. Lett. 2008, 431, 62–65. [Google Scholar] [CrossRef]
- Zoladz, J.; Pilc, A.; Majerczak, J.; Grandys, M.; Zapart-Bukowska, J.; Duda, K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharm. 2008, 59 (Suppl. S7), 119–132. [Google Scholar]
- Freeman, A.; Allnutt, A.; Bagheri, R.; Wong, A.; Ashtary-Larky, D.; Rashidlamir, A.; Figueroa, A. Resistance or Endurance Training Increases Brain-derived Neurotrophic Factor Concentrations In Middle Age Females: 698. Med. Sci. Sports Exerc. 2021, 53 (Suppl. S8), 236. [Google Scholar] [CrossRef]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity—Exercise-Induced Response of Peripheral Brain-Derived Neurotrophic Factor. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctot, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef]
- De Assis, G.G.; Gasanov, E.V.; de Sousa, M.B.C.; Kozacz, A.; Murawska-Cialowicz, E. Brain derived neutrophic factor, a link of aerobic metabolism to neuroplasticity. J. Physiol. Pharm. 2018, 69, 351–358. [Google Scholar] [CrossRef]
- Mata, J.; Thompson, R.J.; Gotlib, I.H. BDNF Genotype Moderates the Relation between Physical Activity and Depressive Symptoms. Health Psychol. 2010, 29, 130–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavrakakis, N.; Oldehinkel, A.J.; Nederhof, E.; Oude Voshaar, R.C.; Verhulst, F.C.; Ormel, J.; de Jonge, P. Plasticity genes do not modify associations between physical activity and depressive symptoms. Health Psychol. 2013, 32, 785–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stults-Kolehmainen, M.A.; Sinha, R. The effects of stress on physical activity and exercise. Sports Med. 2014, 44, 81–121. [Google Scholar] [CrossRef] [PubMed]
- Schule, C.; Zill, P.; Baghai, T.C.; Eser, D.; Zwanzger, P.; Wenig, N.; Rupprecht, R.; Bondy, B. Brain-derived neurotrophic factor Val66Met polymorphism and dexamethasone/CRH test results in depressed patients. Psychoneuroendocrinology 2006, 31, 1019–1025. [Google Scholar] [CrossRef]
- de Assis, G.G.; Gasanov, E.V. BDNF and Cortisol integrative system—Plasticity vs. degeneration: Implications of the Val66Met polymorphism. Front. Neuroendocr. 2019, 55, 100784. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Larson, E.B.; Wang, L.I.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Crane, P.; Kukull, W. Exercise is Associated with Reduced Risk for Incident Dementia among Persons 65 Years of Age and Older. Ann. Intern. Med. 2006, 144, 73–81. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Babyak, M.A.; Doraiswamy, P.M.; Watkins, L.; Hoffman, B.M.; Barbour, K.A.; Herman, S.; Craighead, W.E.; Brosse, A.L.; Waugh, R. Exercise and Pharmacotherapy in the Treatment of Major Depressive Disorder. Psychosom. Med. 2007, 69, 587. [Google Scholar] [CrossRef] [Green Version]
- Hing, B.; Sathyaputri, L.; Potash, J.B. A comprehensive review of genetic and epigenetic mechanisms that regulate BDNF expression and function with relevance to major depressive disorder. Am. J. Med. Genet. 2018, 177, 143–167. [Google Scholar] [CrossRef] [Green Version]
- Svanborg, P.; Ekselius, L. Self-assessment of DSM-IV criteria for major depression in psychiatric out- and inpatients. Nord. J. Psychiatry 2003, 57, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Sonnby, K.; Åslund, C.; Leppert, J.; Nilsson, K.W. Symptoms of ADHD and depression in a large adolescent population: Co-occurring symptoms and associations to experiences of sexual abuse. Nord. J. Psychiatry 2011, 65, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L.; Teicher, M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008, 31, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hosang, G.M.; Shiles, C.; Tansey, K.E.; McGuffin, P.; Uher, R. Interaction between stress and the BDNF Val66Met polymorphism in depression: A systematic review and meta-analysis. BMC Med. 2014, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Hellhammer, D.; Hellhammer, J. Stress: The Brain-Body Connection; Karger Medical and Scientific Publishers: Basel, Switzerland, 2008. [Google Scholar]
- Hero, T.; Gerhards, F.; Thiart, H.; Hellhammer, D.H.; Linden, M. Neuropattern: A new translational tool to detect and treat stress pathology. II. The Teltow study. Stress 2012, 15, 488–494. [Google Scholar]
- Whitaker, R. Translating words, translating cultures, L. Hardwick: Book review. Akroterion 2001, 46, 109–112. [Google Scholar]
- Nelson, B. Translating Cultures, Cultures of Translation. J. Intercult. Stud. 2007, 28, 361–365. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. In Methodology in the Social Sciences, 2nd ed.; Guilford Press: New York, NY, USA, 2018; 692p. [Google Scholar]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Adlard, P.A.; Perreau, V.M.; Cotman, C.W. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neuro. Agi. 2005, 26, 511–520. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Lopes, M.; Fregni, F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: Implications for the role of neuroplasticity in depression. Int. J. Neuropsychopharmacol. 2008, 11, 1169–1180. [Google Scholar] [CrossRef]
- Belsky, J.; Pluess, M. Beyond Diathesis Stress: Differential Susceptibility to Environmental Influences. Psychol. Bull. 2009, 135, 885–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmeister, M.; McInnis, M.G.; Zöllner, S. Psychiatric genetics: Progress amid controversy. Nat. Rev. Genet. 2008, 9, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Boyce, W.T.; Ellis, B.J. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005, 17, 271–301. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Hoeksema, S.; Girgus, J.S. The emergence of gender differences in depression during adolescence. Psychol. Bull. 1994, 115, 424–443. [Google Scholar] [CrossRef]
- Bungum, T.; Dowda, M.; Weston, A.; Trost, S.G.; Pate, R.R. Correlates of physical activity in male and female youth. Pediatric Exerc. Sci. 2000, 12, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Andreska, T.; Aufmkolk, S.; Sauer, M.; Blum, R. High abundance of BDNF within glutamatergic presynapses of cultured hippocampal neurons. Front. Cell. Neurosci. 2014, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Barde, Y.-A.; Matsumoto, T.; Rauskolb, S.; Polack, M.; Klose, J.; Kolbeck, R.; Korte, M. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat. Neurosci. 2008, 11, 131–133. [Google Scholar] [CrossRef]
- Colino-Oliveira, M.; Rombo, D.M.; Dias, R.B.; Ribeiro, J.A.; Sebastiao, A.M. BDNF-induced presynaptic facilitation of GABAergic transmission in the hippocampus of young adults is dependent of TrkB and adenosine A.sub.2A receptors. Purinergic Signal. 2016, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Chiaruttini, C.; Vicario, A.; Li, Z.; Baj, G.; Braiuca, P.; Wu, Y.; Lee, F.; Gardossi, L.; Baraban, J.; Tongiorgi, E. Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. Proc. Natl. Acad. Sci. USA 2009, 106, 16481–16486. [Google Scholar] [CrossRef] [Green Version]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef]
- Kaufman, J.; Yang, B.-Z.; Douglas-Palumberi, H.; Grasso, D.; Lipschitz, D.; Houshyar, S.; Krystal, J.H.; Gelernter, J. Brain-Derived Neurotrophic Factor–5-HTTLPR Gene Interactions and Environmental Modifiers of Depression in Children. Biol. Psychiatry 2006, 59, 673–680. [Google Scholar] [CrossRef]
- Kang, J.-S. Exercise copes with prolonged stress-induced impairment of spatial memory performance by endoplasmic reticulum stress. J. Exerc. Nutr. Bioch. 2015, 19, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto-Gonzalez, J.L.; Holm, M.M.; Vardya, I.; Christensen, T.; Wiborg, O.; Jensen, K. Presynaptic Plasticity as a Hallmark of Rat Stress Susceptibility and Antidepressant Response. PLoS ONE 2015, 10, e0119993. [Google Scholar]
- Yeh, C.M.; Huang, C.C.; Hsu, K.S. Prenatal stress alters hippocampal synaptic plasticity in young rat offspring through preventing the proteolytic conversion of pro-brain-derived neurotrophic factor (BDNF) to mature BDNF. J. Physiol. 2012, 590, 991–1010. [Google Scholar] [CrossRef]
- Boersma, G.J.; Tamashiro, K.L. Individual differences in the effects of prenatal stress exposure in rodents. Neurobiol. Stress 2015, 1, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Fan, W.; Zhang, X.; Dong, E. Gestational stress induces depressive-like and anxiety-like phenotypes through epigenetic regulation of BDNF expression in offspring hippocampus. Epigenetics 2016, 11, 150–162. [Google Scholar] [CrossRef] [Green Version]
- de Assis, G.G.; Hoffman, J.R.; Bojakowski, J.; Murawska-Cialowicz, E.; Cieszczyk, P.; Gasanov, E.V. The Val66 and Met66 Alleles-Specific Expression of BDNF in Human Muscle and Their Metabolic Responsivity. Front. Mol. Neurosci. 2021, 14, 638176. [Google Scholar] [CrossRef] [PubMed]
- de Assis, G.G.; Hoffman, J.R. The BDNF Val66Met Polymorphism is a Relevant, But not Determinant, Risk Factor in the Etiology of Neuropsychiatric Disorders—Current Advances in Human Studies: A Systematic Review. Brain Plast. 2022, 1–10. [Google Scholar] [CrossRef]
- Kraemer, H.C. Determining gene moderation of environmental risk factors for a mental disorder: A “perfect storm” of methodological problems. Int. J. Methods Psychiatr. Res. 2012, 21, 185–194. [Google Scholar] [CrossRef]
- Bender, R.; Lange, S. Adjusting for multiple testing—when and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S. A farewell to Bonferroni: The problems of low statistical power and publication bias. Behav. Ecol. 2004, 15, 1044–1045. [Google Scholar] [CrossRef]
- Roisman, G.I.; Newman, D.A.; Fraley, R.C.; Haltigan, J.D.; Groh, A.M.; Haydon, K.C. Distinguishing differential susceptibility from diathesis–stress: Recommendations for evaluating interaction effects. Dev. Psychopathol. 2012, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Pluess, M. Individual differences in environmental sensitivity. Child Dev. Perspect. 2015, 9, 138–143. [Google Scholar] [CrossRef]






| Wave 1 (n = 1337) | Wave 2 (n = 1269) | Wave 3 (n = 890) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Female (n = 761) | Male (n = 576) | χ2/Z (p) | Female (n = 740) | Male (n = 529) | χ2/Z (p) | Female (n = 566) | Male (n = 324) | χ2/Z (p) | |
| Age, years a (SD) | 14.37 (1.029) | 14.42 (1.038) | –0.197 (0.844) | 17.30 (1.029) | 17.35 (1.040) | –0.356 (0.721) | 20.407 (1.0289) | 20.425 (1.037) | –0.108 (0.914) |
| Number of depressive symptoms, mean (SD) | 2.188 (2.350) | 1.405 (1.807) | –6.081 (<0.001) | 3.57 (2.605) | 1.90 (2.194) | –11.840 (<0.001) | 3.66 (2.840) | 2.25 (2.564) | –7.452 (<0.001) |
| Participants with depression (%) | 123 (16.2) | 36 (6.3) | 30.747 (<0.001) | 244 (33.0) | 70 (13.2) | 64.555 (<0.001) | 196 (34.6) | 60 (18.5) | 26.100 (<0.001) |
| Childhood stress a (SD) | 3.05 (2.046) | 2.72 (1.817) | –2.727 (0.006) | ||||||
| Participants with workout > 30 min (%) | –2.345 (0.019) | 33.396 (<0.001) | 35.211 (<0.001) | ||||||
| Never | 51 (6.7) | 49 (3.7) | 57 (7.7) | 47 (8.9) | 63 (11.1) | 28 (8.6) | |||
| Less than once a month | 36 (4.7) | 24 (4.2) | 54 (7.3) | 27 (33.3) | 108 (19.1) | 43 (13.3) | |||
| 1–3 times a month | 53 (7.0) | 31 (5.4) | 67 (9.1) | 32 (6.0) | 90 (15.9) | 39 (12.0) | |||
| Once a week | 89 (11.7) | 50 (8.7) | 86 (11.6) | 51 (9.6) | 65 (11.5) | 26 (8.0) | |||
| 2–3 times per week | 280 (36.8) | 185 (32.1) | 250 (33.8) | 139 (26.3) | 146 (25.8) | 81 (25.0) | |||
| 4–6 times per week | 213 (28.0) | 196 (34.0) | 168 (22.7) | 152 (28.7) | 78 (13.8) | 84 (25.9) | |||
| Every day | 39 (5.1) | 41 (7.1) | 58 (7.8) | 81 (15.3) | 16 (7.1) | 23 (7.1) | |||
| BDNF rs6265 alleles (%) | 2.235 (0.327) | 2.207 (0.332) | 0.538 (0.764) | ||||||
| G: G | 507 (66.6) | 401 (69.6) | 491 (66.4) | 364 (68.8) | 367 (64.8) | 217 (67.0) | |||
| G: A | 232 (30.5) | 155 (26.9) | 228 (30.8) | 147 (27.8) | 181 (32.0) | 96 (29.6) | |||
| A: A | 22 (2.9) | 20 (3.5) | 21 (2.8) | 18 (3.4) | 18 (3.3) | 11 (3.2) | |||
| Wave 1 (n = 1337) | Model A1 | Model A2 | ||
| p | OR (95% CI) | p | OR (95% CI) | |
| Sex in wave 1 | <0.001 | 2.95 (1.99–4.36) | <0.001 | 2.89 (1.95–4.29) |
| Age in wave 1 | 0.003 | 1.29 (1.09–1.52) | 0.002 | 1.3 (1.1–1.53) |
| Physical activity in wave 1 | 0.003 | 0.86 (0.77–0.95) | <0.001 | 0.8 (0.71–0.9) |
| BDNF rs6265 GG | 0.44 | 0.05 | ||
| BDNF rs6265 GA | 0.32 | 0.82 (0.56–1.21) | 0.02 | 0.33 (0.13–0.84) |
| BDNF rs6265 AA | 0.49 | 1.37 (0.55–3.41) | 0.056 | 1.87 (0.23–15.14) |
| BDNF rs6265 GG × physical activity in wave 1 | 0.08 | |||
| BDNF rs6265 GA × physical activity in wave 1 | 0.03 | 1.3 (1.1–1.53) | ||
| BDNF rs6265 AA × physical activity in wave 1 | 0.076 | 0.92 (0.53–1.59) | ||
| Constant | <0.001 | <0.001 | ||
| Wave 2 (n = 1269) | Model B1 | Model B2 | ||
| p | OR (95% CI) | p | OR (95% CI) | |
| Sex in wave 1 | <0.001 | 3.13 (2.32–4.22) | <0.001 | 3.13 (2.32–4.23) |
| Age in wave 2 | 0.13 | 1.11 (0.97–1.26) | 0.11 | 1.11 (0.98–1.27) |
| Childhood stress | <0.001 | 1.24 (1.16–1.32) | <0.001 | 1.17 (1.08–1.26) |
| BDNF rs6265 GG | 0.41 | 0.01 | ||
| BDNF rs6265 GA | 0.39 | 0.88 (0.65–1.18) | 0.05 | 0.57 (0.33–0.99) |
| BDNF rs6265 AA | 0.27 | 0.6 (0.24–1.49) | 0.02 | 0.01 (0–.44) |
| BDNF rs6265 GG × childhood stress | 0.02 | |||
| BDNF rs6265 GA × childhood stress | 0.07 | 1.14 (0.99–1.31) | ||
| BDNF rs6265 AA × childhood stress | 0.02 | 3.87 (1.22–12.31) | ||
| Constant | <0.001 | <0.001 |
| Model C | Model C2 | |||||
|---|---|---|---|---|---|---|
| p | OR | (95% CI) | p | OR | (95% CI) | |
| Sex in wave 1 | <0.001 | 0.33 | (0.24–0.44) | <0.001 | 3.04 | (2.25–4.12) |
| Age | 0.28 | 1.08 | (0.94–1.23) | 0.23 | 1.08 | (0.95–1.24) |
| Physical activity | 0.001 | 0.87 | (0.81–0.95) | 0.001 | 0.86 | (0.78–0.94) |
| Childhood stress | <0.001 | 1.22 | (1.15–1.30) | <0.001 | 1.16 | (1.07–1.25) |
| BDNF rs6265 GG | 0.46 | 0.052 | ||||
| BDNF rs6265 GA | 0.30 | 1.63 | (0.65–4.11) | 0.07 | 0.45 | (0.19–1.06) |
| BDNF rs6265 AA | 0.44 | 1.45 | (0.57–3.72) | 0.10 | 0 | (0–2.73) |
| rs6265 GG × physical activity | 0.719 | |||||
| rs6265 GA × physical activity | 0.469 | 1.07 | (0.87–1.27) | |||
| rs6265 AA × physical activity | 0.687 | 1.22 | (0.46–3.25) | |||
| rs6265 GG × stress | 0.020 | |||||
| rs6265 GA × stress | 0.057 | 1.15 | (1.00–1.32) | |||
| rs6265 AA × stress | 0.034 | 4.00 | (1.11–14.46) | |||
| Constant | 0.04 | 0.08 | 0.011 | 0.04 |
| Outcome | Interaction Model | Interaction Term | R2 Change | F | p |
|---|---|---|---|---|---|
| Depressive symptoms wave 1 | 1 | x*physical activity wave 1 | 0.006 | 4.345 | 0.013 |
| 1 | x*childhood stress | 0.000 | 0.171 | 0.843 | |
| 2 | x*childhood stress | 0.000 | 0.018 | 0.982 | |
| x*physical activity wave 1 | 0.004 | 3.142 | 0.044 | ||
| x*childhood stress and x*physical activity wave 1 | 0.004 | 1.593 | 0.174 | ||
| 3 | x*physical activity wave 1*childhood stress | 0.004 | 3.290 | 0.038 | |
| Depressive symptoms wave 2 | 1 | x*physical activity wave 2 | 0.002 | 1.479 | 0.228 |
| 1 | x*childhood stress | 0.009 | 6.690 | 0.001 | |
| 2 | x*childhood stress | 0.008 | 5.973 | 0.003 | |
| x*physical activity wave 2 | 0.001 | 0.830 | 0.436 | ||
| x*childhood stress and x*physical activity wave 2 | 0.009 | 3.690 | 0.006 | ||
| 3 | x*physical activity wave 2*childhood stress | 0.003 | 1.966 | 0.141 | |
| Depressive symptoms wave 3 | 1 | x*physical activity wave 3 | 0.003 | 1.555 | 0.212 |
| 1 | x*childhood stress | 0.002 | 1.053 | 0.349 | |
| 2 | x*childhood stress | 0.003 | 1.705 | 0.182 | |
| 2 | x*physical activity wave 3 | 0.005 | 2.330 | 0.098 | |
| x*childhood stress and x*physical activity wave 3 | 0.007 | 1.700 | 0.148 | ||
| 3 | x*physical activity wave 3 *childhood stress | 0.001 | 0.472 | 0.624 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soler, C.T.; Kanders, S.H.; Olofsdotter, S.; Vadlin, S.; Åslund, C.; Nilsson, K.W. Exploration of the Moderating Effects of Physical Activity and Early Life Stress on the Relation between Brain-Derived Neurotrophic Factor (BDNF) rs6265 Variants and Depressive Symptoms among Adolescents. Genes 2022, 13, 1236. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13071236
Soler CT, Kanders SH, Olofsdotter S, Vadlin S, Åslund C, Nilsson KW. Exploration of the Moderating Effects of Physical Activity and Early Life Stress on the Relation between Brain-Derived Neurotrophic Factor (BDNF) rs6265 Variants and Depressive Symptoms among Adolescents. Genes. 2022; 13(7):1236. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13071236
Chicago/Turabian StyleSoler, Catalina Torres, Sofia H. Kanders, Susanne Olofsdotter, Sofia Vadlin, Cecilia Åslund, and Kent W. Nilsson. 2022. "Exploration of the Moderating Effects of Physical Activity and Early Life Stress on the Relation between Brain-Derived Neurotrophic Factor (BDNF) rs6265 Variants and Depressive Symptoms among Adolescents" Genes 13, no. 7: 1236. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13071236