Multi-Oxygenated Organic Compounds in Fine Particulate Matter Collected in the Western Mediterranean Area
Abstract
:1. Introduction
2. Experimental Section
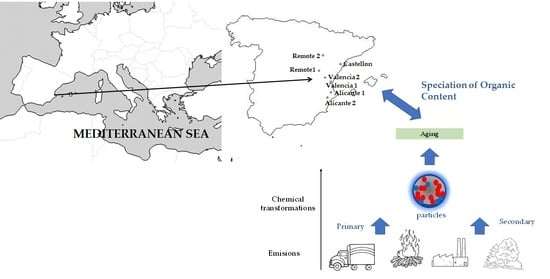
2.1. Location Description
2.2. Sample Collection
2.3. Analysis of PM Composition
2.4. Complementary Experiments
3. Results
3.1. General Description
3.2. Identification and Quantification of Organic Tracers
3.3. Sources Assessment of Organic Tracers
3.3.1. Dicarboxylic Acids
3.3.2. Mono-Carboxylic Acids
3.3.3. Levoglucosan
3.4. Ratios of Specific Tracers
3.4.1. C3/C4 Ratio
3.4.2. C6/C9 and C8/C9 Ratios
3.4.3. C18:0/C16 Ratio
3.4.4. C18:0/C18:1 Ratio
3.4.5. LG/OC
3.5. Major Inorganic Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Yang, L.; Chen, J.; Kawamura, K.; Sato, M.; Tilgner, A.; van Pinxteren, D.; Chen, Y.; Xue, L.; Wang, X.; et al. Molecular distributions of dicarboxylic acids, oxocarboxylic acids and α-dicarbonyls in PM2.5 collected at the top of Mt. Tai, North China, during the wheat burning season of 2014. Atmos. Chem. Phys. 2018, 18, 10741–10758. [Google Scholar] [CrossRef] [Green Version]
- Dongarrà, G.; Manno, E.; Varrica, D.; Lombardo, M.; Vultaggio, M. Study on ambient concentrations of PM10, PM10-2.5, PM2.5 and gaseous pollutants. Trace elements and chemical speciation of atmospheric particulates. Atmos. Environ. 2010, 44, 5244–5257. [Google Scholar] [CrossRef]
- Calvo, A.I.; Alves, C.; Castro, A.; Pont, V.; Vicente, A.M.; Fraile, R. Research on aerosol sources and chemical composition: Past, current and emerging issues. Atmos. Res. 2013, 120–121, 1–28. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of organic aerosols in the atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.J.; Zhang, Y.L.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.M.; Dällenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabha, S.; Saikia, B.K. Advanced micro- and nanoscale characterization techniques for carbonaceous aerosols. Handbook of Nanomaterials in Analytical Chemistry. Mod. Trends Anal. 2020, 449–472. [Google Scholar] [CrossRef]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and global climate modeling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef] [Green Version]
- Querol, X.; Alastuey, A.; Pey, J.; Cusack, M.; Pérez, N.; Mihalopoulos, N.; Theodosi, C.; Gerasopoulos, E.; Kubilay, N.; Koçak, M. Variability in regional background aerosols within the Mediterranean. Atmos. Chem. Phys. 2009, 9, 4575–4591. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.; Arey, J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review. Atmos. Environ. 2003, 37, 197–219. [Google Scholar] [CrossRef]
- Gieré, R.; Querol, X. Solid Particulate Matter in the Atmosphere. Elements 2010, 6, 215–222. [Google Scholar] [CrossRef]
- Tortajada-Genaro, L.A.; Borrás, E. Temperature effect of tapered element oscillating microbalance (TEOM) system measuring semi-volatile organic particulate matter. J. Environ. Monit 2011, 13, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Borrás, E.; Tortajada-Genaro, L.A. Secondary organic aerosol formation from the photo-oxidation of benzene. Atmos. Environ. 2012, 47, 154–163. [Google Scholar] [CrossRef]
- Yonghong, W.; Riva, M.; Xie, H.; Heikkinen, L.; Schallhart, S.; Zha, Q.; Yan, C.; He, X.-C.; Peräkylä, O.; Ehn, M. Formation of highly oxygenated organic molecules from chlorine-atom-initiated oxidation of alpha-pinene. Atmos. Chem. Phys. 2020, 20, 5145–5155. [Google Scholar] [CrossRef]
- Bianchi, F.; Kurtén, T.; Riva, M.; Mohr, C.; Rissanen, M.P.; Roldin, P.; Berndt, T.; Crounse, J.D.; Wennberg, P.O.; Mentel, T.F.; et al. Highly Oxygenated Organic Molecules (HOM) from Gas-Phase Autoxidation Involving Peroxy Radicals: A Key Contributor to Atmospheric Aerosol. Chem. Rev. 2019, 119, 3472–3509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkovich, A.H.; Graber, E.R.; Schkolnik, G.; Rudich, Y.; Maenhaut, W.; Artaxo, P. Low molecular weight organic acids in aerosol particles from Rondonia, Brazil, during the biomass-burning, transition and wet periods. Atmos. Chem. Phys. 2005, 5, 781–797. [Google Scholar] [CrossRef] [Green Version]
- Kourtchev, I.; Warnke, J.; Maenhaut, W.; Hoffmann, T.; Claeys, M. Polar organic marker compounds in PM2.5 aerosol from a mixed forest site in western Germany. Chemosphere 2008, 73, 1308–1314. [Google Scholar] [CrossRef]
- Mirante, F.; Alves, C.; Pio, C.; Pindado, O.; Pérez, R.; Revuelta, M.A.; Artiñano, B. Organic composition of size segregated atmospheric particulate matter, during summer and winter sampling campaigns at representative sites in Madrid, Spain. Atmos. Res. 2013, 132–133, 345–361. [Google Scholar] [CrossRef]
- García, M.I.; van Drooge, B.L.; Rodríguez, S.; Alastuey, A. Speciation of organic aerosols in the Saharan Air Layer and in the free troposphere westerlies. Atmos. Chem. Phys. 2017, 17, 8939–8958. [Google Scholar] [CrossRef] [Green Version]
- Jaoui, M.; Kleindienst, T.E.; Lewandowski, M.; Offenberg, J.H.; Ednby, E.O. Identification and Quantification of Aerosol Polar Oxygenated Compounds Bearing Carboxylic or Hydroxyl Groups. 1. Method Development. Anal. Chem. 2004, 76, 4765–4778. [Google Scholar] [CrossRef]
- Wang, W.; Vas, G.; Dommisse, R.; Loones, K.; Claeys, M. Fragmentation study of diastereoisomeric 2-methyltetrols, oxidation products of isoprene, as their trimethylsilyl ethers, using gas chromatography/ion trap mass spectrometry. Rapid. Comm. Mass. Spectrom. 2004, 18, 1787–1797. [Google Scholar] [CrossRef]
- Lopez-Hilfiker, F.D.; Mohr, C.; Ehn, M.; Rubach, F.; Kleist, E.; Wildt, J.; Mentel, T.M.; Carrasquillo, A.J.; Daumit, K.E.; Hunter, J.F.; et al. Phase partitioning and volatility of secondary organic aerosol components formed from -pinene ozonolysis and OH oxidation: The importance of accretion products and other low volatility compounds. Atmos. Chem. Phys. 2015, 15, 7765–7776. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Takeuchi, M.; Nah, T.; Xu, L.; Canagaratna, M.R.; Stark, H.; Baumann, K.; Canonaco, F.; Prévôt, A.S.H.; Huey, L.G.; et al. Chemical Characterization of Secondary Organic Aerosol at a Rural Site in the Southeastern, U.S.: Insights from Simultaneous HR-ToF-AMS and FIGAERO-CIMS Measurements. Atmos. Chem. Phys. Discuss 2020. in review. [Google Scholar] [CrossRef] [Green Version]
- Krechmer, J.E.; Groessl, M.; Zhang, X.; Junninen, H.; Massoli, P.; Lambe, A.T.; Kimmel, J.R.; Cubison, M.J.; Graf, S.; Lin, Y.-H.; et al. Ion mobility spectrometry–mass spectrometry (IMS–MS) for on and offline analysis of atmospheric gas and aerosol species. Atmos. Meas. Tech. 2016, 9, 3245–3262. [Google Scholar] [CrossRef] [Green Version]
- DeCarlo, P.F.; Kimmel, J.R.; Trimborn, A.; Northway, M.J.; Jayne, J.T.; Aiken, A.C.; Gonin, M.; Fuhrer, K.; Horvath, T.; Docherty, K.; et al. Field-Deployable, High-Resolution, Time-of-Flight Aerosol Mass Spectrometer. Anal. Chem. 2006, 78, 8281–8289. [Google Scholar] [CrossRef]
- Lewandowski, M.; Jaoui, M.; Kleindienst, T.E.; Offenberg, J.H.; Edney, E.O. Composition of PM2.5 during the summer of 2003 in Research Triangle Park, North Carolina. Atmos. Environ. 2007, 41, 4073–4083. [Google Scholar] [CrossRef]
- Ren, G.; Yan, X.; Ma, Y.; Qiao, L.; Chen, Z.; Xin, Y.; Zhou, M.; Shi, Y.; Zheng, K.; Zhu, S.; et al. Characteristics and source apportionment of PM2.5-bound saccharides and carboxylic acids in Central Shanghai, China. Atmos. Res. 2020, 237, 104817. [Google Scholar] [CrossRef]
- Teich, M.; van Pinxteren, D.; Herrmann, H. A one year study of functionalised medium-chain carboxylic acids in atmospheric particles at a rural site in Germany revealing seasonal trends and possible sources. J. Atmos Chem. 2019, 76, 115–132. [Google Scholar] [CrossRef]
- Van Drooge, B.L.; Grimalt, J.O. Particle size-resolved source apportionment of primary and secondary organic tracer compounds at urban and rural locations in Spain. Atmos. Chem. Phys. 2015, 15, 7735–7752. [Google Scholar] [CrossRef] [Green Version]
- Borrás, E.; Tortajada-Genaro, L.A. Determination of oxygenated compounds in secondary organic aerosol from isoprene and toluene smog chamber experiments. Environ. Anal. Chem. 2012, 92, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Millan, M.; Salvador, R.; Mantilla, E.; Artiñano, B. Meteorology and photochemical air pollution in southern Europe: Experimental results from EC research projects. Atmos. Environ. 1996, 30, 1909–1924. [Google Scholar] [CrossRef]
- Millan, M.; Artiñano, B.; Alonso, L.A.; Castro, M.; Fernández-Patier, R.; Goberna, J. Meso-meteorological cycles of air pollution in the Iberian Peninsula (MECAPIP); Air Pollution Research Report 44, EUR 14834; European Commission: Brussels, Belgium, 1992; pp. 1–219. [Google Scholar]
- Viana, M.; Kuhlbusch, T.A.J.; Querol, X.; Alastuey, A.; Harrison, R.M.; Hopke, P.K.; Winiwarter, W.; Vallius, M.; Szidat, S.; Prévôt, A.S.H.; et al. Source apportionment of particulate matter in Europe: A review of methods and results. Aero. Sci. 2008, 39, 827–849. [Google Scholar] [CrossRef]
- Alier, M.; van Drooge, B.L.; Dall’Osto, M.; Querol, X.; Grimalt, J.O.; Tauler, R. Source apportionment of submicron organic aerosol at an urban background and a road site in Barcelona (Spain) during SAPUSS. Atmos. Chem. Phys. 2013, 13, 10353–10371. [Google Scholar] [CrossRef] [Green Version]
- Ministerio de Transportes, Mobilidad y Agenda Urbana. Gobierno de España Home Page. Available online: www.mitma.es (accessed on 23 October 2019).
- Fundación Centro de Estudios Ambientales del Mediterráneo Home Page. Available online: www.ceam.es/ceamet (accessed on 23 October 2019).
- Ministerio para la Transición Ecológica y Reto Demográfico. Gobierno de España Home Page. Available online: www.miteco.gob.es/es (accessed on 23 October 2019).
- Salvador, P.; Artiñano, B.; Querol, X.; Alastuey, A.; Costoya, M. Characterisation of local and external contributions of atmospheric particulate matter at a background coastal site. Atmos. Environ. 2007, 41, 1–17. [Google Scholar] [CrossRef]
- Pey, J.; Querol, X.; Alastuey, A. Variations of levels and composition of PM10 and PM2.5 at an insular site in the Western Mediterranean. Atmos. Res. 2009, 94, 285–299. [Google Scholar] [CrossRef]
- Ram, K.; Sarin, M.M. Day and night variability of EC, OC, WSOC and inorganic ions in urban environment of Indo-Gangetic Plain: Implications to secondary aerosol formation. Atmos. Environ. 2011, 45, 460–468. [Google Scholar] [CrossRef]
- Curry, L.A.; Tsui, W.G.; McNeill, V.F. Technical note: Updated parameterization of the reactive uptake of glyoxal and methylglyoxal by atmospheric aerosols and cloud droplets. Atmos. Chem. Phys. 2018, 18, 9823–9830. [Google Scholar] [CrossRef] [Green Version]
- He, L.Y.; Hu, M.; Huang, X.-F.; Zhang, Y.-H.; Tang, X.-Y. Seasonal pollution characteristics of organic compounds in atmospheric fine particles in Beijing. Sci. Total Environ. 2006, 359, 167–176. [Google Scholar] [CrossRef]
- Van Pinxteren, D.; Neusüß, C.; Herrmann, H. On the abundance and source contributions of dicarboxylic acids in size-resolved aerosol particles at continental sites in central Europe. Atmos. Chem. Phys. 2014, 14, 3913–3928. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.F.; Ho, S.S.H.; Lee, S.C.; Kawamura, K.; Zou, S.C.; Cao, J.J.; Xu, H.M. Summer and winter variations of dicarboxylic acids, fatty acids and benzoic acid in PM2.5 in Pearl Delta River Region, China. Atmos. Chem. Phys. 2011, 11, 2197–2208. [Google Scholar] [CrossRef] [Green Version]
- Elshorbany, Y.; Barnes, I.; Becker, K.; Kleffmann, J.; Wiesen, P. Sources and Cycling of Tropospheric Hydroxyl Radicals—An Overview. Z. Phys. Chem. Int. J. Res. Phys. Chem. Chem. Phys. 2010, 224, 967–987. [Google Scholar] [CrossRef]
- Lelieveld, J.; Gromov, S.; Pozzer, A.; Taraborrelli, D. Global tropospheric hydroxyl distribution, budget and reactivity. Atmos. Chem. Phys. 2016, 16, 12477–12493. [Google Scholar] [CrossRef] [Green Version]
- Capka, L.; Mikuška, P.; Kamil, K. Determination of dicarboxylic acids in atmospheric aerosols using continuous aerosol sampler with on-line connected ion chromatography system. Atmos. Environ. 2020, 222, 117178. [Google Scholar] [CrossRef]
- Oliveira, C.; Pio, C.; Alves, C.; Evtyugina, M.; Santosa, P.; Goncalves, V.; Nunes, T.; Silvestre, A.J.D.; Palmgren, F.; Wahlin, P.; et al. Seasonal distribution of polar organic compounds in the urban atmosphere of two large cities from the North and South of Europe. Atmos. Environ. 2007, 41, 5555–5570. [Google Scholar] [CrossRef]
- Deshmukh, D.K.; Kawamura, K.; De, M.K. Dicarboxylic acids, u-oxocarboxylic acids, a-dicarbonyls, WSOC, OC, EC, and inorganic ions in wintertime size-segregated aerosols from central India: Sources and formation processes. Chemosphere 2016, 161, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Reche, C.; Viana, M.; Amato, F.; Alastuey, A.; Moreno, T.; Hillamo, R.; Teinilä, K.; Saarnio, K.; Secoe, R.; Peñuelas, J.; et al. Biomass burning contributions to urban aerosols in a coastal Mediterranean City. Sci. Total Environ. 2012, 427–428, 175–190. [Google Scholar] [CrossRef]




| Area | CS | VLC1 | VLC2 | AL1 | AL2 | Remote1 | Remote2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urban | Sub-Urban | Urban | Sub-Urban | Industrial | ||||||||||
| Av. | Rang. | Av. | Rang. | Av. | Rang. | Av. | Rang. | Av. | Rang. | Av. | Rang. | Av. | Rang. | |
| SO2 | 3 | (3–5) | 4 | (3–6) | 3 | (3–6) | 3 | (3–3) | 3 | (2–4) | 3 | (2–5) | 4 | (14–3) |
| CO | 0.2 | (0.1–0.3) | 0.2 | (0.1–0.4) | 0.1 | (0.1–0.4) | 0.2 | (0.1–0.4) | 0.2 | (0.1–0.3) | b.d.l | b.d.l | b.d.l | b.d.l |
| NO | 9 | (2–25) | 15 | (2–57) | 22 | (3–72) | 3 | (2–7) | 17 | (3–46) | 1 | (2–9) | 2,5 | (2–10) |
| NO2 | 26 | (14–52) | 30 | (10–57) | 44 | (16–76) | 15 | (4–33) | 35 | (10–49) | 5 | (4–25) | 4.3 | (9–4) |
| NOX | 39 | (17–90) | 56 | (15–43) | 78 | (20–174) | 19 | (6–43) | 63 | (11–98) | 6 | (7–98) | 7.1 | (6–16) |
| O3 | 47 | (23–69) | 41 | (9–69) | 37 | (0–71) | 55 | (14–82) | 50 | (5–86) | 73 | (20–110) | 88 | (47–138) |
| PM10 | 22 | (9–34) | 22 | (10–33) | 37 | (13–63) | 26 | (8–53) | 31 | (11–54) | 14 | (1–64) | 7 | (1–10) |
| PM2.5 | 10 | (5–15) | 15 | (5–27) | 27 | (24–30) | 13 | (6–24) | 21 | (4–29) | 7 | (1–46) | 3.4 | (0.1–5) |
| Cl− | 0.2 | (0–2) | 0.1 | (0–0.1) | b.d.l | b.d.l | 0,1 | (0.1–1.3) | 0.1 | (0–0.6) | b.d.l | b.d.l | b.d.l | b.d.l |
| NO3− | 0.6 | (0–6) | 0.2 | (0–1) | b.d.l | b.d.l | 1,1 | (0.1–6) | 8 | (0–10) | b.d.l | b.d.l | b.d.l | b.d.l |
| SO4−2 | 2 | (0.4–6) | 3 | (0.7–7) | b.d.l | b.d.l | 3 | (0.7–8) | 10 | (0.8–14) | b.d.l | b.d.l | b.d.l | b.d.l |
| NH4+ | 0.4 | (0–1.6) | 0.6 | (0–1.6) | b.d.l | b.d.l | 0.6 | (0.2–1.7) | 0.5 | (0.3–1.1) | b.d.l | b.d.l | b.d.l | b.d.l |
| O.M | 3 | (1.1–6) | 3.0 | (1.0–4) | 1.1 | (0.3–2) | 4.6 | (1.8–10) | 1.4 | (0.8–2) | b.d.l | b.d.l | b.d.l | b.d.l |
| Family | Code | Detected Compounds | Range | Average | Median |
|---|---|---|---|---|---|
| n-Alkanols | o | 8 | 0.22–1207 | 307 | 29 |
| Anhydrosugar | an | 1 | 0–2274 | 1947 | 186 |
| Aldehydes | a | 7 | 0.01–64 | 10 | 21 |
| MCA | m | 22 | 0–989 | 123 | 58 |
| DCA | d | 18 | 0–998 | 114 | 37 |
| Hydroxy-MCA | o-m | 3 | 0–382 | 77 | 49 |
| Keto-MCA | a-m | 3 | 0–48 | 14 | 34 |
| Keto-DCA | a-d | 1 | 18–835 | 215 | 174 |
| Hydroxy-aldehyde | o-a | 7 | 0.2–218 | 12 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrás, E.; Tortajada-Genaro, L.A.; Sanz, F.; Muñoz, A. Multi-Oxygenated Organic Compounds in Fine Particulate Matter Collected in the Western Mediterranean Area. Atmosphere 2021, 12, 94. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos12010094
Borrás E, Tortajada-Genaro LA, Sanz F, Muñoz A. Multi-Oxygenated Organic Compounds in Fine Particulate Matter Collected in the Western Mediterranean Area. Atmosphere. 2021; 12(1):94. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos12010094
Chicago/Turabian StyleBorrás, Esther, Luis Antonio Tortajada-Genaro, Francisco Sanz, and Amalia Muñoz. 2021. "Multi-Oxygenated Organic Compounds in Fine Particulate Matter Collected in the Western Mediterranean Area" Atmosphere 12, no. 1: 94. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos12010094