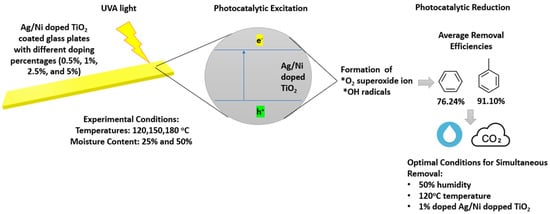
Photocatalytic Reduction of VOCs with Ag/Ni-Doped Photocatalyst in Different Temperature and Humidity Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Photocatalyst
2.2. Characterization of Ag/Ni-Doped Photocatalyst
2.3. Experimental Setup
2.4. Analysis Methods
3. Results and Discussion
3.1. Photocatalyst Characterization
3.1.1. SEM-EDX Analysis Results
3.1.2. XRD Analysis Results
3.1.3. Coating Properties
3.2. Photocatalytic Reduction of VOCs
3.2.1. Effect of Temperature
3.2.2. Effect of Photocatalysts Doping Percentage
3.2.3. Effect of Humidity
3.2.4. Comparison of the Effects
3.3. Unwanted Peaks and By-Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yücedağ, C.; Kaya, L.G. Impacts of Air Pollutants to Plants. J. Grad. Sch. Nat. Appl. Sci. Mehmet Akif Ersoy Univ. 2016, 7, 67–74. [Google Scholar]
- Holman, C. 8—Sources of Air Pollution. In Air Pollution and Health; SHolgate, T., Samet, J.M., Koren, H.S., Maynard, R.L., Eds.; Academic Press: London, UK, 1999; pp. 115–148. [Google Scholar]
- EPA Technical Overview of Volatile Organic Compounds. 2017. Available online: https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds (accessed on 15 April 2022).
- Rymen, T.A.A. Tracing Organic Gaseous Pollutants in the Atmosphere: The Gas Chromatographic Survey of Vinyl Chloride-and Paint industry. Int. J. Environ. Anal. Chem. 1979, 6, 1–23. [Google Scholar] [CrossRef]
- Manahan, S.E. Fundamentals of Environmental Chemistry; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Gholami, M.; Nassehinia, H.R.; Jonidi-Jafari, A.; Nasseri, S.; Esrafili, A. Comparison of Benzene & Toluene removal from synthetic polluted air with use of Nano photocatalytic TiO2/ZNO process. J. Environ. Health Sci. Eng. 2014, 12, 1–8. [Google Scholar]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Rahimi, N.; Pax, R.A.; Gray, E.M. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Dijy, C.; Divya, D. Reduction of air pollution from vehicles using titanium dioxide. Int. Res. J. Eng. Technol. 2015, 2, 1308–1314. [Google Scholar]
- Zhong, L.; Haghighat, F.; Blondeau, P.; Kozinski, J. Modeling and physical interpretation of photocatalytic oxidation Efficiency in indoor air applications. Build. Environ. 2010, 45, 2689–2697. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Mennig, M. Sol-Gel Technologies for Glass Producers and Users; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chapuis, Y.; Klvana, D.; Guy, C.; Kirchnerova, J. Photocatalytic oxidation of volatile organic compounds using fuorescent visible light. J. Air Waste Manag. Assoc. 2002, 52, 845–854. [Google Scholar] [CrossRef]
- Huang, H.; Liu, G.; Zhan, Y.; Xu, Y.; Lu, H.; Huang, H.; Feng, Q.; Wu, M. Photocatalytic oxidation of gaseous benzene under VUV irradiation over TiO2/zeolites catalysts. Catal. Today 2017, 281, 649–655. [Google Scholar] [CrossRef]
- Ji, J.; Xu, Y.; Huang, H.; He, M.; Liu, S.; Liu, G.; Xie, R.; Feng, Q.; Shu, Y.; Zhan, Y.; et al. Mesoporous TiO2 under VUV irradiation: Enhanced photocatalytic oxidation for VOCs degradation at room temperature. Chem. Eng. J. 2017, 327, 490–499. [Google Scholar] [CrossRef]
- Passalia, C.; Alfano, O.M.; Brandi, R.J. Integral design methodology of photocatalytic reactors for air pollution remediation. Molecules 2017, 22, 945. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, D.V. Titanium Dioxide in gas-Phase Photocatalytic Oxidation of Aromatic and Heteroatom Organic Substances: Deactivation and Reactivation of Photocatalyst. Theor. Exp. Chem. 2014, 50, 133–154. [Google Scholar] [CrossRef]
- Dursun, S.; Ayturan, Z.C. Simultaneous Removal of Gaseous Benzene and Toluene with Photocatalytic Oxidation Process at High Temperatures under UVC Irradiation. Environ. Sci. Pollut. Res. 2022, 29, 38232–38247. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.; Martin, J.C.; Ávila, O.; Gil-Llambias, F.J. Influence of the Bentonite/Titania Ratio on the Textural Characteristics of Incorporated Ceramics for Photocatalytic Destruction of Volatile Organic Compounds. In Studies in Surface Science and Catalysis; Llewellyn, P.L., Rodriquez-Reinoso, F., Rouqerol, J., Seaton, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 233–240. [Google Scholar]
- Persson, H. Photocatalytic Oxidation for VOC Abatement. Master’s Thesis, Department of Chemical Engineering and Technology, KTH Royal Institute of Technology, Stockholm, Sweden, 2015. [Google Scholar]
- Han, Y.; Zhang, J.; Zhao, Y. Visible-light-induced photocatalytic oxidation of nitric oxide and sulfur dioxide: Discrete kinetics and mechanism. Energy 2016, 103, 725–734. [Google Scholar] [CrossRef]
- Łabuz, P.; Gryboś, J.; Pietrzyk, P.; Sobańska, K.; Macyk, W.; Sojka, Z. Photogeneration of reactive oxygen species over ultrafine TiO2 particles functionalized with rutin–ligand induced sensitization and crystallization effects. Res. Chem. Intermed. 2019, 45, 5781–5800. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Zhu, Y.; Lamson, J.J.; Zhao, R. Determination and risk assessment of by-products resulting from photocatalytic oxidation of toluene. Appl. Catal. B 2009, 89, 570–576. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Zhu, Y.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Zorn, M.E.; Tompkins, D.T.; Zeltner, W.A.; Anderson, M.A. Photocatalytic oxidation of acetone vapor on TiO2/ZrO2 thin films. Appl. Catal. B Environ. 1999, 23, 1–8. [Google Scholar] [CrossRef]
- Wu, J.-F.; Hung, C.-H.; Yuan, C.-S. Kinetic modeling of promotion and inhibition of temperature on photocatalytic degradation of benzene vapor. J. Photochem. Photobiol. A Chem. 2005, 170, 299–306. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Gatto, S.; Pirola, C.; Naldoni, A.; Di Michele, A.; Cerrato, G.; Crocellà, V.; Capucci, V. Photocatalytic degradation of acetone, acetaldehyde and toluene in gas-phase: Comparison between nano and micro-sized TiO2. Appl. Catal. B Environ. 2014, 146, 123–130. [Google Scholar] [CrossRef]
- Hussain, M.; Russo, N.; Saracco, G. Photocatalytic abatement of VOCs by novel optimized TiO2 nanoparticles. Chem. Eng. J. 2011, 166, 138–149. [Google Scholar] [CrossRef]
- Khan, R.; Kim, T.-J. Preparation and application of visible-light-responsive Ni-doped and SnO2-coupled TiO2 nanocomposite photocatalysts. J. Hazard. Mater. 2009, 163, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ding, J.; Bao, J.; Gao, C.; Qi, Z.; Yang, X.; He, B.; Li, C. Photocatalytic degradation of gaseous toluene on Fe-TiO2 under visible light irradiation: A study on the structure, activity and deactivation mechanism. Appl. Surf. Sci. 2012, 258, 5031–5037. [Google Scholar] [CrossRef]
- Dhada, I.; Nagar, P.K.; Sharma, M. Challenges of TiO2-based photooxidation of volatile organic compounds: Designing, coating, and regenerating catalyst. Ind. Eng. Chem. Res. 2015, 54, 5381–5387. [Google Scholar] [CrossRef]
- Li, X.; Zou, X.; Qu, Z.; Zhao, Q.; Wang, L. Photocatalytic degradation of gaseous toluene over Ag-doping TiO2 nanotube powder prepared by anodization coupled with impregnation method. Chemosphere 2011, 83, 674–679. [Google Scholar] [CrossRef]
- Restek Pro EZGC Chromatogram Modeler. Available online: https://ez.restek.com/proezgc/en (accessed on 30 June 2022).
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Luo, Y.; Ollis, D.F. Heterogeneous photocatalytic oxidation of trichloroethylene and toluene mixtures in air: Kinetic promotion and inhibition, time-dependent catalyst activity. J. Catal. 1996, 163, 1–11. [Google Scholar] [CrossRef]
- D’hennezel, O.; Pichat, P.; Ollis, D.F. Benzene and toluene gas-phase photocatalytic degradation over H2O and HCL pretreated TiO2: By-products and mechanisms. J. Photochem. Photobiol. A—Chem. 1998, 118, 197–204. [Google Scholar] [CrossRef]
- Sauer, M.L.; Hale, M.A.; Ollis, D.F. Heterogeneous photocatalytic oxidation of dilute toluene–chlorocarbon mixtures in air. J. Photochem. Photobiol. A—Chem. 1995, 88, 169–178. [Google Scholar] [CrossRef]
- Einaga, H.; Futamura, S.; Ibusuki, T. Heterogeneous photocatalytic oxidation of benzene, toluene, cyclohexene and cyclohexane in humidifed air: Comparison of decomposition behavior on photoirradiated TiO2 catalyst. Appl. Catal. B Environ. 2002, 38, 215–225. [Google Scholar] [CrossRef]











| Samples with Different Doping Percentages | Parameters | Measured Values | Thin Film Thickness (µm) |
|---|---|---|---|
| 0.5% | Viscosity (mPa.s) | 1.5 | 5.9 |
| Surface Tension (dyn/cm) | 23.77 | ||
| Density (g/cm3) | 0.86 | ||
| Dip Speed (mm/s) | 2 | ||
| 1% | Viscosity (mPa.s) | 1.7 | 6.4 |
| Surface Tension (dyn/cm) | 21.606 | ||
| Density (g/cm3) | 0.87 | ||
| Dip Speed (mm/s) | 2 | ||
| 2.5% | Viscosity (mPa.s) | 2 | 7.1 |
| Surface Tension (dyn/cm) | 22.99 | ||
| Density (g/cm3) | 0.865 | ||
| Dip Speed (mm/s) | 2 | ||
| 5% | Viscosity (mPa.s) | 1.4 | 5.7 |
| Surface Tension (dyn/cm) | 21.57 | ||
| Density (g/cm3) | 0.867 | ||
| Dip Speed (mm/s) | 2 |
| Reactions |
|---|
| Photonic Excitation Reaction: TiO2 + hv → h+ + e− |
| Oxidation Reaction: OH− + h+ → OH* |
| Reduction Reaction: O2 + e− → O2− |
| Ionization of Water: H2O → OH− + H+ |
| Protonization of Super-oxide: O2*− + H+ → HOO* |
| Electron Removal: HOO* + e− → HOO− |
| Formation of H2O2: HOO− + H+ → H2O2 |
| OH* + pollutant + O2 → products CO2, H2O, vb.) |
| Pollutant | Photocatalyst | Removal Efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| at 120 °C, 25% Humidity | at 120 °C, 50% Humidity | at 150 °C, 25% Humidity | at 150 °C, 50% Humidity | at 180 °C, 25% Humidity | at 180 °C, 50% Humidity | Avg. | ||
| Benzene | Photocatalyst 1 | 77.04 | 71.71 | 30.55 | 54.32 | 84.17 | 90.15 | 67.99 |
| Photocatalyst 2 | 95.39 | 89.81 | 83.35 | 88.38 | 89.63 | 82.53 | 88.18 | |
| Photocatalyst 3 | 81.23 | 83.60 | 94.12 | 87.34 | 68.95 | 79.64 | 82.48 | |
| Photocatalyst 4 | 74.35 | 83.43 | 71.47 | 71.45 | 40.48 | 56.72 | 66.32 | |
| Avg. | 82.00 | 82.14 | 69.87 | 75.37 | 70.81 | 77.26 | ||
| Toluene | Photocatalyst 1 | 95.81 | 96.32 | 96.48 | 95.36 | 89.73 | 86.61 | 93.39 |
| Photocatalyst 2 | 90.35 | 92.35 | 93.52 | 92.28 | 89.75 | 89.97 | 91.38 | |
| Photocatalyst 3 | 85.49 | 85.62 | 93.74 | 87.15 | 94.48 | 94.46 | 90.16 | |
| Photocatalyst 4 | 96.81 | 96.27 | 94.48 | 94.46 | 91.12 | 68.74 | 90.32 | |
| Avg. | 92.12 | 92.65 | 94.56 | 92.32 | 91.27 | 84.95 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayturan, Z.C.; Dursun, S. Photocatalytic Reduction of VOCs with Ag/Ni-Doped Photocatalyst in Different Temperature and Humidity Environments. Atmosphere 2024, 15, 70. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos15010070
Ayturan ZC, Dursun S. Photocatalytic Reduction of VOCs with Ag/Ni-Doped Photocatalyst in Different Temperature and Humidity Environments. Atmosphere. 2024; 15(1):70. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos15010070
Chicago/Turabian StyleAyturan, Zeynep Cansu, and Sukru Dursun. 2024. "Photocatalytic Reduction of VOCs with Ag/Ni-Doped Photocatalyst in Different Temperature and Humidity Environments" Atmosphere 15, no. 1: 70. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos15010070