Climate Change and Water Exploitation as Co-Impact Sources on River Benthic Macroinvertebrates
Abstract
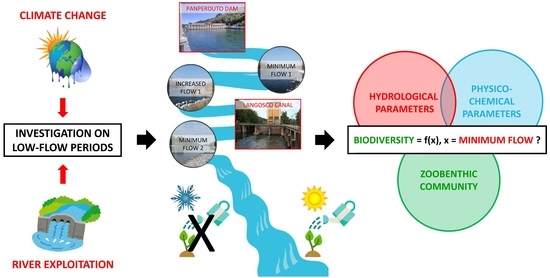
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Long-term Analysis of Lake Maggiore Outflows
3.2. Analysis of Low-Flow Periods Downstream of Water Withdrawals in the Ticino River
3.3. Analysis of Benthic Macroinvertebrate Communities during the Low-Flow Periods
4. Discussion
4.1. Effects of Climate Change on Low-Flow Periods
4.2. Effects of Low Flows on Benthic Macroinvertebrates
4.3. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, H.V.; Torresan, S.; Critto, A.; Marcomini, A. Alteration of freshwater ecosystem services under global change – A review focusing on the Po River basin (Italy) and the Red River basin (Vietnam). Sci. Total Environ. 2019, 652, 1347–1365. [Google Scholar] [CrossRef]
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; Vicente-Serrano, S.M.; Volaire, F.; Boone, A.; Le Page, M.; Llasat, M.C.; Albergel, C.; Burak, S.; et al. Challenges for drought assessment in the Mediterranean region under future climate scenarios. Earth-Sci. Rev. 2020, 210, 103348. [Google Scholar] [CrossRef]
- Wilby, R.L.; Harris, I. A framework for assessing uncertainties in climate change impacts: Low-flow scenarios for the River Thames, UK. Water Resour. Res. 2006, 42, 02419. [Google Scholar] [CrossRef]
- Giuntoli, I.; Vidal, J.-P.; Prudhomme, C.; Hannah, D.M. Future hydrological extremes: The uncertainty from multiple global climate and global hydrological models. Earth Syst. Dyn. 2015, 6, 267–285. [Google Scholar] [CrossRef] [Green Version]
- Vetter, T.; Huang, S.; Aich, V.; Yang, T.; Wang, X.; Krysanova, V.; Hattermann, F. Multi-model climate impact assessment and intercomparison for three large-scale river basins on three continents. Earth Syst. Dyn. 2015, 6, 17–43. [Google Scholar] [CrossRef] [Green Version]
- Vidal, J.-P.; Hingray, B.; Magand, C.; Sauquet, E.; Ducharne, A. Hierarchy of climate and hydrological uncertainties in transient low-flow projections. Hydrol. Earth Syst. Sci. 2016, 20, 3651–3672. [Google Scholar] [CrossRef] [Green Version]
- Amraoui, N.; Sbai, M.A.; Stollsteiner, P. Assessment of Climate Change Impacts on Water Resources in the Somme River Basin (France). Water Resour. Manag. 2019, 33, 2073–2092. [Google Scholar] [CrossRef]
- De Niel, J.; Van Uytven, E.; Willems, P. Uncertainty Analysis of Climate Change Impact on River Flow Extremes Based on a Large Multi-Model Ensemble. Water Resour. Manag. 2019, 33, 4319–4333. [Google Scholar] [CrossRef]
- Lytle, D.A.; Poff, N.L. Adaptation to natural flow regimes. Trends Ecol. Evol. 2004, 19, 94–100. [Google Scholar] [CrossRef]
- Acreman, M.C.; Ferguson, A.J.D. Environmental flows and the European Water Framework Directive. Freshw. Biol. 2010, 55, 32–48. [Google Scholar] [CrossRef]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C. The Natural Flow Regime. Bioscience 1997, 47, 769–784. [Google Scholar] [CrossRef]
- Bunn, S.E.; Arthington, A.H. Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poff, N.L.; Zimmerman, J.K.H. Ecological responses to altered flow regimes: A literature review to inform the science and management of environmental flows. Freshw. Biol. 2010, 55, 194–205. [Google Scholar] [CrossRef]
- European Commission. Report from the Commission to the European Parliament and the Council on the implementation of the Water Framework Directive (2000/60/EC) and the Floods Directive (2007/60/EC), Second River Basin Management Plans, First Flood Risk Management Plans. COM(2019) 95 final. Belgium, 2019; p. 10. Available online: https://ec.europa.eu/info/sites/default/files/com_report_wfd_fd_2019_en_1.pdf (accessed on 15 January 2021).
- Acreman, M.C.; Dunbar, M.J. Defining environmental river flow requirements—A review. Hydrol. Earth Syst. Sci. 2004, 8, 861–876. [Google Scholar] [CrossRef]
- Bragg, O.M.; Black, A.; Duck, R.W.; Rowan, J. Approaching the physical-biological interface in rivers: A review of methods for ecological evaluation of flow regimes. Prog. Phys. Geogr. Earth Environ. 2005, 29, 506–531. [Google Scholar] [CrossRef]
- Quadroni, S.; Crosa, G.; Gentili, G.; Espa, P. Response of stream benthic macroinvertebrates to current water management in Alpine catchments massively developed for hydropower. Sci. Total Environ. 2017, 609, 484–496. [Google Scholar] [CrossRef]
- Salmaso, F.; Crosa, G.; Espa, P.; Gentili, G.; Quadroni, S.; Zaccara, S. Benthic macroinvertebrates response to water management in a lowland river: Effects of hydro-power vs irrigation off-stream diversions. Environ. Monit. Assess. 2017, 190, 33. [Google Scholar] [CrossRef] [PubMed]
- Poff, N.L. Beyond the natural flow regime? Broadening the hydro-ecological foundation to meet environmental flows challenges in a non-stationary world. Freshw. Biol. 2018, 63, 1011–1021. [Google Scholar] [CrossRef]
- Arthington, A.H.; Bhaduri, A.; Bunn, S.; Jackson, S.; Tharme, R.E.; Tickner, D.; Young, B.; Acreman, M.; Baker, N.; Capon, S.; et al. The Brisbane Declaration and Global Action Agenda on Environmental Flows (2018). Front. Environ. Sci. 2018, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Steel, A.E.; Peek, R.A.; Lusardi, R.A.; Yarnell, S.M. Associating metrics of hydrologic variability with benthic macroinvertebrate communities in regulated and unregulated snowmelt-dominated rivers. Freshw. Biol. 2017, 63, 844–858. [Google Scholar] [CrossRef]
- Wilby, R.; Orr, H.; Watts, G.; Battarbee, R.; Berry, P.; Chadd, R.; Dugdale, S.; Dunbar, M.; Elliott, J.; Extence, C. Evidence needed to manage freshwater ecosystems in a changing climate: Turning adaptation principles into practice. Sci. Total Environ. 2010, 408, 4150–4164. [Google Scholar] [CrossRef]
- Acreman, M.; Overton, I.; King, J.; Wood, P.; Cowx, I.; Dunbar, M.J.; Kendy, E.; Young, W. The changing role of ecohydrological science in guiding environmental flows. Hydrol. Sci. J. 2014, 59, 433–450. [Google Scholar] [CrossRef]
- Arthington, A.H.; Kennen, J.G.; Stein, E.D.; Webb, J.A. Recent advances in environmental flows science and water management—Innovation in the Anthropocene. Freshw. Biol. 2018, 63, 1022–1034. [Google Scholar] [CrossRef] [Green Version]
- Billi, P.; Fazzini, M. Global change and river flow in Italy. Glob. Planet. Chang. 2017, 155, 234–246. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Buffagni, A.; Erba, S.; Birk, S.; Cazzola, M.; Feld, C.; Ofenbock, T.; van de Bund, W. Towards European Inter-Calibration for the Water Framework Directive: Procedures and Examples for Different River Types from the EC Project Star: Contract N. EVK1-CT 2001-00089: Matrix of Possible Class Boundaries of Grades of" Ecological Status" Associated with Different Methods Stressors; Provincia Autonoma di Trento: Bari, Italy, 2005; p. 468. [Google Scholar]
- Campaioli, S.; Ghetti, P.F.; Minelli, A.; Ruffo, S. Manuale per il Riconoscimento dei Macroinvertebrati Delle Acque Dolci Italiane; Provincia Autonoma di Trento: Bari, Italy, 1994; p. 357. [Google Scholar]
- Sansoni, G. Atlante per il Riconoscimento dei Macroinvertebrati Bentonici dei Corsi d’acqua Italiani; Provincia Autonoma di Trento: Bari, Italy, 1988; p. 191. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 1998. [Google Scholar]
- Richter, B.D.; Baumgartner, J.V.; Powell, J.; Braun, D.P. A Method for Assessing Hydrologic Alteration within Ecosystems. Conserv. Biol. 1996, 10, 1163–1174. [Google Scholar] [CrossRef] [Green Version]
- Hamed, K.; Rao, A.R. A modified Mann-Kendall trend test for autocorrelated data. J. Hydrol. 1998, 204, 182–196. [Google Scholar] [CrossRef]
- Schmidt-Kloiber, A.; Hering, D. www.freshwaterecology.info—An online tool that unifies, standardises and codifies more than 20,000 European freshwater organisms and their ecological preferences. Ecol. Indic. 2015, 53, 271–282. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B.; Novak, J.A.; Higgins, M.J.; Wessell, K.J.; Lessard, J.L. Development and application of a macroinvertebrate functional-group approach in the bioassessment of remnant river oxbows in southwest Florida. J. N. Am. Benthol. Soc. 2002, 21, 290–310. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.C.; Sample, J.E.; Moe, J.S.; Petrin, Z.; Meissner, T.; Hering, D. Unravelling the effect of flow regime on macroinvertebrates and benthic algae in regulated versus unregulated streams. Ecohydrology 2018, 11, e1996. [Google Scholar] [CrossRef]
- Quadroni, S.; Salmaso, F.; Gentili, G.; Crosa, G.; Espa, P. Response of benthic macroinvertebrates to different hydropower off-stream diversion schemes. Ecohydrology 2020, e2267. [Google Scholar] [CrossRef]
- Clausen, B.; Biggs, B.J.F. Relationships between benthic biota and hydrological indices in New Zealand streams. Freshw. Biol. 1997, 38, 327–342. [Google Scholar] [CrossRef]
- Salmi, T.; Määttä, A.; Anttila, P.; Ruoho-Airola, T.; Amnell, T. Detecting Trends of Annual Values of Atmospheric Pollutants by the Mann–Kendall Test and Sen’s Slope Estimates—the Excel Template Application MAKESENS; Ilmatieteen Laitos: Helsinki, Finland, 2002; p. 35. [Google Scholar]
- Mahdi, E.; McLeod, A.I. Package “portes”: Portmanteau Tests for Univariate and Multivariate Time Series Models. R package version 5.0. 2020. Available online: https://cran.r-project.org/web/packages/portes/portes.pdf (accessed on 30 September 2021).
- Patakamuri, S.K.; O’Brien, N. Package “modifiedmk”: Modified Versions of Mann Kendall and Spearman’s Rho Trend Tests. R package version 3.0. 2021. Available online: https://cran.r-project.org/web/packages/modifiedmk/modifiedmk.pdf (accessed on 30 September 2021).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data anal-ysis. Palaeontol. Electronics 2001, 4, 9. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 13 January 2021).
- Addinsoft. XLSTAT, A Complete statistical Add-in for Microsoft Excel; Addinsoft: New York, NY, USA, 2014. [Google Scholar]
- Meaurio, M.; Zabaleta, A.; Boithias, L.; Epelde, A.M.; Sauvage, S.; Sánchez-Pérez, J.-M.; Srinivasan, R.; Antiguedad, I. Assessing the hydrological response from an ensemble of CMIP5 climate projections in the transition zone of the Atlantic region (Bay of Biscay). J. Hydrol. 2017, 548, 46–62. [Google Scholar] [CrossRef]
- EEA (European Environment Agency). Climate change impacts and vulnerability in Europe 2016. In EEA Report No 1/2017; EEA: Copenhagen, Denmark, 2017; p. 419. [Google Scholar]
- Vansteenkiste, T.; Tavakoli, M.; Ntegeka, V.; De Smedt, F.; Batelaan, O.; Pereira, F.; Willems, P. Intercomparison of hydrological model structures and calibration approaches in climate scenario impact projections. J. Hydrol. 2014, 519, 743–755. [Google Scholar] [CrossRef]
- Wang, X. Advances in separating effects of climate variability and human activity on stream discharge: An overview. Adv. Water Resour. 2014, 71, 209–218. [Google Scholar] [CrossRef]
- Cui, T.; Tian, F.; Yang, T.; Wen, J.; Khan, M.Y.A. Development of a comprehensive framework for assessing the impacts of climate change and dam construction on flow regimes. J. Hydrol. 2020, 590, 125358. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, X.; Zhao, T. Annual shifts of flow regime alteration: New insights from the Chaishitan Reservoir in China. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Falcucci, A.; Maiorano, L.; Boitani, L. Changes in land-use/land-cover patterns in Italy and their implications for biodiversity conservation. Landsc. Ecol. 2007, 22, 617–631. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Lana-Renault, N. Hydrological and erosive consequences of farmland abandonment in Europe, with special reference to the Mediterranean region – A review. Agric. Ecosyst. Environ. 2011, 140, 317–338. [Google Scholar] [CrossRef]
- Ward, J.V.; A Stanford, J. Thermal Responses in the Evolutionary Ecology of Aquatic Insects. Annu. Rev. EÈntomol. 1982, 27, 97–117. [Google Scholar] [CrossRef]
- Dolédec, S. Seasonal dynamics of benthic macroinvertebrate communities in the Lower Ardèche River (France). Hydrobiologia 1989, 182, 73–89. [Google Scholar] [CrossRef]
- Cortes, R.M.V.; Ferreira, M.T.; Oliveira, S.V.; Oliveira, D. Macroinvertebrate community structure in a regulated river segment with different flow conditions. River Res. Appl. 2002, 18, 367–382. [Google Scholar] [CrossRef]
- Bonada, N.; Rieradevall, M.; Prat, N. Macroinvertebrate community structure and biological traits related to flow permanence in a Mediterranean river network. Hydrobiologia 2007, 589, 91–106. [Google Scholar] [CrossRef]
- Krajenbrink, H.J.; Acreman, M.; Dunbar, M.J.; Hannah, D.; Laizé, C.L.; Wood, P.J. Macroinvertebrate community responses to river impoundment at multiple spatial scales. Sci. Total Environ. 2019, 650, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Wills, T.C.; Baker, E.A.; Nuhfer, A.J.; Zorn, T.G. Response of the benthic macroinvertebrate community in a northern Michigan stream to reduced summer streamflows. River Res. Appl. 2006, 22, 819–836. [Google Scholar] [CrossRef] [Green Version]
- Rader, R.B.; Belish, T.A. Influence of mild to severe flow alterations on invertebrates in three mountain streams. Regul. Rivers: Res. Manag. 1999, 15, 353–363. [Google Scholar] [CrossRef]
- Dewson, Z.S.; James, A.B.W.; Death, R.G. A review of the consequences of decreased flow for instream habitat and macroinvertebrates. J. North Am. Benthol. Soc. 2007, 26, 401–415. [Google Scholar] [CrossRef]
- Chinnayakanahalli, K.J.; Hawkins, C.P.; Tarboton, D.; Hill, R.A. Natural flow regime, temperature and the composition and richness of invertebrate assemblages in streams of the western United States. Freshw. Biol. 2011, 56, 1248–1265. [Google Scholar] [CrossRef]
- Nukazawa, K.; Shirasaka, K.; Kajiwara, S.; Saito, T.; Irie, M.; Suzuki, Y. Gradients of flow regulation shape community structures of stream fishes and insects within a catchment subject to typhoon events. Sci. Total Environ. 2020, 748, 141398. [Google Scholar] [CrossRef]
- Wei, G.; Yang, Z.; Cui, B.; Li, B.; Chen, H.; Bai, J.; Dong, S. Impact of Dam Construction on Water Quality and Water Self-Purification Capacity of the Lancang River, China. Water Resour. Manag. 2009, 23, 1763–1780. [Google Scholar] [CrossRef]
- Salmaso, F.; Quadroni, S.; Gentili, G.; Crosa, G. Thermal regime of a highly regulated Italian river (Ticino River) and implications for aquatic communities. J. Limnol. 2016, 76. [Google Scholar] [CrossRef] [Green Version]
- Snelder, T.H.; Lamouroux, N. Co-variation of fish assemblages, flow regimes and other habitat factors in French rivers. Freshw. Biol. 2010, 55, 881–892. [Google Scholar] [CrossRef]
- Booker, D.J.; Snelder, T.H.; Greenwood, M.J.; Crow, S.K. Relationships between invertebrate communities and both hydrological regime and other environmental factors across New Zealand’s rivers. Ecohydrology 2015, 8, 13–32. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, Á.; De La Puente, M. Multimetric assessment of nutrient enrichment in impounded rivers based on benthic macroinvertebrates. Environ. Monit. Assess. 2004, 96, 233–249. [Google Scholar] [CrossRef]
- Miracle, M.R.; Moss, B.; Vicente, E.; Romo Pérez, S.; Rueda, J.; Bécares Mantecón, E.; Gyllström, M. Response of ma-croinvertebrates to experimental nutrient and fish additions in European localities at different latitudes. Limnetica 2006, 25, 585–612. [Google Scholar]
- Racchetti, E.; Salmaso, F.; Pinardi, M.; Quadroni, S.; Soana, E.; Sacchi, E.; Severini, E.; Celico, F.; Viaroli, P.; Bartoli, M. Is Flood Irrigation a Potential Driver of River-Groundwater Interactions and Diffuse Nitrate Pollution in Agricultural Watersheds? Water 2019, 11, 2304. [Google Scholar] [CrossRef] [Green Version]
- Theodoropoulos, C.; Vourka, A.; Stamou, A.; Rutschmann, P.; Skoulikidis, N. Response of freshwater macroinvertebrates to rainfall-induced high flows: A hydroecological approach. Ecol. Indic. 2017, 73, 432–442. [Google Scholar] [CrossRef]
- Basset, A.; Glazier, D.S. Resource limitation and intraspecific patterns of weight × length variation among spring detritivores. Hydrobiol. 1995, 316, 127–137. [Google Scholar] [CrossRef]
- Haapala, A.; Muotka, T.; Laasonen, P. Distribution of benthic macroinvertebrates and leaf litter in relation to streambed retentivity: Implications for headwater stream restoration. Boreal. Environ. Res. 2003, 8, 19–30. [Google Scholar]
- Robinson, C.T. Long-term changes in community assembly, resistance, and resilience following experimental floods. Ecol. Appl. 2012, 22, 1949–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornaroli, R.; Calabrese, S.; Marazzi, F.; Zaupa, S.; Mezzanotte, V. The influence of multiple controls on structural and functional characteristics of macroinvertebrate community in a regulated Alpine river. Ecohydrology 2019, 12, e2069. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Rothhaupt, K.-O.; Mörtl, M.; Cantonati, M.; Tóth, L.G.-; Fischer, P. Ecological effects of water-level fluctuations in lakes: An urgent issue. In Ecological Effects of Water-Level Fluctuations in Lakes; Springer: Dordrecht, The Netherlands, 2008; Volume 204, pp. 1–4. [Google Scholar]
- Jourdan, J.; O’Hara, R.B.; Bottarin, R.; Huttunen, K.-L.; Kuemmerlen, M.; Monteith, D.; Muotka, T.; Ozoliņš, D.; Paavola, R.; Pilotto, F.; et al. Effects of changing climate on European stream invertebrate communities: A long-term data analysis. Sci. Total Environ. 2018, 621, 588–599. [Google Scholar] [CrossRef]
- Mastore, M.; Rossetti, S.B.; Giovannardi, S.; Scari’, G.; Brivio, M.F. Inducible factors with antimicrobial activity after immune challenge in the haemolymph of Red Palm Weevil (Insecta). Innate Immun. 2015, 21, 392–405. [Google Scholar] [CrossRef] [Green Version]
- Barbaro, A.D.L.; Gariboldi, M.B.; Mastore, M.; Brivio, M.F.; Giovannardi, S. In Vivo Effects of A Pro-PO System Inhibitor on the Phagocytosis of Xenorhabdus Nematophila in Galleria Mellonella Larvae. Insects 2019, 10, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastore, M.; Quadroni, S.; Toscano, A.; Mottadelli, N.; Brivio, M.F. Susceptibility to entomopathogens and modulation of basal immunity in two insect models at different temperatures. J. Therm. Biol. 2019, 79, 15–23. [Google Scholar] [CrossRef]
- Brivio, M.F.; Mastore, M. When Appearance Misleads: The Role of the Entomopathogen Surface in the Relationship with Its Host. Insects 2020, 11, 387. [Google Scholar] [CrossRef]
- Bruno, D.; Belmar, O.; Maire, A.; Morel, A.; Dumont, B.; Datry, T. Structural and functional responses of invertebrate communities to climate change and flow regulation in alpine catchments. Glob. Chang. Biol. 2019, 25, 1612–1628. [Google Scholar] [CrossRef] [Green Version]






| Ljung–Box | Mann-Kendall | Sen’s Estimate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrological parameters | n | χ2 | p | Z | p | Q | Qmin95 | Qmax95 | B | Bmin95 | Bmax95 |
| Base flow | 77 | 0.18 | 0.669 | −2.10 | 0.036 | −0.001 | −0.003 | 0.000 | 0.4 | 0.5 | 0.4 |
| Lo pulse # | 77 | 15.43 | <0.001 | −4.89 * | < 0.001 * | −0.070 | −0.103 | −0.039 | 6.3 | 7.7 | 4.7 |
| Lo pulse L | 77 | 3.55 | 0.060 | 4.82 | <0.001 | 0.351 | 0.167 | 0.568 | −0.1 | 5.8 | −3.1 |
| Fall rate | 77 | 0.19 | 0.666 | −3.27 | 0.001 | −0.119 | −0.197 | −0.049 | −12.1 | −9.5 | −15.3 |
| Reversals | 77 | 34.69 | <0.001 | −3.09 * | 0.002 * | −0.863 | −1.096 | −0.625 | 98 | 106 | 87 |
| XLowP L | 54 | 0.05 | 0.820 | 3.97 | <0.001 | 0.324 | 0.134 | 0.590 | 1.7 | 5.8 | −3.8 |
| QM-Sep/QM-year | 77 | 0.58 | 0.447 | −2.08 | 0.029 | −0.004 | −0.008 | 0.000 | 1.0 | 1.3 | 0.9 |
| Taxon | AD = 65.8 | MA–IF1 | AD = 65.9 | MA–MF1 | AD = 62.3 | MA–MF2 | |||
|---|---|---|---|---|---|---|---|---|---|
| C (%) | I-Warm | NI-Cold | C (%) | I-Warm | NI-Cold | C (%) | I-Warm | NI-Cold | |
| Leuctra | 2.2 | 46 | 0.2 | 2.3 | 73 | 0.1 | 2.5 | 64 | 0.4 |
| Baetis | 16.0 | 425 | 229 | 14.4 | 678 | 231 | 16.7 | 537 | 201 |
| Caenis | 4.3 | 45 | 95 | 2.0 | 61 | 115 | 4.0 | 81 | 89 |
| Ecdyonurus | 1.1 | 24 | 20 | 1.7 | 66 | 28 | 2.2 | 51 | 26 |
| Ephemerella | 13.1 | 23 | 361 | 7.7 | 45 | 505 | 7.3 | 28 | 219 |
| Hydropsychidae | 17.2 | 299 | 362 | 22.3 | 1080 | 487 | 16.8 | 493 | 331 |
| Hydroptilidae | 1.0 | 15 | 12 | 1.0 | 33 | 4 | 0.9 | 20 | 1 |
| Leptoceridae | 0.9 | 9 | 13 | 0.4 | 7 | 3 | 0.2 | 3 | 2 |
| Psychomyiidae | 3.9 | 31 | 77 | 1.5 | 19 | 66 | 2.0 | 23 | 62 |
| Rhyacophilidae | 1.4 | 19 | 27 | 1.3 | 48 | 53 | 0.4 | 9 | 12 |
| Chironomidae | 17.6 | 306 | 422 | 33.9 | 307 | 2390 | 35.0 | 519 | 1200 |
| Simuliidae | 4.4 | 93 | 24 | 3.8 | 55 | 127 | 4.3 | 61 | 81 |
| Elmidae | 1.8 | 30 | 43 | 1.9 | 30 | 94 | 0.6 | 11 | 17 |
| Neritidae | 1.1 | 24 | 10 | 0.05 | 0.3 | 1 | 0.004 | 0.1 | 0 |
| Asellidae | 0.3 | 3 | 4 | 1.2 | 42 | 15 | 0.1 | 3 | 1 |
| Gammaridae | 4.5 | 74 | 11 | 0.1 | 3 | 2 | 1.5 | 40 | 20 |
| Lumbricidae | 0.8 | 19 | 13 | 0.5 | 21 | 13 | 0.5 | 10 | 10 |
| Naididae | 1.4 | 1 | 26 | 1.2 | 6 | 100 | 2.4 | 26 | 58 |
| Dugesia | 0.8 | 15 | 4 | 0.6 | 21 | 12 | 0.7 | 16 | 9 |
| Hydracarina | 2.3 | 31 | 15 | 0.6 | 13 | 10 | 0.5 | 9 | 6 |
| Period | Model Equation | Adj. R2 | AIC | F | p |
|---|---|---|---|---|---|
| I-warm | N = 964 + 30.6 × DURLF-max | 0.11 | 440 | 4.6 | 0.041 |
| H = 1.51 + 0.03 × Qmin | 0.17 | −66 | 7.1 | 0.013 | |
| % EPT = 1.04 − 0.54 × NO3−−N | 0.19 | −105 | 7.8 | 0.009 | |
| A/H = 1.34 − 0.03 × T | 0.20 | −119 | 8.3 | 0.008 | |
| TDc = 0.19 + 0.0006 × INCL | 0.27 | −176 | 11.5 | 0.002 | |
| LC = −0.50 + 2.25 × NO3−−N | 0.18 | −18 | 7.6 | 0.010 | |
| NI-cold | N = −15373 + 2234 × pH | 0.17 | 485 | 6.8 | 0.014 |
| S = 25.3 − 0.03 × QP75 | 0.13 | 113 | 5.5 | 0.027 | |
| EPT = 10.1 − 0.002 × ΔQ | 0.23 | 39 | 9.8 | 0.004 | |
| H = 1.29 + 0.02 × Qmin | 0.18 | −57 | 7.2 | 0.01 | |
| C/D = 0.94 − 0.009 × INCL | 0.48 | −121 | 27.9 | <0.0001 | |
| % EPT = 0.58 − 0.0002 × ΔQ | 0.13 | −98 | 5.2 | 0.030 | |
| TDc = 0.16 + 0.0002 × INCMax | 0.24 | −180 | 10.0 | 0.004 | |
| LC = −0.03 + 0.006 × ΔQ | 0.28 | 87 | 12.0 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmaso, F.; Crosa, G.; Espa, P.; Quadroni, S. Climate Change and Water Exploitation as Co-Impact Sources on River Benthic Macroinvertebrates. Water 2021, 13, 2778. https://0-doi-org.brum.beds.ac.uk/10.3390/w13192778
Salmaso F, Crosa G, Espa P, Quadroni S. Climate Change and Water Exploitation as Co-Impact Sources on River Benthic Macroinvertebrates. Water. 2021; 13(19):2778. https://0-doi-org.brum.beds.ac.uk/10.3390/w13192778
Chicago/Turabian StyleSalmaso, Francesca, Giuseppe Crosa, Paolo Espa, and Silvia Quadroni. 2021. "Climate Change and Water Exploitation as Co-Impact Sources on River Benthic Macroinvertebrates" Water 13, no. 19: 2778. https://0-doi-org.brum.beds.ac.uk/10.3390/w13192778