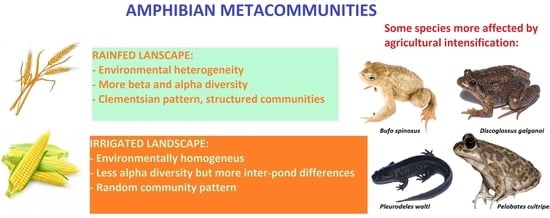
Amphibian Metacommunity Responses to Agricultural Intensification in a Mediterranean Landscape
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Amphibian Sampling
2.3. Environmental Variables
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ceballos, G.; Ehrlich, P.R. The misunderstood sixth mass extinction. Science 2018, 360, 1080–1081. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.H.C.; Muths, E.; Schmidt, B.R.; Petrovan, S.O. Amphibian conservation in the Anthropocene. Biol. Conserv. 2019, 236, 543–547. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Martin-Guay, M.O.; Paquette, A.; Dupras, J.; Rivest, D. The new Green Revolution: Sustainable intensification of agriculture by intercropping. Sci. Total Environ. 2018, 615, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balestrieri, A.; Gazzola, A.; Formenton, G.; Canova, L. Long-term impact of agricultural practices on the diversity of small mammal communities: A case study based on owl pellets. Environ. Monit. Assess. 2019, 191. [Google Scholar] [CrossRef]
- Habel, J.C.; Ulrich, W.; Biburger, N.; Seibold, S.; Schmitt, T. Agricultural intensification drives butterfly decline. Insect Conserv. Divers. 2019, 12, 289–295. [Google Scholar] [CrossRef]
- Morgado, R.; Santana, J.; Porto, M.; Sánchez-Oliver, J.S.; Reino, L.; Herrera, J.M.; Rego, F.; Beja, P.; Moreira, F. A Mediterranean silent spring? The effects of olive farming intensification on breeding bird communities. Agric. Ecosyst. Environ. 2020, 288, 106694. [Google Scholar] [CrossRef]
- Uchida, K.; Ushimaru, A. Biodiversity declines due to abandonment and intensification of agricultural lands: Patterns and mechanisms. Ecol. Monogr. 2014, 84, 637–658. [Google Scholar] [CrossRef]
- Betts, M.G.; Wolf, C.; Pfeifer, M.; Banks-Leite, C.; Arroyo-Rodríguez, V.; Bandini Ribeiro, D.; Barlow, J.; Eigenbrod, F.; Faria, D.; Fletcher, R.J., Jr.; et al. Extinction filters mediate the global effects of habitat fragmentation on animals. Science 2019, 366, 1236–1239. [Google Scholar] [CrossRef] [Green Version]
- Fahrig, L. Ecological Responses to Habitat Fragmentation Per Se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Córdova-Lepe, F.; Del Valle, R.; Ramos-Jiliberto, R. The process of connectivity loss during habitat fragmentation and their consequences on population dynamics. Ecol. Model. 2018, 376, 68–75. [Google Scholar] [CrossRef]
- Lewis-Phillips, J.; Brooks, S.J.; Sayer, C.D.; Patmore, I.R.; Hilton, G.M.; Harrison, A.; Robson, H.; Axmacher, J.C. Ponds as insect chimneys: Restoring overgrown farmland ponds benefits birds through elevated productivity of emerging aquatic insects. Biol. Conserv. 2020, 241, 108253. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity organisation, spatial extent and dispersal in aquatic systems: Patterns, processes and prospects. Freshw. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]
- Chaparro, G.; Horváth, Z.; O’Farrell, I.; Ptacnik, R.; Hein, T. Plankton metacommunities in floodplain wetlands under contrasting hydrological conditions. Freshw. Biol. 2018, 60, 380–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez Cobelas, M.; Rojo, C.; Angeler, D.C. Mediterranean Limnology: Current status, gaps and the future. J. Limnol. 2005, 64, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.; Beja, P. Mediterranean amphibians and the loss of temporary ponds: Are there alternative breeding habitats? Biol. Conserv. 2013, 165, 179–186. [Google Scholar] [CrossRef]
- Ruhí, A.; Sebastian, O.S.; Feo, C.; Franch, M.; Gascón, S.; Richter-Boix, À.; Boix, D.; Llorente, G. Man-made Mediterranean temporary ponds as a tool for amphibian conservation. Ann. Limnol. Int. J. Limnol. 2012, 48, 81–93. [Google Scholar] [CrossRef]
- Bolle, H.J. (Ed.) Mediterranean Climate. Variability and Trends; Springer: Berlin, Germany, 2003; 320p. [Google Scholar]
- Beklioglu, M.; Romo, S.; Kagalou, I.; Quintana, X.; Becares, E. State of the art in the functioning of shallow Mediterranean lakes: Workshop conclusions. Hydrobiologia 2007, 584, 317–326. [Google Scholar] [CrossRef]
- Harmanny, K.S.; Malek, Ž. Adaptations in irrigated agriculture in the Mediterranean region: An overview and spatial analysis of implemented strategies. Reg. Environ. Change 2019, 19, 1401–1416. [Google Scholar] [CrossRef] [Green Version]
- Giralt, D.; Pantoja, J.; Morales, M.B.; Traba, J.; Bota, G. Landscape-Scale Effects of Irrigation on a Dry Cereal Farmland Bird Community. Front. Ecol. Evol. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- De Frutos, A.; Olea, P.P.; Mateo-Tomás, P. Responses of medium- and large-sized bird diversity to irrigation in dry cereal agroecosystems across spatial scales. Agric. Ecosyst. Environ. 2015, 207, 141–152. [Google Scholar] [CrossRef]
- Clemente-Orta, G.; Madeira, F.; Batuecas, I.; Sossai, I.; Juárez-Escario, A.; Albajes, R. Changes in landscape composition influence the abundance of insects on maize: The role of fruit orchards and alfalfa crops. Agric. Ecosyst. Environ. 2020, 291, 106805. [Google Scholar] [CrossRef]
- Cox, N.; Chanson, J.; Stuart, S. The Status and Distribution of Reptiles and Amphibians of the Mediterranean Basin; IUCN: Gland, Switzerland; Cambridge, UK, 2006; 42p. [Google Scholar]
- Beja, P.; Alcazar, R. Conservation of Mediterranean temporary ponds under agricultural intensification: An evaluation using amphibians. Biol. Conserv. 2003, 114, 317–326. [Google Scholar] [CrossRef]
- Fortuna, M.A.; Gómez-Rodríguez, C.; Bascompte, J. Spatial network structure and amphibian persistence in stochastic environments. Proc. R. Soc. Lond. B Biol. Sci. 2005, 273, 1429–1434. [Google Scholar] [CrossRef] [Green Version]
- Caballero-Díaz, C.; Sánchez-Montes, G.; Butler, H.M.; Vredenburg, V.T.; Martínez-Solano, Í. The role of artificial breeding sites in amphibian conservation: A case study in rural areas in central Spain. Herpetol. Conserv. Biol. 2020, 15, 87–104. [Google Scholar]
- Valdez, J.W.; Gould, J.; Garnham, J.I. Global assessment of artificial habitat use by amphibian species. Biol. Conserv. 2021, 257, 109129. [Google Scholar] [CrossRef]
- Jakob, C.; Poizat, G.; Veith, M.; Seitz, A.; Crivelli, A.J. Breeding phenology and larval distribution of amphibians in a Mediterranean pond network with unpredictable hydrology. Hydrobiologia 2003, 499, 51–61. [Google Scholar] [CrossRef]
- Richter-Boix, A.; Llorente, G.A.; Montori, A. Structure and dynamics of an amphibian metacommunity in two regions. J. Anim. Ecol. 2007, 76, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Rodríguez, F.; Juan, M.; Gallego, I.; Lusi, M.; Fenoy, E.; León, D.; Peñalver, P.; Toja, J.; Casas, J.J. Diversity in Mediterranean farm ponds: Trade-offs and synergies between irrigation modernisation and biodiversity conservation. Freshw. Biol. 2003, 58, 63–78. [Google Scholar] [CrossRef]
- Aspe, C.; Gilles, A.; Jacqué, M. Irrigation canals as tools for climate change adaptation and fish biodiversity management in Southern France. Reg. Environ. Change 2014, 16, 1975–1984. [Google Scholar] [CrossRef] [Green Version]
- Riedener, E.; Rusterholz, H.P.; Baur, B. Land-use abandonment owing to irrigation cessation affects the biodiversity of hay meadows in an arid mountain region. Agric. Ecosyst. Environ. 2016, 185, 144–152. [Google Scholar] [CrossRef]
- Fernández Aláez, M.; Fernández Aláez, C.; Rodríguez, S.; Bécares, E. Evaluation of the state of conservation of shallow ponds in the province of Leon (Northwest Spain) using botanical criteria. Limnetica 1999, 17, 107–117. [Google Scholar]
- Fernandez-Aláez, C.; Fernández-Aláez, M.; Trigal, C.; Luis, B. Hydrochemistry of northwest Spain ponds and its relationships to groundwaters. Limnetica 2006, 25, 433–452. [Google Scholar]
- Pozo, R.; Fernandez-Aláez, M.; Fernández-Aláez, C. Composición de las comunidades de macrófitos y establecimiento del estado de conservación de charcas y lagunas de la Depresión del Duero (noroeste de España) en base a criterios botánicos. Limnetica 2012, 31, 47–58. [Google Scholar]
- Logue, J.B.; Mouquet, N.; Peter, H.; Hillebrand, H. Empirical approaches to metacommunities: A review and comparison with theory. Trends Ecol. Evol. 2011, 26, 482–491. [Google Scholar] [CrossRef]
- Boissinot, A.; Besnard, A.; Lourdais, O. Amphibian diversity in farmlands: Combined influences of breeding-site and landscape attributes in western France. Agric. Ecosyst. Environ. 2019, 269, 51–61. [Google Scholar] [CrossRef]
- Hecnar, S.J.; M’Closkey, R.T. Species richness patterns of amphibians in southwestern Ontario ponds. J. Biogeogr. 1998, 25, 763–772. [Google Scholar] [CrossRef]
- Piha, H.; Luoto, M.; Merila, J. Amphibian Occurrence Is Influenced by Current and Historic Landscape Characteristics. Ecol. Appl. 2007, 17, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Indermaur, L.; Schaub, M.; Jokela, J.; Tockner, K.; Schmidt, B.R. Differential response to abiotic conditions and predation risk rather than competition avoidance determine breeding site selection by anurans. Ecography 2010, 33, 887–895. [Google Scholar] [CrossRef]
- Cayuela, H.; Valenzuela-Sánchez, A.; Teulier, L.; Martínez-Solano, Í.; Léna, J.P.; Merilä, J.; Muths, E.; Shine, R.; Quay, L.; Denoël, M.; et al. Determinants and consequences of dispersal in vertebrates with complex life cycles: A review of pond-breeding amphibians. Quart. Rev. Biol. 2020, 95, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Montes, G.; Wang, J.; Ariño, A.H.; Martínez-Solano, Í. Mountains as barriers to gene flow in amphibians: Quantifying the differential effect of a major mountain ridge on the genetic structure of four sympatric species with different life history traits. J. Biogeogr. 2018, 45, 318–331. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 30 January 2021).
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Marion, Z.H.; Fordyce, J.A.; Fitzpatrick, B.M. Pairwise beta diversity resolves an underappreciated source of confusion in calculating species turnover. Ecology 2017, 98, 933–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socolar, J.B.; Gilroy, J.J.; Kunin, W.E.; Edwards, D.P. How Should Beta-Diversity Inform Biodiversity Conservation? Trends Ecol. Evol. 2016, 31, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J. Species Association Analysis. 2016. Available online: https://cran.r-project.org/web/packages/spaa/spaa.pdf (accessed on 30 January 2021).
- Leibold, M.A.; Mikkelson, G.M. Coherence, species turnover, and boundary clumping: Elements of meta-community structure. Oikos 2002, 97, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Presley, S.J.; Higgins, C.L.; Willig, M.R. A comprehensive framework for the evaluation of metacommunity structure. Oikos 2010, 119, 908–917. [Google Scholar] [CrossRef]
- Dallas, T. Metacom: An R package for the analysis of metacommunity structure. Ecography 2014, 37, 402–405. [Google Scholar] [CrossRef] [Green Version]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002; 500p. [Google Scholar]
- Collins, S.J.; Fahrig, L. Responses of anurans to composition and configuration of agricultural landscapes. Agric. Ecosyst. Environ. 2017, 239, 399–409. [Google Scholar] [CrossRef]
- Recuero, E. Sapo de Espuelas—Pelobates cultripes. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Martínez Solano, I., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2014. Available online: http://www.vertebradosibericos.org/ (accessed on 30 January 2021).
- Martínez-Solano, I. Sapillo pintojo ibérico—Discoglossus galganoi. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Martínez-Solano, I., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2014. Available online: http://www.vertebradosibericos.org/ (accessed on 30 January 2021).
- Ortiz, M.E.; Marco, A.; Saiz, N.; Lizana, M. Impact of ammonium nitrate on growth and survival of six European amphibians. Arch. Environ. Contam. Toxicol. 2004, 47, 234–239. [Google Scholar] [CrossRef]
- Polo-Cavia, N.; Burraco, P.; Gomez-Mestre, I. Low levels of chemical anthropogenic pollution may threaten amphibians by impairing predator recognition. Aquat. Toxicol. 2016, 172, 30–35. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Bécares, E.; Fernández-Aláez, C.; Fernández-Aláez, M.; Mayo, R.; Jiménez, J.J. Chemical pollution in inland shallow ponds in the Mediterranean region (NW Spain): PAHs, insecticides and herbicides in water and sediments. Sci. Total Environ. 2016, 544, 797–810. [Google Scholar] [CrossRef]
- Tavares, H.N.; Da Silva, F.R. Species turnover drives the spatial distribution of frog beta diversity in farmland ponds. J. Trop. Ecol. 2019, 35, 199–202. [Google Scholar] [CrossRef]
- Boix, D.; Caria, M.C.; Gascón, S.; Mariani, M.A.; Sala, J.; Ruhí, A.; Compte, J.; Bagella, S. Contrasting intra-annual patterns of six biotic groups with different dispersal mode and ability in Mediterranean temporary ponds. Mar. Freshw. Res. 2017, 68, 1044–1060. [Google Scholar] [CrossRef]
- Ficetola, G.F.; De Bernardi, F. Amphibians in a human-dominated landscape: The community structure is related to habitat features and isolation. Biol. Conserv. 2004, 119, 219–230. [Google Scholar] [CrossRef]
- Santana, J.; Porto, M.; Reino, L.; Moreira, F.; Ribeiro, P.F.; Santos, J.L.; Rotenberry, J.T.; Beja, P. Using beta diversity to inform agricultural policies and conservation actions on Mediterranean farmland. J. Appl. Ecol. 2017, 54, 1825–1835. [Google Scholar] [CrossRef] [Green Version]
- Sueyoshi, M.; Ishiyama, N.; Nakamura, F. β-diversity decline of aquatic insects at the microhabitat scale associated with agricultural land use. Landsc. Ecol. Eng. 2016, 12, 187–196. [Google Scholar] [CrossRef]
- Johnson, R.K.; Angeler, D.G. Effects of agricultural land use on stream assemblages: Taxon-specific responses of alpha and beta diversity. Ecol. Indic. 2014, 45, 386–393. [Google Scholar] [CrossRef]
- Clements, F.E. Plant Succession. An Analysis of the Development of Vegetation; Carnegie Institution of Washington: Washington, DC, USA, 1917. [Google Scholar] [CrossRef] [Green Version]
- Lewinsohn, T.M.; Inácio Prado, P.; Jordano, P.; Bascompte, J.; Olesen, J.M. Structure in plant-animal interaction assemblages. Oikos 2006, 113, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Brasil, L.S.; Vieira, T.B.; de Oliveira-Junior, J.M.B.; Dias-Silva, K.; Juen, L. Elements of metacommunity structure in Amazonian Zygoptera among streams under different spatial scales and environmental conditions. Ecol. Evol. 2019, 7, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, D.J.; Fitzgerald, L.A. Disassembly of a dune-dwelling lizard community due to landscape fragmentation. Ecosphere 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Gómez-Mestre, I. Sapo corredor—Epidalea calamita. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Martínez-Solano, I., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2014. Available online: http://www.vertebradosibericos.org/ (accessed on 30 January 2021).
- Price, S.J.; Marks, D.R.; Howe, R.W.; Hanowski, J.A.M.; Niemi, G.J. The importance of spatial scale for conservation and assessment of anuran populations in coastal wetlands of the western Great Ponds, USA. Landsc. Ecol. 2005, 20, 441–454. [Google Scholar] [CrossRef]
- Rubbo, M.J.; Kiesecker, J.M. Amphibian Breeding Distribution in an Urbanized Landscape. Conserv. Biol. 2005, 19, 504–511. [Google Scholar] [CrossRef]
- Couto, A.P.; Ferreira, E.; Torres, R.T.; Fonseca, C. Local and landscape drivers of pond-breeding amphibian diversity at the northern edge of the Mediterranean. Herpetologica 2017, 73, 10–17. [Google Scholar] [CrossRef]
- Pyron, R.A. Global amphibian declines have winners and losers. Proc. Natl. Acad. Sci. USA 2018, 115, 3739–3741. [Google Scholar] [CrossRef] [Green Version]
- Nowakowski, A.J.; Thompson, M.E.; Donnelly, M.A.; Todd, B.D. Amphibian sensitivity to habitat modification is associated with population trends and species traits. Glob. Ecol. Biogeogr. 2017, 26, 700–712. [Google Scholar] [CrossRef] [Green Version]
- Capellà-Marzo, B.; Sánchez-Montes, G.; Martínez-Solano, I. Contrasting demographic trends and asymmetric migration rates in a spatially structured amphibian population. Integr. Zool. 2020, 15, 482–497. [Google Scholar] [CrossRef] [PubMed]





| Sorensen | Simpson | Nestedness | |
|---|---|---|---|
| All ponds Rainfed | 0.75 | 0.5 | 0.25 |
| All ponds Irrigated | 0.6 | 0.42 | 0.18 |
| All ponds Rainfed + Irrigated | 0.81 | 0.61 | 0.2 |
| Mean Pairwise Rainfed | 0.177 | 0.092 | 0.084 |
| Mean Pairwise Irrigated | 0.368 | 0.166 | 0.202 |
| Pairwise U Mann-Whitney | 16,996 | 30,014 | 21,802 |
| Pairwise p-value | 2.20 × 10−16 * | 0.06 | 3.478 × 10−11 * |
| Coherence z | Coherence p-Value | Turnover z | Turnover p-Value | Morisita Index | Morisita p-Value | Structure | |
|---|---|---|---|---|---|---|---|
| Rainfed | 2.05 | 0.04 * | 1.78 | 0.075 | 2 | 0.0012 * | Clementsian |
| Irrigated | 0.62 | 0.53 | 1.7 | 0.09 | NA | NA | Random |
| All ponds | 0.96 | 0.34 | 2.41 | 0.015 * | NA | NA | Random |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albero, L.; Martínez-Solano, Í.; Arias, A.; Lizana, M.; Bécares, E. Amphibian Metacommunity Responses to Agricultural Intensification in a Mediterranean Landscape. Land 2021, 10, 924. https://0-doi-org.brum.beds.ac.uk/10.3390/land10090924
Albero L, Martínez-Solano Í, Arias A, Lizana M, Bécares E. Amphibian Metacommunity Responses to Agricultural Intensification in a Mediterranean Landscape. Land. 2021; 10(9):924. https://0-doi-org.brum.beds.ac.uk/10.3390/land10090924
Chicago/Turabian StyleAlbero, Luis, Íñigo Martínez-Solano, Ana Arias, Miguel Lizana, and Eloy Bécares. 2021. "Amphibian Metacommunity Responses to Agricultural Intensification in a Mediterranean Landscape" Land 10, no. 9: 924. https://0-doi-org.brum.beds.ac.uk/10.3390/land10090924