Generation of Monoclonal Antibodies Specific for Native LL37 and Citrullinated LL37 That Discriminate the Two LL37 Forms in the Skin and Circulation of Cutaneous/Systemic Lupus Erythematosus and Rheumatoid Arthritis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antigens Used in this Study
2.2. Anti-LL37 Phage Display Selection Method
2.3. ELISA for Detecting Antibody Specificity Using Peptide Antigens
2.4. Human Studies
2.5. ELISA for Detection of Native LL37 and cit-LL37 in Human Blood
2.6. Detection of IFN-a in SLE Blood
2.7. Immunohistochemistry (IHC)
2.8. Statistical Analyses
3. Results
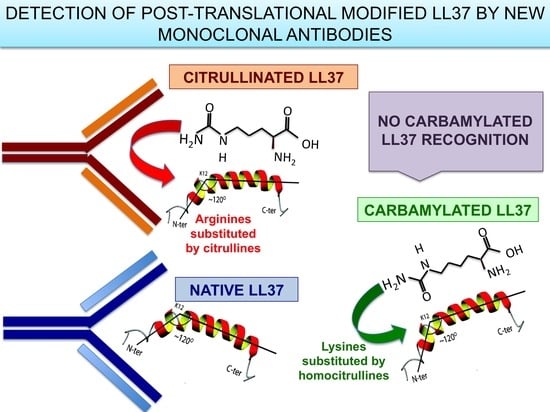
3.1. MRB137 and MRB138 Recognize Exclusively Native LL37
3.2. MRB139, MRB141 and MRB142 Recognize Exclusively cit-LL37 and Do Not Recognize carb-LL37, Nor Citrullines or Homocitrullines Themselves
3.3. MRB140 Recognizes Both Native and cit-LL37 But Not carb-LL37
3.4. MRB137, MRB138 MRB139, MRB140, MRB141 and MRB142 Do Not Cross-Recognize Unrelated Autoantigens in Their Native or Citrullinated Form
3.5. MRB138 and MRB139 Discriminate Expression of Native and cit-LL37 in Human LE Skin
3.6. Mab137 and MRB142 Can Be Used in ELISA Tests to Assess LL37 and cit-LL37 Expression in Body Fluids
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boman, H.G. Antibacterial peptides: Key components needed in immunity. Cell 1991, 65, 205–207. [Google Scholar] [CrossRef]
- Zanetti, M. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 2005, 7, 179–196. [Google Scholar] [PubMed]
- Johansson, J.; Gudmundsson, G.H.; Rottenberg, M.E.; Berndt, K.D.; Agerberth, B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 1998, 273, 3718–3724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- van Harten, R.M.; van Woudenbergh, E.; van Dijk, A.; Haagsman, H.P. Cathelicidins: Immunomodulatory Antimicrobials. Vaccines 2018, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [Green Version]
- Harder, J.; Schröder, J.M. Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J. Leukoc. Biol. 2005, 77, 476–486. [Google Scholar] [CrossRef] [Green Version]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, D.H.; Lee, K.J.; Cho, C.S.; Bang, S.I.; Cho, B.K.; Park, H.J. LL-37 suppresses sodium nitroprusside-induced apoptosis of systemic sclerosis dermal fibroblasts. Exp. Dermatol. 2011, 20, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Asano, Y.; Nakamura, K.; Yamashita, T.; Saigusa, R.; Ichimura, Y.; Toyama, T.; Taniguchi, T.; Yoshizaki, A.; Tamaki, Z.; et al. A potential contribution of antimicrobial peptide LL-37 to tissue fibrosis and vasculopathy in systemic sclerosis. Br. J. Dermatol. 2016, 175, 1195–1203. [Google Scholar] [CrossRef]
- Hoffmann, M.H.; Bruns, H.; Bäckdahl, L.; Neregård, P.; Niederreiter, B.; Herrmann, M.; Catrina, A.I.; Agerberth, B.; Holmdahl, R. The cathelicidins LL-37 and rCRAMP are associated with pathogenic events of arthritis in humans and rats. Ann. Rheum. Dis. 2013, 72, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Kienhöfer, D.; Hahn, J.; Schubert, I.; Reinwald, C.; Ipseiz, N.; Lang, S.C.; Borràs, È.B.; Amann, K.; Sjöwall, C.; Barron, A.E.; et al. No evidence of pathogenic involvement of cathelicidins in patient cohorts and mouse models of lupus and arthritis. PLoS ONE 2014, 9, e115474. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, F.; Pufe, T.; Conradi, L.; Varoga, D.; Tsokos, M.; Papendieck, J.; Petersen, W. Antimicrobial peptides are expressed and produced in healthy and inflamed human synovial membranes. J. Pathol. 2002, 198, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621–5635. [Google Scholar] [CrossRef]
- Frasca, L.; Palazzo, R.; Chimenti, M.S.; Alivernini, S.; Tolusso, B.; Bui, L.; Botti, E.; Giunta, A.; Bianchi, L.; Petricca, L.; et al. Anti-LL37 antibodies are present in psoriatic arthritis (PsA) patients: New biomarkers in PsA. Front. Immunol. 2018, 9, 1936. [Google Scholar] [CrossRef] [Green Version]
- Lande, R.; Palazzo, R.; Gestermann, N.; Jandus, C.; Falchi, M.; Spadaro, F.; Riccieri, V.; James, E.A.; Butera, A.; Boirivant, M.; et al. Native/citrullinated LL37-specific T-cells help autoantibody production in Systemic Lupus Erythematosus. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells though TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef]
- Chamilos, G.; Gregorio, J.; Meller, S.; Lande, R.; Kontoyiannis, D.P.; Modlin, R.L.; Gilliet, M. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood 2012, 120, 3699–3707. [Google Scholar] [CrossRef] [Green Version]
- Gestermann, N.; Di Domizio, J.; Lande, R.; Demaria, O.; Frasca, L.; Feldmeyer, L.; Di Lucca, J.; Gilliet, M. Netting neutrophils activate autoreactive B cells in Lupus. J. Immunol. 2018, 200, 3364–3371. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, N.W.; Jin, F.; Lande, R.; Curk, T.; Xian, W.; Lee, C.; Frasca, L.; Frenkel, D.; Dobnikar, J.; Gilliet, M.; et al. Liquid-crystalline ordering of antimicrobial peptide-DNA complexes controls TLR9 activation. Nat. Mater. 2015, 14, 696–700. [Google Scholar] [CrossRef]
- Kilsgård, O.; Andersson, P.; Malmsten, M.; Nordin, S.L.; Linge, H.M.; Eliasson, M.; Sörenson, E.; Erjefält, J.S.; Bylund, J.; Olin, A.I.; et al. Peptidylarginine deiminases present in the airways during tobacco smoking and inflammation can citrullinate the host defense peptide LL-37, resulting in altered activities. Am. J. Respir. Cell Mol. Biol. 2012, 46, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Koro, C.; Hellvard, A.; Delaleu, N.; Binder, V.; Scavenius, C.; Bergum, B.; Główczyk, I.; Roberts, H.M.; Chapple, I.L.; Grant, M.M.; et al. Carbamylated LL-37 as a modulator of the immune response. Innate Immun. 2016, 22, 218–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.; Bryzek, D.; Dobosz, E.; Scavenius, C.; Svoboda, P.; Rapala-Kozik, M.; Lesner, A.; Frydrych, I.; Enghild, J.; Mydel, P.; et al. A novel biological role for peptidyl-arginine deiminases: Citrullination of cathelicidin LL-37 controls the immunostimulatory potential of cell-free DNA. J. Immunol. 2018, 200, 2327–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziel, J.; Bryzek, D.; Sroka, A.; Maresz, K.; Glowczyk, I.; Bielecka, E.; Kantyka, T.; Pyrć, K.; Svoboda, P.; Pohl, J.; et al. Citrullination alters immunomodulatory function of LL-37 essential for prevention of endotoxin-induced Sepsis. J. Immunol. 2014, 192, 5363–5372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheul, M.K.; van Veelen, P.A.; van Delft, M.A.M.; de Ru, A.; Janssen, G.M.C.; Rispens, T.; Toes, R.E.M.; Trouw, L.A. Pitfalls in the detection of citrullination and carbamylation. Autoimmun. Rev. 2018, 17, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, B.; Mittereder, N.; Chaerkady, R.; Strain, M.; An, L.L.; Rahman, S.; Ma, W.; Low, C.P.; Chan, D.; et al. Spontaneous secretion of the citrullination enzyme PAD2 and cell surface exposure of PAD4 by neutrophils. Front. Immunol. 2017, 8, 1200–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, V.; Fert-Bober, J.; Nigrovic, P.A.; Darrah, E.; Haque, U.J.; Lee, D.M.; van Eyk, J.; Rosen, A.; Andrade, F. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci. Transl. Med. 2013, 30, 209ra150. [Google Scholar] [CrossRef] [Green Version]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef] [Green Version]
- Van Steendam, K.; Tilleman, K.; De Ceuleneer, M.; De Keyser, F.; Elewaut, D.; Deforce, D. Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthr. Res. Ther. 2010, 12, R132. [Google Scholar] [CrossRef] [Green Version]
- van Heemst, J.; Jansen, D.T.S.L.; Polydorides, S.; Moustakas, A.K.; Bax, M.; Feitsma, A.L.; Bontrop-Elferink, D.G.; Baarse, M.; van der Woude, D.; Wolbink, G.-J.; et al. Crossreactivity to vinculin and microbes provides a molecular basis for HLA-based protection against rheumatoid arthritis. Nat Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kinloch, A.J.; Cascino, M.D.; Dai, J.; Bermea, R.S.; Ko, K.; Vesselits, M.; Dragone, L.L.; Vaknin, N.M.; Legendre, M.; Markovitz, D.M.; et al. Anti-vimentin antibodies: A unique antibody class associated with therapy-resistant lupus nephritis. Lupus 2020, 29, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, E.; Yalavarthi, S.; Berthier, C.C.; Hodgin, J.B.; Khandpur, R.; Lin, A.M.; Rubin, C.J.; Zhao, W.; Olsen, S.H.; Klinker, M.; et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011, 187, 538–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuter, A.; Jaouhar, M.; Skrygan, M.; Tigges, C.; Stücker, M.; Altmeyer, P.; Gläser, R.; Gambichler, T. Expression of antimicrobial peptides in different subtypes of cutaneous lupus erythematosus. J. Am. Acad. Dermatol. 2011, 65, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Zhang, F.Z.; Li, P.; Bi, L.Q. LL-37 expression in the skin in systemic lupus erythematosus. Lupus 2011, 20, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.; Palucka, A.K.; Arce, E.; Cantrell, V.; Borvak, J.; Banchereau, J.; Pascual, V. Interferon and Granulopoiesis Signatures in Systemic Lupus Erythematosus Blood. J. Exp. Med. 2003, 197, 711–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirioni, O.; Giacometti, A.; Ghiselli, R.; Bergnach, C.; Orlando, F.; Silvestri, C.; Mocchegiani, F.; Licci, A.; Skerlavaj, B.; Rocchi, M.; et al. LL-37 protects rats against lethal sepsis caused by Gram-negative bacteria. Antimicrob. Agents Chemother. 2006, 50, 1672–1679. [Google Scholar] [CrossRef] [Green Version]
- Kissel, T.; Reijm, S.; Slot, L.M.; Cavallari, M.; Wortel, C.M.; Vergroesen, R.D.; Stoeken-Rijsbergen, G.; Kwekkeboom, J.C.; Kampstra, A.; Levarht, E.; et al. Antibodies and B cells recognising citrullinated proteins display a broad cross-reactivity towards other post-translational modifications. Ann. Rheum. Dis. 2020, 79, 472–480. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lande, R.; Palazzo, R.; Hammel, P.; Pietraforte, I.; Surbeck, I.; Gilliet, M.; Chizzolini, C.; Frasca, L. Generation of Monoclonal Antibodies Specific for Native LL37 and Citrullinated LL37 That Discriminate the Two LL37 Forms in the Skin and Circulation of Cutaneous/Systemic Lupus Erythematosus and Rheumatoid Arthritis Patients. Antibodies 2020, 9, 14. https://0-doi-org.brum.beds.ac.uk/10.3390/antib9020014
Lande R, Palazzo R, Hammel P, Pietraforte I, Surbeck I, Gilliet M, Chizzolini C, Frasca L. Generation of Monoclonal Antibodies Specific for Native LL37 and Citrullinated LL37 That Discriminate the Two LL37 Forms in the Skin and Circulation of Cutaneous/Systemic Lupus Erythematosus and Rheumatoid Arthritis Patients. Antibodies. 2020; 9(2):14. https://0-doi-org.brum.beds.ac.uk/10.3390/antib9020014
Chicago/Turabian StyleLande, Roberto, Raffaella Palazzo, Philippe Hammel, Immacolata Pietraforte, Isabelle Surbeck, Michel Gilliet, Carlo Chizzolini, and Loredana Frasca. 2020. "Generation of Monoclonal Antibodies Specific for Native LL37 and Citrullinated LL37 That Discriminate the Two LL37 Forms in the Skin and Circulation of Cutaneous/Systemic Lupus Erythematosus and Rheumatoid Arthritis Patients" Antibodies 9, no. 2: 14. https://0-doi-org.brum.beds.ac.uk/10.3390/antib9020014