α-Amino Phosphonic Acid as the Oxidized Ore Collector: Flexible Intra-Molecular Proton Transfer Providing an Improved Flotation Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Flotation Test
2.3. Adsorption Test
2.4. Zeta Potential Measurement
2.5. FTIR Spectroscopic Measurement
2.6. X-ray Photoelectron Spectroscopy
2.7. DFT Calculation
3. Result and Discussion
3.1. Micro-Flotation Test of Single Minerals
3.2. Micro-Flotation Test of Artificial Mixed Mineral
3.3. Adsorption of APA and OPA on the Surface of Ilmenite
3.4. Zeta Potential Measurements
3.5. FTIR Analysis
3.6. XPS Analysis
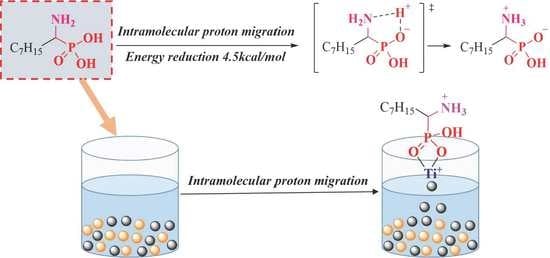
3.7. Computation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Demmer, C.S.; Krogsgaard-Larsen, N.; Bunch, L. Review on modern advances of chemical methods for the introduction of a phosphonic acid group. Chem. Rev. 2011, 111, 7981–8006. [Google Scholar] [CrossRef]
- Paniagua, S.A.; Giordano, A.J.; Smith, O.L.; Barlow, S.; Li, H.; Armstrong, N.R.; Pemberton, J.E.; Brédas, J.L.; Ginger, D.; Marder, S.R. Phosphonic Acids for Interfacial Engineering of Transparent Conductive Oxides. Chem. Rev. 2016, 116, 7117–7158. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, Y. The development of a composite collector for the flotation of rutile. Miner. Eng. 1999, 12, 1419–1430. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, C.; Wu, W. A new synthetic chelating collector for the flotation of oxidized-lead mineral. Minerals 2007, 1, 9–13. [Google Scholar] [CrossRef]
- Liu, C.; Ai, G.; Song, S. The effect of amino trimethylene phosphonic acid on the flotation separation of pentlandite from lizardite. Powder Technol. 2018, 336, 527–532. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Xu, H.; Jia, H.; Liu, G. Flotation behavior and adsorption mechanism of α-hydroxyoctyl phosphinic acid to malachite. Miner. Eng. 2015, 71, 188–193. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Zhao, G.; Wang, S.; Liu, G. Adsorption of α-hydroxyoctyl phosphonic acid to ilmenite/water interface and its application in flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 67–73. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, J.; Sun, W.; Hu, Y.; Cao, J.; Gao, Z. Selective flotation of chalcopyrite from pyrite using diphosphonic acid as collector. Miner. Eng. 2019, 140, 105890. [Google Scholar] [CrossRef]
- Gong, G.; Wang, P.; Liu, J.; Han, Y.; Zhu, Y. Effect and mechanism of Cu(II) on flotation separation of cassiterite from fluorite. Sep. Purif. Technol. 2020, 238, 116401. [Google Scholar] [CrossRef]
- Fan, H.; Tan, W.; Liu, G. 1-Hydroxydodecylidene-1,1-diphosphonic acid flotation of bastnäsite: Performance and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125623. [Google Scholar] [CrossRef]
- Kasomo, R.M.; Li, H.; Chen, Q.; Soraya, D.A.; Leopold, M.; Weng, X.; Mwangi, A.D.; Kiamba, E.; Ge, W.; Song, S. Behavior and mechanism of sodium sulfite depression of almandinefrom rutile in flotation system. Powder Technol. 2020, 374, 49–57. [Google Scholar] [CrossRef]
- Huang, K.; Huang, X.; Jia, Y.; Wang, S.; Cao, Z.; Zhong, H. A novel surfactant styryl phosphonate mono-iso-octyl ester with improved adsorption capacity and hydrophobicity for cassiterite flotation. Miner. Eng. 2019, 142, 105895. [Google Scholar] [CrossRef]
- Tan, X.; He, F.Y.; Shang, Y.B.; Yin, W.Z. Flotation behavior and adsorption mechanism of (1-hydroxy-2-methyl-2-octenyl) phosphonic acid to cassiterite. Trans. Nonferrous Met. Soc. China Engl. Ed. 2016, 26, 2469–2478. [Google Scholar] [CrossRef]
- Susumu, K.; Oh, E.; Delehanty, J.B.; Blanco-Canosa, J.B.; Johnson, B.J.; Jain, V.; Hervey, W.J.; Algar, W.R.; Boeneman, K.; Dawson, P.E.; et al. Multifunctional compact zwitterionic ligands for preparing robust biocompatible semiconductor quantum dots and gold nanoparticles. J. Am. Chem. Soc. 2011, 133, 9480–9496. [Google Scholar] [CrossRef]
- Soroka, M. The synthesis of 1-aminoalkylphosphonic acids. A revised mechanism of the reaction of phosphorus trichloride, amides and aldehydes or ketones in acetic acid (Oleksyszyn reaction). Liebigs Ann. Der Chem. 1990, 1990, 331–334. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Zhao, Y.; Bai, H.; Huang, M.; Zhang, T.; Song, S. High-performance two-dimensional montmorillonite supported-poly(acrylamide-co-acrylic acid) hydrogel for dye removal. Environ. Pollut. 2020, 257, 113574. [Google Scholar] [CrossRef]
- Zhao, G.; Zhong, H.; Qiu, X.; Wang, S.; Gao, Y.; Dai, Z.; Huang, J.; Liu, G. The DFT study of cyclohexyl hydroxamic acid as a collector in scheelite flotation. Miner. Eng. 2013, 49, 54–60. [Google Scholar] [CrossRef]
- Yan, W.; Shi, X.; Zhong, C. Secondary amines as lewis bases in nitroalkene activation. Asian J. Org. Chem. 2013, 2, 904–914. [Google Scholar] [CrossRef]
- Ryu, S. A Density Functional Study of Amine Catalysts for CO2 Fixation into Cyclic Carbonates. Bull. Korean Chem. Soc. 2019, 40, 1033–1038. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Li, G.; Wu, Y.; Jiang, T. A further study on adsorption interaction of humic acid on natural magnetite, hematite and quartz in iron ore pelletizing process: Effect of the solution pH value. Powder Technol. 2015, 271, 155–166. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Deng, W.; Jiang, H.; Gao, Z.; Hu, Y. Adsorption mechanism of new mixed anionic/cationic collectors in a spodumene-feldspar flotation system. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Dong, F.; Gao, Z.; Wu, H.; Wang, Z. Anisotropic adsorption of oleate on diaspore and kaolinite crystals: Implications for their flotation separation. Appl. Surf. Sci. 2014, 321, 331–338. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Wu, H.; Tian, J.; Liu, J.; Gao, Z.; Wang, L. Surface crystal chemistry of spodumene with different size fractions and implications for flotation. Sep. Purif. Technol. 2016, 169, 33–42. [Google Scholar] [CrossRef]
- Fan, X.; Waters, K.E.; Rowson, N.A.; Parker, D.J. Modification of ilmenite surface chemistry for enhancing surfactants adsorption and bubble attachment. J. Colloid Interface Sci. 2009, 329, 167–172. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Rezai, B. Chemical and mineralogical composition of ilmenite: Effects on physical and surface properties. Miner. Eng. 2015, 70, 64–76. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Tian, J.; Lu, Z.; Sun, W.; Hu, Y. Adsorption of Pb(II)/benzohydroxamic acid collector complexes for ilmenite flotation. Miner. Eng. 2018, 126, 16–23. [Google Scholar] [CrossRef]
- Liu, X.; Huang, G.Y.; Li, C.X.; Cheng, R.J. Depressive effect of oxalic acid on titanaugite during ilmenite flotation. Miner. Eng. 2015, 79, 62–67. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Huang, G.; Li, C. Low-temperature performance of cationic collector undecyl propyl ether amine for ilmenite flotation. Miner. Eng. 2017, 114, 50–56. [Google Scholar] [CrossRef]
- Islam, K.N.; Bakar, M.Z.B.A.; Ali, M.E.; Hussein, M.Z.B.; Noordin, M.M.; Loqman, M.Y.; Miah, G.; Wahid, H.; Hashim, U. A novel method for the synthesis of calcium carbonate (aragonite) nanoparticles from cockle shells. Powder Technol. 2013, 235, 70–75. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Bai, H.; Zhang, T.; Ibarra-Galvan, V.; Song, S. Methylene blue removal from water using the hydrogel beads of poly(vinyl alcohol)-sodium alginate-chitosan-montmorillonite. Carbohydr. Polym. 2018, 198, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Y.; Yi, H.; Chen, T.; Kang, S.; Li, H.; Song, S. Preparation and characterization of self-assembly hydrogels with exfoliated montmorillonite nanosheets and chitosan. Nanotechnology 2018, 29, 025605. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Ai, Z.; Li, M.; Li, H.; Peng, W.; Zhao, Y.; Song, S. Adsorption toward Pb(II) occurring on three-dimensional reticular-structured montmorillonite hydrogel surface. Appl. Clay Sci. 2021, 210, 106153. [Google Scholar] [CrossRef]
- Elizondo-Álvarez, M.A.; Uribe-Salas, A.; Bello-Teodoro, S. Chemical stability of xanthates, dithiophosphinates and hydroxamic acids in aqueous solutions and their environmental implications. Ecotoxicol. Environ. Saf. 2021, 207, 111509. [Google Scholar] [CrossRef] [PubMed]
- Adden, N.; Gamble, L.J.; Castner, D.G.; Hoffmann, A.; Gross, G.; Menzel, H. Phosphonic acid monolayers for binding of bioactive molecules to titanium surfaces. Langmuir 2006, 22, 8197–8204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daou, T.J.; Begin-Colin, S.; Grenèche, J.M.; Thomas, F.; Derory, A.; Bernhardt, P.; Legaré, P.; Pourroy, G. Phosphate adsorption properties of magnetite-based nanoparticles. Chem. Mater. 2007, 19, 4494–4505. [Google Scholar] [CrossRef]
- Zorn, G.; Adadi, R.; Brener, R.; Yakovlev, V.A.; Gotman, I.; Gutmanas, E.Y.; Sukenik, C.N. Tailoring the surface of NiTi alloy using PIRAC nitriding followed by anodization and phosphonate monolayer deposition. Chem. Mater. 2008, 20, 5368–5374. [Google Scholar] [CrossRef]
- Mahjoubi, H.; Kinsella, J.M.; Murshed, M.; Cerruti, M. Surface modification of poly (d,l-lactic acid) scaffolds for orthopedic applications: A biocompatible, nondestructive route via diazonium chemistry. ACS Appl. Mater. Interfaces 2014, 6, 9975–9987. [Google Scholar] [CrossRef]
- Zorn, G.; Gotman, I.; Gutmanas, E.Y.; Adadi, R.; Salitra, G.; Sukenik, C.N. Surface modification of Ti45Nb alloy with an alkylphosphonic acid self-assembled monolayer. Chem. Mater. 2005, 17, 4218–4226. [Google Scholar] [CrossRef]
- Jurado-López, B.; Vieira, R.S.; Rabelo, R.B.; Beppu, M.M.; Casado, J.; Rodríguez-Castellón, E. Formation of complexes between functionalized chitosan membranes and copper: A study by angle resolved XPS. Mater. Chem. Phys. 2017, 185, 152–161. [Google Scholar] [CrossRef]









| Molecular Formula and Name | Target Minerals | Finder |
|---|---|---|
 | Pentlandite | Liu et al. [5]. |
 | Malachite and Ilmenite | Li et al., Liu et al. [6,7]. |
 | Chalcopyrite | Huang et al. [8]. |
 | Bastnasite | Fan et al. [9]. |
 | Cassiterite and Rutile | Gong et al., Kasomo et al. [10,11]. |
 | Cassiterite | Huang et al. [12]. |
 | Cassiterite | Tan et al. [13]. |
| Ilmenite | Element | TiO2 | Fe2O3 | MgO | SiO2 | Al2O3 | MnO | CaO |
| Content (mass %) | 47.77 | 47.75 | 1.48 | 1.26 | 0.56 | 0.53 | 0.48 | |
| Titanaugite | Element | SiO2 | Al2O3 | TiO2 | Fe2O3 | MgO | Na2O | CaO |
| Content (mass %) | 40.3 | 17.36 | 1.07 | 11.17 | 7.08 | 2.19 | 13.0 |
| Sample | Element (Mass %) | |||||
|---|---|---|---|---|---|---|
| C(1S) | O(1S) | Ti(2p) | Fe(2p) | N(1s) | P(2p) | |
| Ilmenite | 34.6 | 52.46 | 5.19 | 7.75 | - | - |
| Ilmenite + APA | 40.08 | 46.6 | 4.45 | 6.5 | 1.08 | 1.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Wang, J.; Sun, Y.; Cheng, S.; Gao, H.; Wang, H.; Cao, J. α-Amino Phosphonic Acid as the Oxidized Ore Collector: Flexible Intra-Molecular Proton Transfer Providing an Improved Flotation Efficiency. Minerals 2022, 12, 918. https://0-doi-org.brum.beds.ac.uk/10.3390/min12080918
Chen P, Wang J, Sun Y, Cheng S, Gao H, Wang H, Cao J. α-Amino Phosphonic Acid as the Oxidized Ore Collector: Flexible Intra-Molecular Proton Transfer Providing an Improved Flotation Efficiency. Minerals. 2022; 12(8):918. https://0-doi-org.brum.beds.ac.uk/10.3390/min12080918
Chicago/Turabian StyleChen, Pan, Jinggang Wang, Yameng Sun, Shaoyi Cheng, Huanzhi Gao, Hongbin Wang, and Jian Cao. 2022. "α-Amino Phosphonic Acid as the Oxidized Ore Collector: Flexible Intra-Molecular Proton Transfer Providing an Improved Flotation Efficiency" Minerals 12, no. 8: 918. https://0-doi-org.brum.beds.ac.uk/10.3390/min12080918