The Specificities of Elite Female Athletes: A Multidisciplinary Approach
Abstract
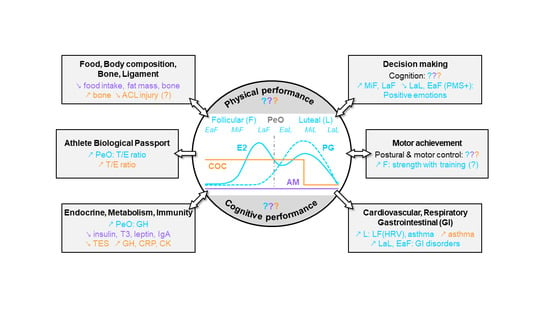
:1. Introduction
2. Hormonal Status and Performance in Elite Female Athletes
2.1. Hormonal Status and Physical Performance
2.1.1. Elite NMC Athletes
- Short intense exercise
- Incremental maximal test and endurance exercise
2.1.2. Elite AM Athletes
2.1.3. Elite COC Athletes
- Short intense exercise
- Incremental maximal test and endurance exercise
2.2. Hormonal Status and Cognitive Performance
2.2.1. Elite NMC Athletes
2.2.2. Elite AM Athletes
2.2.3. Elite COC Athletes
3. Possible Mechanisms: Performance and Health Risks
3.1. Food Intake, Body Composition, Bone, and Ligament
3.1.1. Food Intake and Body Composition
- NMC women
- AM women
- COC women
3.1.2. Bone Health and Ligament Injury Risk
- NMC women
- AM women
- COC women
3.2. CNS and Neuromuscular Responses (Part 1): Decision Making
3.2.1. Cognitive Function
- NMC women
- COC women
3.2.2. Emotions and Social Interactions
- NMC women
- COC women
3.3. CNS and Neuromuscular Responses (Part 2): Motor Achievement
3.3.1. Postural Control
- NMC women
- COC women
3.3.2. Motor Control
- NMC women
- COC women
3.3.3. Strength and Resistance Training
- NMC women
- COC women
3.4. Cardiovascular, Respiratory, Gastrointestinal, and Urinary Function
3.4.1. Cardiovascular Response
- NMC women
- AM women
- COC women
3.4.2. Respiratory Response
- NMC women
- AM women
- COC women
3.4.3. Gastrointestinal and Urinary Function
- Gastrointestinal function
- Urinary function
3.5. Endocrine Response, Metabolism and Immunity
3.5.1. Anabolic Hormones
- NMC women (Table 3)
- AM women (Table 3)
- COC women (Table 3)
3.5.2. Other Hormones
- NMC women
- AM women
- COC women
3.5.3. Metabolism and Immunity
- NMC women
- AM women
- COC women
3.6. Anti-Doping: Athlete Biological Passport (ABP)
3.6.1. ABP: Steroidal Module
- NMC women
- COC women
- Women with other drug administration
3.6.2. ABP: Hematological Module
- NMC women
- Women with other drug administration
4. Practical Considerations and Perspectives
4.1. Summary of Current Knowledge and Limitations
4.1.1. Impact of Hormonal Status on Physical and Cognitive Performance
4.1.2. Impact of Hormonal Status on Health Risks
4.1.3. Limitations
4.2. New Approach to Ensure Optimal Performance and Health
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5αAdiol | urinary 5α-androstane-3α:17β-diol |
| 5βAdiol | urinary 5β-androstane-3α,17β-diol |
| A | urinary androsterone |
| ABP | athlete biological passport |
| ABPS | abnormal blood profil score |
| ACL | anterior cruciate ligament |
| ACTH | adrenocorticotropic hormone |
| AM | amenorrhea |
| BMD | bone mineral density |
| CCK | cholecystokinin |
| CHO | carbohydrate |
| CK | creatine kinase |
| CNS | central nervous system |
| COC | combined oral contraceptive |
| CRF | cortisol-releasing factor |
| CRP | C-reactive protein |
| DHEA | dehydroepiandrosterone |
| DXA | dual-energy absorptiometry |
| E | urinary epitestosterone |
| E2 | estradiol |
| EaF | early follicular phase |
| EaL | early luteal phase |
| Etio | urinary etiocholanolone |
| F | follicular phase |
| FAT | female athlete triad |
| FEV1 | forced expiratory volume in one second |
| FSH | follicle-stimulating hormone |
| GI | gastrointestinal symptom |
| GLP-1 | glucagon-like-peptide-1 |
| GH | growth hormone |
| GHRH | growth hormone-releasing hormone |
| GnRH | gonadotrophin-releasing hormone |
| HBG | hemoglobin |
| HF | high-frequency peak (HRV) |
| HR | heart rate |
| HRV | heart rate variability |
| ICTP | type 1 carboxyterminal telopeptide |
| IgA | immunoglobulin A |
| IGF-1 | insulin-like growth factor |
| IGFBP-1 | insulin-like growth factor-binding protein-1 |
| IGFBP-3 | insulin-like growth factor-binding protein-3 |
| IL-6 | interleukin-6 |
| IOC | International Olympic Committee |
| IUD | intrauterine device |
| L | luteal phase |
| LaF | late follicular phase |
| LaL | late luteal phase |
| LF | low-frequency peak (HRV) |
| LH | luteinizing hormone |
| MC | menstrual cycle |
| MiF | mid-follicular phase |
| MiL | mid-luteal phase |
| NMC | normal menstrual cycle |
| NO | nitric oxide |
| OFFS | OFF-hr Score |
| PaCO2 | arterial partial pressure of carbon dioxide |
| PC20 | provocative concentration of methacholine that results in a 20% drop in FEV1 |
| PeO | peri-ovulatory time |
| PETCO2 | end-tidal carbon dioxide |
| PG | progesterone |
| PMA | premenstrual asthma |
| PMS | premenstrual syndrome |
| POMS | profile of mood state |
| PRL | prolactin |
| RED-S | relative energy deficiency in sport |
| RET% | reticulocytes percentage |
| SaO2 | blood oxygen saturation |
| SHBG | sex hormone-binding globulin |
| T3 | tri-iodothyronine |
| T | urinary testosterone |
| T/E | urinary testosterone/epitestosterone ratio |
| TES | blood testosterone |
| TNFα | tumor necrosis factor alpha |
| TSH | thyrotropin |
| UI | urinary incontinence |
| URTI | upper respiratory tract infection |
| VE | ventilation |
| VE/VCO2 | carbon dioxide ventilatory equivalent |
| VE/VO2 | oxygen ventilatory equivalent |
| VO2 | oxygen consumption |
| VO2max | maximal oxygen consumption |
References
- Hargreaves, J. Sporting Females: Critical Issues in the History and Sociology of Women’s Sport. Available online: https://www.routledge.com/Sporting-Females-Critical-Issues-in-the-History-and-Sociology-of-Womens/Hargreaves/p/book/9780415070287 (accessed on 26 April 2021).
- Sims, S.T.; Heather, A.K. Myths and Methodologies: Reducing Scientific Design Ambiguity in Studies Comparing Sexes and/or Menstrual Cycle Phases. Exp. Physiol. 2018, 103, 1309–1317. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.L.; Greaves, L.; Repta, R. Better Science with Sex and Gender: Facilitating the Use of a Sex and Gender-Based Analysis in Health Research. Int. J. Equity Health 2009, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- De Souza, M.J. Menstrual Disturbances in Athletes: A Focus on Luteal Phase Defects. Med. Sci. Sports Exerc. 2003, 35, 1553–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiMarco, C.S. Speroff L, Glass RH, Kase NG, Editors. Clinical Gynecologic Endocrinology and Infertility. 6th Ed. Baltimore: Lippincott, Williams & Wilkins, 1999:1–1200. Fertil. Steril. 2000, 74, 425–426. [Google Scholar] [CrossRef]
- Nattiv, A.; Agostini, R.; Drinkwater, B.; Yeager, K.K. The Female Athlete Triad. The Inter-Relatedness of Disordered Eating, Amenorrhea, and Osteoporosis. Clin. Sports Med. 1994, 13, 405–418. [Google Scholar] [CrossRef]
- Oxfeldt, M.; Dalgaard, L.B.; Jørgensen, A.A.; Hansen, M. Hormonal Contraceptive Use, Menstrual Dysfunctions, and Self-Reported Side Effects in Elite Athletes in Denmark. Int. J. Sports Physiol. Perform. 2020, 15, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Torstveit, M.K.; Sundgot-Borgen, J. The Female Athlete Triad: Are Elite Athletes at Increased Risk? Med. Sci. Sports Exerc. 2005, 37, 184–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Santiago, K.A.; Abutalib, Z.; Temme, K.E.; Hulme, A.; Goolsby, M.A.; Esopenko, C.L.; Casey, E.K. Menstrual Irregularity, Hormonal Contraceptive Use, and Bone Stress Injuries in Collegiate Female Athletes in the United States. PM & R 2020. [Google Scholar] [CrossRef]
- Larsen, B.; Morris, K.; Quinn, K.; Osborne, M.; Minahan, C. Practice Does Not Make Perfect: A Brief View of Athletes’ Knowledge on the Menstrual Cycle and Oral Contraceptives. J. Sci. Med. Sport 2020, 23, 690–694. [Google Scholar] [CrossRef]
- Martin, D.; Sale, C.; Cooper, S.B.; Elliott-Sale, K.J. Period Prevalence and Perceived Side Effects of Hormonal Contraceptive Use and the Menstrual Cycle in Elite Athletes. Int. J. Sports Physiol. Perform. 2018, 13, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, Y.; Eijkemans, M.J.C.; Coelingh Bennink, H.J.T.; Blankenstein, M.A.; Fauser, B.C.J.M. The Effect of Combined Oral Contraception on Testosterone Levels in Healthy Women: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2014, 20, 76–105. [Google Scholar] [CrossRef] [Green Version]
- Schaumberg, M.A.; Emmerton, L.M.; Jenkins, D.G.; Burton, N.W.; Janse de Jonge, X.A.K.; Skinner, T.L. Use of Oral Contraceptives to Manipulate Menstruation in Young, Physically Active Women. Int. J. Sports Physiol. Perform. 2018, 13, 82–87. [Google Scholar] [CrossRef] [PubMed]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The Effects of Menstrual Cycle Phase on Exercise Performance in Eumenorrheic Women: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Sale, K.J.; McNulty, K.L.; Ansdell, P.; Goodall, S.; Hicks, K.M.; Thomas, K.; Swinton, P.A.; Dolan, E. The Effects of Oral Contraceptives on Exercise Performance in Women: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1785–1812. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Williams, N.I.; Olmsted-Kramer, L.C.; Leidy, H.J.; Putukian, M. Neuromuscular Performance and Knee Laxity Do Not Change across the Menstrual Cycle in Female Athletes. Knee Surg. Sports Traumatol. Arthr. 2006, 14, 817–822. [Google Scholar] [CrossRef]
- Tsampoukos, A.; Peckham, E.A.; James, R.; Nevill, M.E. Effect of Menstrual Cycle Phase on Sprinting Performance. Eur. J. Appl. Physiol. 2010, 109, 659–667. [Google Scholar] [CrossRef]
- Julian, R.; Hecksteden, A.; Fullagar, H.H.K.; Meyer, T. The Effects of Menstrual Cycle Phase on Physical Performance in Female Soccer Players. PLoS ONE 2017, 12, e0173951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tounsi, M.; Jaafar, H.; Aloui, A.; Souissi, N. Soccer-Related Performance in Eumenorrheic Tunisian High-Level Soccer Players: Effects of Menstrual Cycle Phase and Moment of Day. J. Sports Med. Phys. Fit. 2018, 58, 497–502. [Google Scholar] [CrossRef]
- Köse, B. Analysis of the Effect of Menstrual Cycle Phases on Aerobic-Anaerobic Capacity and Muscle Strength. J. Educ. Train. Stud. 2018, 6, 23. [Google Scholar] [CrossRef]
- Romero-Moraleda, B.; Coso, J.D.; Gutiérrez-Hellín, J.; Ruiz-Moreno, C.; Grgic, J.; Lara, B. The Influence of the Menstrual Cycle on Muscle Strength and Power Performance. J. Hum. Kinet. 2019, 68, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Štefanovský, M.; Péterová, A.; Vanderka, M.; Lengvarský, L. Influence of Selected Phases of the Menstrual Cycle on Performance in Special Judo Fitness Test and Wingate Test. Acta Gymnica 2016, 46, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.; Hughes, F.; Young, K.; Scruton, A.; Keiller, D.; Caddy, O.; Baker, J.; Barnes, R. The Effects of Menstrual Cycle Phase on the Development of Peak Torque under Isokinetic Conditions. Isokinet. Exerc. Sci. 2013, 21. [Google Scholar] [CrossRef]
- Burrows, M.; Bird, S. Velocity at VO2 Max and Peak Treadmill Velocity Are Not Influenced within or across the Phases of the Menstrual Cycle. Eur. J. Appl. Physiol. 2005, 93, 575–580. [Google Scholar] [CrossRef]
- Vaiksaar, S.; Jürimäe, J.; Mäestu, J.; Purge, P.; Kalytka, S.; Shakhlina, L.; Jürimäe, T. No Effect of Menstrual Cycle Phase and Oral Contraceptive Use on Endurance Performance in Rowers. J. Strength Cond. Res. 2011, 25, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Kishali, N.F.; Imamoglu, O.; Katkat, D.; Atan, T.; Akyol, P. Effects of Menstrual Cycle on Sports Performance. Int. J. Neurosci. 2006, 116, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Vanheest, J.L.; Rodgers, C.D.; Mahoney, C.E.; De Souza, M.J. Ovarian Suppression Impairs Sport Performance in Junior Elite Female Swimmers. Med. Sci. Sports Exerc. 2014, 46, 156–166. [Google Scholar] [CrossRef]
- Tornberg, Å.B.; Melin, A.; Koivula, F.M.; Johansson, A.; Skouby, S.; Faber, J.; Sjödin, A. Reduced Neuromuscular Performance in Amenorrheic Elite Endurance Athletes. Med. Sci. Sports Exerc. 2017, 49, 2478–2485. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, K.E.; Holtzman, B.; Cooper, K.M.; Flynn, E.F.; Bruinvels, G.; Tenforde, A.S.; Popp, K.L.; Simpkin, A.J.; Parziale, A.L. Low Energy Availability Surrogates Correlate with Health and Performance Consequences of Relative Energy Deficiency in Sport. Br. J. Sports Med. 2019, 53, 628–633. [Google Scholar] [CrossRef]
- Peters, C.; Burrows, M. Androgenicity of the Progestin in Oral Contraceptives Does Not Affect Maximal Leg Strength. Contraception 2006, 74, 487–491. [Google Scholar] [CrossRef]
- Lebrun, C.M.; Petit, M.A.; McKenzie, D.C.; Taunton, J.E.; Prior, J.C. Decreased Maximal Aerobic Capacity with Use of a Triphasic Oral Contraceptive in Highly Active Women: A Randomised Controlled Trial. Br. J. Sports Med. 2003, 37, 315–320. [Google Scholar] [CrossRef]
- Rechichi, C.; Dawson, B. Effect of Oral Contraceptive Cycle Phase on Performance in Team Sport Players. J. Sci. Med. Sport 2009, 12, 190–195. [Google Scholar] [CrossRef]
- Rechichi, C.; Dawson, B. Oral Contraceptive Cycle Phase Does Not Affect 200-m Swim Time Trial Performance. J. Strength Cond. Res. 2012, 26, 961–967. [Google Scholar] [CrossRef]
- Redman, L.M.; Weatherby, R.P. Measuring Performance during the Menstrual Cycle: A Model Using Oral Contraceptives. Med. Sci. Sports Exerc. 2004, 36, 130–136. [Google Scholar] [CrossRef]
- Reilly, T.; Whitley, H. Effects of Menstrual Cycle Phase and Oral Contraceptive Use on Endurance Exercise. J. Sports Sci. 1994, 2, 150. [Google Scholar]
- Rickenlund, A.; Carlström, K.; Ekblom, B.; Brismar, T.B.; Von Schoultz, B.; Hirschberg, A.L. Effects of Oral Contraceptives on Body Composition and Physical Performance in Female Athletes. J. Clin. Endocrinol. Metab. 2004, 89, 4364–4370. [Google Scholar] [CrossRef] [Green Version]
- Rechichi, C.; Dawson, B.; Goodman, C. Oral Contraceptive Phase Has No Effect on Endurance Test. Int. J. Sports Med. 2008, 29, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Sunderland, C.; Nevill, M. Effect of the Menstrual Cycle on Performance of Intermittent, High-Intensity Shuttle Running in a Hot Environment. Eur. J. Appl. Physiol. 2003, 88, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Cockerill, I.M.; Nevill, A.M.; Byrne, N.C. Mood, Mileage and the Menstrual Cycle. Br. J. Sports Med. 1992, 26, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.; Knight, C.J.; Forrest Née Whyte, L.J. Elite Female Athletes’ Experiences and Perceptions of the Menstrual Cycle on Training and Sport Performance. Scand. J. Med. Sci. Sports 2021, 31, 52–69. [Google Scholar] [CrossRef]
- Bruinvels, G.; Burden, R.; Brown, N.; Richards, T.; Pedlar, C. The Prevalence and Impact of Heavy Menstrual Bleeding (Menorrhagia) in Elite and Non-Elite Athletes. PLoS ONE 2016, 11, e0149881. [Google Scholar] [CrossRef]
- Bruinvels, G.; Goldsmith, E.; Blagrove, R.; Simpkin, A.; Lewis, N.; Morton, K.; Suppiah, A.; Rogers, J.P.; Ackerman, K.E.; Newell, J.; et al. Prevalence and Frequency of Menstrual Cycle Symptoms Are Associated with Availability to Train and Compete: A Study of 6812 Exercising Women Recruited Using the Strava Exercise App. Br. J. Sports Med. 2021, 55, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Solli, G.S.; Sandbakk, S.B.; Noordhof, D.A.; Ihalainen, J.K.; Sandbakk, Ø. Changes in Self-Reported Physical Fitness, Performance, and Side Effects Across the Phases of the Menstrual Cycle Among Competitive Endurance Athletes. Int. J. Sports Physiol. Perform. 2020, 15, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.; Vaisberg, M.; de Araújo, M.P.; Martins, M.A.; Capel, T.; Bachi, A.L.L.; de Bella, Z.I.K.J.-D. Relationship between Anxiety and Interleukin 10 in Female Soccer Players with and Without Premenstrual Syndrome (PMS). Rev. Bras. Ginecol. Obs. 2017, 39, 602–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, R.; Vaisberg, M.; Bachi, A.L.L.; de Dos Santos, J.M.B.; de Paula Vieira, R.; Luna-Junior, L.A.; Araújo, M.P.; Parmigiano, T.R.; Borges, F.; Di-Bella, Z.I.K.J. Premenstrual Syndrome, Inflammatory Status, and Mood States in Soccer Players. Neuroimmunomodulation 2019, 26, 1–6. [Google Scholar] [CrossRef]
- Findlay, R.J.; Macrae, E.H.R.; Whyte, I.Y.; Easton, C.; Forrest Née Whyte, L.J. How the Menstrual Cycle and Menstruation Affect Sporting Performance: Experiences and Perceptions of Elite Female Rugby Players. Br. J. Sports Med. 2020, 54, 1108–1113. [Google Scholar] [CrossRef]
- Baskaran, C.; Plessow, F.; Ackerman, K.E.; Singhal, V.; Eddy, K.T.; Misra, M. A Cross-Sectional Analysis of Verbal Memory and Executive Control across Athletes with Varying Menstrual Status and Non-Athletes. Psychiatry Res. 2017, 258, 605–606. [Google Scholar] [CrossRef]
- Baskaran, C.; Cunningham, B.; Plessow, F.; Singhal, V.; Woolley, R.; Ackerman, K.E.; Slattery, M.; Lee, H.; Lawson, E.A.; Eddy, K.; et al. Estrogen Replacement Improves Verbal Memory and Executive Control in Oligomenorrheic/Amenorrheic Athletes in a Randomized Controlled Trial. J. Clin. Psychiatry 2017, 78, e490–e497. [Google Scholar] [CrossRef]
- Brennan, I.M.; Feltrin, K.L.; Nair, N.S.; Hausken, T.; Little, T.J.; Gentilcore, D.; Wishart, J.M.; Jones, K.L.; Horowitz, M.; Feinle-Bisset, C. Effects of the Phases of the Menstrual Cycle on Gastric Emptying, Glycemia, Plasma GLP-1 and Insulin, and Energy Intake in Healthy Lean Women. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G602–G610. [Google Scholar] [CrossRef] [Green Version]
- Hormes, J.M.; Timko, C.A. All Cravings Are Not Created Equal. Correlates of Menstrual versus Non-Cyclic Chocolate Craving. Appetite 2011, 57, 1–5. [Google Scholar] [CrossRef]
- Bryant, M.; Truesdale, K.P.; Dye, L. Modest Changes in Dietary Intake across the Menstrual Cycle: Implications for Food Intake Research. Br. J. Nutr. 2006, 96, 888–894. [Google Scholar] [CrossRef] [Green Version]
- McNeil, J.; Doucet, É. Possible Factors for Altered Energy Balance across the Menstrual Cycle: A Closer Look at the Severity of PMS, Reward Driven Behaviors and Leptin Variations. Eur. J. Obs. Gynecol. Reprod. Biol. 2012, 163, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, I.; Ben Saâda, W.; Sifaou, A.; Haouat, E.; Kandara, H.; Ben Salem, L.; Ben Slama, C. Change in Women’s Eating Habits during the Menstrual Cycle. Ann. Endocrinol. 2017, 78, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Tucci, S.A.; Murphy, L.E.; Boyland, E.J.; Halford, J.C. Influence of premenstrual syndrome and oral contraceptive effects on food choice during the follicular and luteal phase of the menstrual cycle. Endocrinol. Nutr. 2009, 56, 170–175. [Google Scholar] [CrossRef]
- Rossi, K.A. Nutritional Aspects of the Female Athlete. Clin. Sports Med. 2017, 36, 627–653. [Google Scholar] [CrossRef] [PubMed]
- Thein-Nissenbaum, J.M.; Rauh, M.J.; Carr, K.E.; Loud, K.J.; McGuine, T.A. Associations between Disordered Eating, Menstrual Dysfunction, and Musculoskeletal Injury among High School Athletes. J. Orthop. Sports Phys. 2011, 41, 60–69. [Google Scholar] [CrossRef]
- Jürimäe, J.; Vaiksaar, S.; Mäestu, J.; Purge, P.; Jürimäe, T. Adiponectin and Bone Metabolism Markers in Female Rowers: Eumenorrheic and Oral Contraceptive Users. J. Endocrinol. Investig. 2011, 34, 835–839. [Google Scholar] [CrossRef]
- Rael, B.; Romero-Parra, N.; Alfaro-Magallanes, V.M.; Barba-Moreno, L.; Cupeiro, R.; Janse de Jonge, X.; Peinado, A.B.; IronFEMME Study Group. Body Composition Over the Menstrual and Oral Contraceptive Cycle in Trained Females. Int. J. Sports Physiol. Perform. 2020, 375–381. [Google Scholar] [CrossRef]
- Stachoń, A.J. Menstrual Changes in Body Composition of Female Athletes. Coll. Antropol. 2016, 40, 111–122. [Google Scholar]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC Consensus Statement: Beyond the Female Athlete Triad--Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Slusarz, K.; Guereca, G.; Pierce, L.; Slattery, M.; Mendes, N.; Herzog, D.B.; Misra, M. Higher Ghrelin and Lower Leptin Secretion Are Associated with Lower LH Secretion in Young Amenorrheic Athletes Compared with Eumenorrheic Athletes and Controls. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E800–E806. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, K.A.; Buckman, M.T.; Peake, G.T.; Riedesel, M.L. Body Composition of Oligo/Amenorrheic Athletes. Med. Sci. Sports Exerc. 1983, 15, 215–217. [Google Scholar] [CrossRef]
- Loucks, A.B.; Horvath, S.M. Athletic Amenorrhea: A Review. Med. Sci. Sports Exerc. 1985, 17, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Singhal, V.; de Lourdes Eguiguren, M.; Eisenbach, L.; Clarke, H.; Slattery, M.; Eddy, K.; Ackerman, K.E.; Misra, M. Body Composition, Hemodynamic, and Biochemical Parameters of Young Female Normal-Weight Oligo-Amenorrheic and Eumenorrheic Athletes and Nonathletes. Ann. Nutr. Metab. 2014, 65, 264–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melin, A.K.; Ritz, C.; Faber, J.; Skouby, S.; Pingel, J.; Sundgot-Borgen, J.; Sjödin, A.; Tornberg, Å.B. Impact of Menstrual Function on Hormonal Response to Repeated Bouts of Intense Exercise. Front. Physiol. 2019, 10, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, M.; Junak, M. Assessment of Dietary Choices of Young Women in the Contexts of Hormonal Contraceptives. Rocz. Panstw. Zakl. Hig. 2017, 68, 69–76. [Google Scholar]
- Nakamura, M.; Nose-Ogura, S. Effect of Administration of Monophasic Oral Contraceptive on the Body Composition and Aerobic and Anaerobic Capacities of Female Athletes. J. Obs. Gynaecol. Res. 2021, 47, 792–799. [Google Scholar] [CrossRef]
- Casazza, G.A.; Suh, S.-H.; Miller, B.F.; Navazio, F.M.; Brooks, G.A. Effects of Oral Contraceptives on Peak Exercise Capacity. J. Appl. Physiol. 2002, 93, 1698–1702. [Google Scholar] [CrossRef] [Green Version]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.; Cooper, S.B.; Tang, J.C.Y.; Fraser, W.D.; Sale, C.; Elliott-Sale, K.J. Bone Metabolic Marker Concentrations across the Menstrual Cycle and Phases of Combined Oral Contraceptive Use. Bone 2021, 145, 115864. [Google Scholar] [CrossRef]
- Monson, J.P.; Drake, W.M.; Carroll, P.V.; Weaver, J.U.; Rodriguez-Arnao, J.; Savage, M.O. Influence of Growth Hormone on Accretion of Bone Mass. Horm. Res. 2002, 58 (Suppl. 1), 52–56. [Google Scholar] [CrossRef]
- De Souza, M.J.; Williams, N.I. Beyond Hypoestrogenism in Amenorrheic Athletes: Energy Deficiency as a Contributing Factor for Bone Loss. Curr. Sports Med. Rep. 2005, 4, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Dolan, E.; Elliott-Sale, K.J.; Sale, C. Reduced Energy Availability: Implications for Bone Health in Physically Active Populations. Eur. J. Nutr. 2018, 57, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Chikamura, C.; Ishikawa, H.; Aoi, S.; Ikeda, H.; Harada, T.; Katada, K.; Ishizaki, F.; Yatsuya, H.; Ono, Y. Factors Predicting Bone Mineral Density (BMD) Changes in Young Women over A One-Year Study: Changes in Body Weight and Bone Metabolic Markers during the Menstrual Cycle and Their Effects on BMD. Acta Med. Okayama 2012, 66, 9. [Google Scholar]
- Mozzanega, B.; Gizzo, S.; Bernardi, D.; Salmaso, L.; Patrelli, T.S.; Mioni, R.; Finos, L.; Nardelli, G.B. Cyclic Variations of Bone Resorption Mediators and Markers in the Different Phases of the Menstrual Cycle. J. Bone Min. Metab. 2013, 31, 461–467. [Google Scholar] [CrossRef]
- Krahenbühl, T.; de Guimarães, R.F.; de Barros Filho, A.A.; Gonçalves, E.M. Bone geometry and physical activity in children and adolescents: Systematic review. Rev. Paul. Pediatr. 2018, 36, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanhers, C.; Courteix, D.; Valente-Dos-Santos, J.; Ferry, B.; Gracia-Marco, L.; Pereira, B.; Borda, I.M.; Lespessailles, E.; Duclos, M. Gonadal Hormones May Predict Structural Bone Fragility in Elite Female Soccer Player. J. Sports Sci. 2020, 38, 827–837. [Google Scholar] [CrossRef]
- Hewett, T.E.; Zazulak, B.T.; Myer, G.D. Effects of the Menstrual Cycle on Anterior Cruciate Ligament Injury Risk: A Systematic Review. Am. J. Sports Med. 2007, 35, 659–668. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Castillo, T.N.; Korotkova, T.A.; Kennedy, A.C.; Kim, H.J.; Stewart, D.R. Trends in Serum Relaxin Concentration among Elite Collegiate Female Athletes. Int. J. Womens Health 2011, 3, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Khowailed, I.A.; Petrofsky, J.; Lohman, E.; Daher, N.; Mohamed, O. 17β-Estradiol Induced Effects on Anterior Cruciate Ligament Laxness and Neuromuscular Activation Patterns in Female Runners. J. Womens Health 2015, 24, 670–680. [Google Scholar] [CrossRef] [Green Version]
- Herzberg, S.D.; Motu’apuaka, M.L.; Lambert, W.; Fu, R.; Brady, J.; Guise, J.-M. The Effect of Menstrual Cycle and Contraceptives on ACL Injuries and Laxity: A Systematic Review and Meta-Analysis. Orthop. J. Sports Med. 2017, 5, 2325967117718781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somerson, J.S.; Isby, I.J.; Hagen, M.S.; Kweon, C.Y.; Gee, A.O. The Menstrual Cycle May Affect Anterior Knee Laxity and the Rate of Anterior Cruciate Ligament Rupture: A Systematic Review and Meta-Analysis. JBJS Rev. 2019, 7, e2. [Google Scholar] [CrossRef] [PubMed]
- Belanger, M.J.; Moore, D.C.; Crisco, J.J.; Fadale, P.D.; Hulstyn, M.J.; Ehrlich, M.G. Knee Laxity Does Not Vary with the Menstrual Cycle, before or after Exercise. Am. J. Sports Med. 2004, 32, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Beynnon, B.D.; Bernstein, I.M.; Belisle, A.; Brattbakk, B.; Devanny, P.; Risinger, R.; Durant, D. The Effect of Estradiol and Progesterone on Knee and Ankle Joint Laxity. Am. J. Sports Med. 2005, 33, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Brook, E.M.; Tenforde, A.S.; Broad, E.M.; Matzkin, E.G.; Yang, H.Y.; Collins, J.E.; Blauwet, C.A. Low Energy Availability, Menstrual Dysfunction, and Impaired Bone Health: A Survey of Elite Para Athletes. Scand. J. Med. Sci. Sports 2019, 29, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Thein-Nissenbaum, J.; Hammer, E. Treatment Strategies for the Female Athlete Triad in the Adolescent Athlete: Current Perspectives. Open Access J. Sports Med. 2017, 8, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maïmoun, L.; Coste, O.; Georgopoulos, N.A.; Roupas, N.D.; Mahadea, K.K.; Tsouka, A.; Mura, T.; Philibert, P.; Gaspari, L.; Mariano-Goulart, D.; et al. Despite a High Prevalence of Menstrual Disorders, Bone Health Is Improved at a Weight-Bearing Bone Site in World-Class Female Rhythmic Gymnasts. J. Clin. Endocrinol. Metab. 2013, 98, 4961–4969. [Google Scholar] [CrossRef] [Green Version]
- Singhal, V.; Reyes, K.C.; Pfister, B.; Ackerman, K.; Slattery, M.; Cooper, K.; Toth, A.; Gupta, N.; Goldstein, M.; Eddy, K.; et al. Bone Accrual in Oligo-Amenorrheic Athletes, Eumenorrheic Athletes and Non-Athletes. Bone 2019, 120, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Christo, K.; Prabhakaran, R.; Lamparello, B.; Cord, J.; Miller, K.K.; Goldstein, M.A.; Gupta, N.; Herzog, D.B.; Klibanski, A.; Misra, M. Bone Metabolism in Adolescent Athletes with Amenorrhea, Athletes with Eumenorrhea, and Control Subjects. Pediatrics 2008, 121, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Hadji, P.; Colli, E.; Regidor, P.-A. Bone Health in Estrogen-Free Contraception. Osteoporos Int. 2019, 30, 2391–2400. [Google Scholar] [CrossRef]
- Kuohung, W.; Borgatta, L.; Stubblefield, P. Low-Dose Oral Contraceptives and Bone Mineral Density: An Evidence-Based Analysis. Contraception 2000, 61, 77–82. [Google Scholar] [CrossRef]
- Lopez, L.M.; Grimes, D.A.; Schulz, K.F.; Curtis, K.M.; Chen, M. Steroidal Contraceptives: Effect on Bone Fractures in Women. Cochrane Database Syst. Rev. 2014, CD006033. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.D.; Scholes, D.; LaCroix, A.Z.; Ichikawa, L.E.; Barlow, W.E.; Ott, S.M. Longitudinal Changes in Bone Density in Relation to Oral Contraceptive Use. Contraception 2003, 68, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Almstedt, H.C.; Cook, M.M.; Bramble, L.F.; Dabir, D.V.; LaBrie, J.W. Oral Contraceptive Use, Bone Mineral Density, and Bone Turnover Markers over 12 Months in College-Aged Females. J. Bone Min. Metab. 2020, 38, 544–554. [Google Scholar] [CrossRef]
- Brajic, T.S.; Berger, C.; Schlammerl, K.; Macdonald, H.; Kalyan, S.; Hanley, D.A.; Adachi, J.D.; Kovacs, C.S.; Prior, J.C.; CaMos Research Group. Combined Hormonal Contraceptives Use and Bone Mineral Density Changes in Adolescent and Young Women in a Prospective Population-Based Canada-Wide Observational Study. J. Musculoskelet. Neuronal. Interact. 2018, 18, 227–236. [Google Scholar]
- Cobb, K.L.; Bachrach, L.K.; Sowers, M.; Nieves, J.; Greendale, G.A.; Kent, K.K.; Brown, B.W.; Pettit, K.; Harper, D.M.; Kelsey, J.L. The Effect of Oral Contraceptives on Bone Mass and Stress Fractures in Female Runners. Med. Sci. Sports Exerc. 2007, 39, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, H.; Soleimany, G.; Movaseghi, S.; Dadgostar, E.; Lotfian, S. The Effect of Hormone Therapy on Bone Mineral Density and Cardiovascular Factors among Iranian Female Athletes with Amenorrhea/Oligomenorrhea: A Randomized Clinical Trial. Med. J. Islam Repub. Iran. 2018, 32, 27. [Google Scholar] [CrossRef] [Green Version]
- Singhal, V.; Ackerman, K.E.; Bose, A.; Flores, L.P.T.; Lee, H.; Misra, M. Impact of Route of Estrogen Administration on Bone Turnover Markers in Oligoamenorrheic Athletes and Its Mediators. J. Clin. Endocrinol. Metab. 2019, 104, 1449–1458. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Singhal, V.; Baskaran, C.; Slattery, M.; Campoverde Reyes, K.J.; Toth, A.; Eddy, K.T.; Bouxsein, M.L.; Lee, H.; Klibanski, A.; et al. Oestrogen Replacement Improves Bone Mineral Density in Oligo-Amenorrhoeic Athletes: A Randomised Clinical Trial. Br. J. Sports Med. 2019, 53, 229–236. [Google Scholar] [CrossRef]
- Hicks-Little, C.A.; Thatcher, J.R.; Hauth, J.M.; Goldfuss, A.J.; Cordova, M.L. Menstrual Cycle Stage and Oral Contraceptive Effects on Anterior Tibial Displacement in Collegiate Female Athletes. J. Sports Med. Phys. Fit. 2007, 47, 255–260. [Google Scholar]
- Konopka, J.A.; Hsue, L.; Chang, W.; Thio, T.; Dragoo, J.L. The Effect of Oral Contraceptive Hormones on Anterior Cruciate Ligament Strength. Am. J. Sports Med. 2020, 48, 85–92. [Google Scholar] [CrossRef]
- Samuelson, K.; Balk, E.M.; Sevetson, E.L.; Fleming, B.C. Limited Evidence Suggests a Protective Association Between Oral Contraceptive Pill Use and Anterior Cruciate Ligament Injuries in Females: A Systematic Review. Sports Health 2017, 9, 498–510. [Google Scholar] [CrossRef]
- Nose-Ogura, S.; Yoshino, O.; Yamada-Nomoto, K.; Nakamura, M.; Harada, M.; Dohi, M.; Okuwaki, T.; Osuga, Y.; Kawahara, T.; Saito, S. Oral Contraceptive Therapy Reduces Serum Relaxin-2 in Elite Female Athletes. J. Obs. Gynaecol. Res. 2017, 43, 530–535. [Google Scholar] [CrossRef] [Green Version]
- Sherwin, B.B. Estrogen and Cognitive Functioning in Women. Endocr. Rev. 2003, 24, 133–151. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Su, C. Progesterone and Neuroprotection. Horm. Behav. 2013, 63, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Hampson, E. Regulation of Cognitive Function by Androgens and Estrogens. Curr. Opin. Behav. Sci. 2018, 23, 49–57. [Google Scholar] [CrossRef]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone Receptors: Form and Function in Brain. Front. Neuroendocr. 2008, 29, 313–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, R.D.; Watson, P.D.; Duff, M.C.; Cohen, N.J. The Role of the Hippocampus in Flexible Cognition and Social Behavior. Front. Hum. Neurosci. 2014, 8, 742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toffoletto, S.; Lanzenberger, R.; Gingnell, M.; Sundström-Poromaa, I.; Comasco, E. Emotional and Cognitive Functional Imaging of Estrogen and Progesterone Effects in the Female Human Brain: A Systematic Review. Psychoneuroendocrinology 2014, 50, 28–52. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J. Sex Hormones Affect Neurotransmitters and Shape the Adult Female Brain during Hormonal Transition Periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Comasco, E.; Frokjaer, V.G.; Sundström-Poromaa, I. Functional and Molecular Neuroimaging of Menopause and Hormone Replacement Therapy. Front. Neurosci. 2014, 8, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotomayor-Zárate, R.; Cruz, G.; Renard, G.; Espinosa, P.; Ramirez, V. Sex Hormones and Brain Dopamine Functions. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14. [Google Scholar] [CrossRef]
- Maccoby, E.; Jackiln, C. Sex Differences in Intellectual Functioning. In Proceedings of the Invitational Conference on Testing Problems; American Psychological Association (APA): New York, NY, USA, 1972; pp. 37–55. [Google Scholar]
- Kimura, D. Sex and Cognition|The MIT Press. Available online: https://mitpress.mit.edu/books/sex-and-cognition (accessed on 26 April 2021).
- Sundström Poromaa, I.; Gingnell, M. Menstrual Cycle Influence on Cognitive Function and Emotion Processing-from a Reproductive Perspective. Front. Neurosci. 2014, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundström-Poromaa, I. The Menstrual Cycle Influences Emotion but Has Limited Effect on Cognitive Function. Vitam Horm 2018, 107, 349–376. [Google Scholar] [CrossRef] [PubMed]
- Beltz, A.M.; Moser, J.S. Ovarian Hormones: A Long Overlooked but Critical Contributor to Cognitive Brain Structures and Function. Ann. N. Y. Acad. Sci. 2020, 1464, 156–180. [Google Scholar] [CrossRef]
- Le, J.; Thomas, N.; Gurvich, C. Cognition, The Menstrual Cycle, and Premenstrual Disorders: A Review. Brain Sci. 2020, 10, 198. [Google Scholar] [CrossRef] [Green Version]
- Andreano, J.M.; Cahill, L. Sex Influences on the Neurobiology of Learning and Memory. Learn. Mem. 2009, 16, 248–266. [Google Scholar] [CrossRef] [Green Version]
- Courvoisier, D.S.; Renaud, O.; Geiser, C.; Paschke, K.; Gaudy, K.; Jordan, K. Sex Hormones and Mental Rotation: An Intensive Longitudinal Investigation. Horm. Behav. 2013, 63, 345–351. [Google Scholar] [CrossRef]
- Hampson, E.; Levy-Cooperman, N.; Korman, J.M. Estradiol and Mental Rotation: Relation to Dimensionality, Difficulty, or Angular Disparity? Horm. Behav. 2014, 65, 238–248. [Google Scholar] [CrossRef]
- Maki, P.M.; Rich, J.B.; Shayna Rosenbaum, R. Implicit Memory Varies across the Menstrual Cycle: Estrogen Effects in Young Women. Neuropsychologia 2002, 40, 518–529. [Google Scholar] [CrossRef]
- Griksiene, R.; Ruksenas, O. Effects of Hormonal Contraceptives on Mental Rotation and Verbal Fluency. Psychoneuroendocrinology 2011, 36, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, K.L.; Rubin, L.H.; Maki, P.M. Effects of Menstrual Cycle Phase and Oral Contraceptive Use on Verbal Memory. Horm. Behav. 2008, 54, 286–293. [Google Scholar] [CrossRef]
- Zhu, X.; Kelly, T.H.; Curry, T.E.; Lal, C.; Joseph, J.E. Altered Functional Brain Asymmetry for Mental Rotation: Effect of Estradiol Changes across the Menstrual Cycle. Neuroreport 2015, 26, 814–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pletzer, B.; Kronbichler, M.; Ladurner, G.; Nuerk, H.-C.; Kerschbaum, H. Menstrual Cycle Variations in the BOLD-Response to a Number Bisection Task: Implications for Research on Sex Differences. Brain Res. 2011, 1420, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Hussain, D.; Hanafi, S.; Konishi, K.; Brake, W.G.; Bohbot, V.D. Modulation of Spatial and Response Strategies by Phase of the Menstrual Cycle in Women Tested in a Virtual Navigation Task. Psychoneuroendocrinology 2016, 70, 108–117. [Google Scholar] [CrossRef]
- Scheuringer, A.; Pletzer, B. Sex Differences and Menstrual Cycle Dependent Changes in Cognitive Strategies during Spatial Navigation and Verbal Fluency. Front. Psychol. 2017, 8, 381. [Google Scholar] [CrossRef]
- Solís-Ortiz, S.; Corsi-Cabrera, M. Sustained Attention Is Favored by Progesterone during Early Luteal Phase and Visuo-Spatial Memory by Estrogens during Ovulatory Phase in Young Women. Psychoneuroendocrinology 2008, 33, 989–998. [Google Scholar] [CrossRef]
- Gulinello, M.; Gong, Q.H.; Smith, S.S. Progesterone Withdrawal Increases the Alpha4 Subunit of the GABA(A) Receptor in Male Rats in Association with Anxiety and Altered Pharmacology—A Comparison with Female Rats. Neuropharmacology 2002, 43, 701–714. [Google Scholar] [CrossRef]
- Rosenberg, L.; Park, S. Verbal and Spatial Functions across the Menstrual Cycle in Healthy Young Women. Psychoneuroendocrinology 2002, 27, 835–841. [Google Scholar] [CrossRef]
- Hatta, T.; Nagaya, K. Menstrual Cycle Phase Effects on Memory and Stroop Task Performance. Arch. Sex. Behav. 2009, 38, 821–827. [Google Scholar] [CrossRef]
- Jacobs, E.; D’Esposito, M. Estrogen Shapes Dopamine-Dependent Cognitive Processes: Implications for Women’s Health. J. Neurosci. 2011, 31, 5286–5293. [Google Scholar] [CrossRef] [Green Version]
- Rumberg, B.; Baars, A.; Fiebach, J.; Ladd, M.E.; Forsting, M.; Senf, W.; Gizewski, E.R. Cycle and Gender-Specific Cerebral Activation during a Verb Generation Task Using FMRI: Comparison of Women in Different Cycle Phases, under Oral Contraception, and Men. Neurosci. Res. 2010, 66, 366–371. [Google Scholar] [CrossRef]
- Solis-Ortiz, S.; Guevara, M.A.; Corsi-Cabrera, M. Performance in a Test Demanding Prefrontal Functions Is Favored by Early Luteal Phase Progesterone: An Electroencephalographic Study. Psychoneuroendocrinology 2004, 29, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; Pratt, J.; Hommel, B. Estrogen Modulates Inhibition of Return in Healthy Human Females. Neuropsychologia 2012, 50, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Thimm, M.; Weis, S.; Hausmann, M.; Sturm, W. Menstrual Cycle Effects on Selective Attention and Its Underlying Cortical Networks. Neuroscience 2014, 258, 307–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler-Chiurazzi, E.B.; Singh, M.; Simpkins, J.W. Reprint of: From the 90’s to Now: A Brief Historical Perspective on More than Two Decades of Estrogen Neuroprotection. Brain Res. 2016, 1645, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Hampson, E.; Morley, E.E. Estradiol Concentrations and Working Memory Performance in Women of Reproductive Age. Psychoneuroendocrinology 2013, 38, 2897–2904. [Google Scholar] [CrossRef]
- Warren, A.M.; Gurvich, C.; Worsley, R.; Kulkarni, J. A Systematic Review of the Impact of Oral Contraceptives on Cognition. Contraception 2014, 90, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A. Natural and Synthetic Sex Hormones: Effects on Higher-Order Cognitive Function and Prepulse Inhibition. Biol. Psychol. 2013, 93, 17–23. [Google Scholar] [CrossRef]
- Islam, F.; Sparkes, C.; Roodenrys, S.; Astheimer, L. Short-Term Changes in Endogenous Estrogen Levels and Consumption of Soy Isoflavones Affect Working and Verbal Memory in Young Adult Females. Nutr. Neurosci. 2008, 11, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Beltz, A.M.; Hampson, E.; Berenbaum, S.A. Oral Contraceptives and Cognition: A Role for Ethinyl Estradiol. Horm. Behav. 2015, 74, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; De Tommaso, M.; Cianci, A.; Colacurci, N.; Rella, L.; Loiudice, L.; Cicinelli, M.V.; Livrea, P. Oral Contraceptive Therapy Modulates Hemispheric Asymmetry in Spatial Attention. Contraception 2011, 84, 634–636. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D. Masculinizing Effects on Otoacoustic Emissions and Auditory Evoked Potentials in Women Using Oral Contraceptives. Hear. Res. 2000, 142, 23–33. [Google Scholar] [CrossRef]
- Griksiene, R.; Monciunskaite, R.; Arnatkeviciute, A.; Ruksenas, O. Does the Use of Hormonal Contraceptives Affect the Mental Rotation Performance? Horm. Behav. 2018, 100, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wharton, W.; Hirshman, E.; Merritt, P.; Doyle, L.; Paris, S.; Gleason, C. Oral Contraceptives and Androgenicity: Influences on Visuospatial Task Performance in Younger Individuals. Exp. Clin. Psychopharmacol. 2008, 16, 156–164. [Google Scholar] [CrossRef]
- Gurvich, C.; Warren, A.M.; Worsley, R.; Hudaib, A.-R.; Thomas, N.; Kulkarni, J. Effects of Oral Contraceptive Androgenicity on Visuospatial and Social-Emotional Cognition: A Prospective Observational Trial. Brain Sci. 2020, 10, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihalik, J.P.; Ondrak, K.S.; Guskiewicz, K.M.; McMurray, R.G. The Effects of Menstrual Cycle Phase on Clinical Measures of Concussion in Healthy College-Aged Females. J. Sci. Med. Sport 2009, 12, 383–387. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Ertman, N.; Lakhani, Y.S.; Cahill, L. Hormonal Contraception Usage Is Associated with Altered Memory for an Emotional Story. Neurobiol. Learn. Mem. 2011, 96, 378–384. [Google Scholar] [CrossRef] [Green Version]
- Holloway, J.L.; Beck, K.D.; Servatius, R.J. Facilitated Acquisition of the Classically Conditioned Eyeblink Response in Females Is Augmented in Those Taking Oral Contraceptives. Behav. Brain Res. 2011, 216, 301–307. [Google Scholar] [CrossRef]
- Rosen, M.L.; López, H.H. Menstrual Cycle Shifts in Attentional Bias for Courtship Language. Evol. Hum. Behav. 2009, 30, 131–140. [Google Scholar] [CrossRef]
- Farage, M.A.; Osborn, T.W.; MacLean, A.B. Cognitive, Sensory, and Emotional Changes Associated with the Menstrual Cycle: A Review. Arch. Gynecol. Obs. 2008, 278, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Derntl, B.; Windischberger, C.; Robinson, S.; Lamplmayr, E.; Kryspin-Exner, I.; Gur, R.C.; Moser, E.; Habel, U. Facial Emotion Recognition and Amygdala Activation Are Associated with Menstrual Cycle Phase. Psychoneuroendocrinology 2008, 33, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.A.; Makhanova, A.; Marcinkowska, U.M.; Jasienska, G.; McNulty, J.K.; Eckel, L.A.; Nikonova, L.; Maner, J.K. Progesterone and Women’s Anxiety across the Menstrual Cycle. Horm. Behav. 2018, 102, 34–40. [Google Scholar] [CrossRef]
- Baker, F.C.; Driver, H.S. Circadian Rhythms, Sleep, and the Menstrual Cycle. Sleep. Med. 2007, 8, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Golub, S. Lifting the Curse of Menstruation: A Feminist Appraisal of the Influence of Menstruation on Women’s Lives; Routledge: New York, NY, USA, 2017; ISBN 978-1-317-95973-1. [Google Scholar]
- Woods, N.F.; Most, A.; Dery, G.K. Prevalene of Perimenstrual Symptoms. Am. J. Public Health 1982, 72, 1257–1264. [Google Scholar] [CrossRef] [Green Version]
- Alonso, C.; Coe, C. Disruptions of Social Relationships Accentuate the Association between Emotional Distress and Menstrual Pain in Young Women. Health Psychol. 2001, 20, 411–416. [Google Scholar] [CrossRef]
- Chen, H.-M.; Chen, C.-H. Related Factors and Consequences of Menstrual Distress in Adolescent Girls with Dysmenorrhea. Kaohsiung J. Med. Sci. 2005, 21, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Dorn, L.D.; Negriff, S.; Huang, B.; Pabst, S.; Hillman, J.; Braverman, P.; Susman, E.J. Menstrual Symptoms in Adolescent Girls: Association with Smoking, Depressive Symptoms, and Anxiety. J. Adolesc. Health 2009, 44, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Iacovides, S.; Avidon, I.; Baker, F.C. What We Know about Primary Dysmenorrhea Today: A Critical Review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [Green Version]
- Matteson, K.A.; Zaluski, K.M. Menstrual Health as a Part of Preventive Health Care. Obs. Gynecol. Clin. N. Am. 2019, 46, 441–453. [Google Scholar] [CrossRef]
- Koikawa, N.; Takami, Y.; Kawasaki, Y.; Kawana, F.; Shiroshita, N.; Ogasawara, E.; Kasai, T. Changes in the Objective Measures of Sleep between the Initial Nights of Menses and the Nights during the Midfollicular Phase of the Menstrual Cycle in Collegiate Female Athletes. J. Clin. Sleep Med. 2020, 16, 1745–1751. [Google Scholar] [CrossRef]
- Shechter, A.; Boivin, D.B. Sleep, Hormones, and Circadian Rhythms throughout the Menstrual Cycle in Healthy Women and Women with Premenstrual Dysphoric Disorder. Int. J. Endocrinol. 2010, 2010, e259345. [Google Scholar] [CrossRef] [PubMed]
- Woosley, J.A.; Lichstein, K.L. Dysmenorrhea, the Menstrual Cycle, and Sleep. Behav. Med. 2014, 40, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Girdler, S.S.; Pedersen, C.A.; Stern, R.A.; Light, K.C. Menstrual Cycle and Premenstrual Syndrome: Modifiers of Cardiovascular Reactivity in Women. Health Psychol. 1993, 12, 180–192. [Google Scholar] [CrossRef]
- Romans, S.E.; Kreindler, D.; Einstein, G.; Laredo, S.; Petrovic, M.J.; Stanley, J. Sleep Quality and the Menstrual Cycle. Sleep. Med. 2015, 16, 489–495. [Google Scholar] [CrossRef]
- Andersch, B.; Milsom, I. An Epidemiologic Study of Young Women with Dysmenorrhea. Am. J. Obs. Gynecol. 1982, 144, 655–660. [Google Scholar] [CrossRef]
- Chantler, I.; Mitchell, D.; Fuller, A. Actigraphy Quantifies Reduced Voluntary Physical Activity in Women with Primary Dysmenorrhea. J. Pain 2009, 10, 38–46. [Google Scholar] [CrossRef]
- Eryilmaz, G.; Ozdemir, F.; Pasinlioglu, T. Dysmenorrhea Prevalence among Adolescents in Eastern Turkey: Its Effects on School Performance and Relationships with Family and Friends. J. Pediatr. Adolesc. Gynecol. 2010, 23, 267–272. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Rangel-Flores, E.; Carrillo-Alarcón, L.C.; Veras-Godoy, H.A. Prevalence and Impact of Primary Dysmenorrhea among Mexican High School Students. Int. J. Gynecol. Obstet. 2009, 107, 240–243. [Google Scholar] [CrossRef]
- Patel, V.; Tanksale, V.; Sahasrabhojanee, M.; Gupte, S.; Nevrekar, P. The Burden and Determinants of Dysmenorrhoea: A Population-Based Survey of 2262 Women in Goa, India. BJOG 2006, 113, 453–463. [Google Scholar] [CrossRef]
- Pitangui, A.C.R.; de Gomes, M.R.A.; Lima, A.S.; Schwingel, P.A.; dosAlbuquerque, A.P.S.; de Araújo, R.C. Menstruation Disturbances: Prevalence, Characteristics, and Effects on the Activities of Daily Living among Adolescent Girls from Brazil. J. Pediatric. Adolesc. Gynecol. 2013, 26, 148–152. [Google Scholar] [CrossRef]
- Lewis, C.A. Effects of Hormonal Contraceptives on Mood: A Focus on Emotion Recognition and Reactivity, Reward Processing, and Stress Response. Curr. Psychiatry Rep. 2019, 15, 115. [Google Scholar] [CrossRef] [Green Version]
- Robakis, T.; Williams, K.E.; Nutkiewicz, L.; Rasgon, N.L. Hormonal Contraceptives and Mood: Review of the Literature and Implications for Future Research. Curr. Psychiatry Rep. 2019, 21, 57. [Google Scholar] [CrossRef]
- Schaffir, J.; Worly, B.L.; Gur, T.L. Combined Hormonal Contraception and Its Effects on Mood: A Critical Review. Eur. J. Contracept. Reprod. Health Care 2016, 21, 347–355. [Google Scholar] [CrossRef]
- Ekenros, L.; Bäckström, T.; Hirschberg, A.L.; Fridén, C. Changes in Premenstrual Symptoms in Women Starting or Discontinuing Use of Oral Contraceptives. Gynecol. Endocrinol. 2019, 35, 422–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, T.; Graham, C.A.; Bancroft, J.; Tanner, A.; Doll, H.A. The Effects of Oral Contraceptives on Androgen Levels and Their Relevance to Premenstrual Mood and Sexual Interest: A Comparison of Two Triphasic Formulations Containing Norgestimate and Either 35 or 25 Microg of Ethinyl Estradiol. Contraception 2007, 76, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.A.; Bancroft, J.; Doll, H.A.; Greco, T.; Tanner, A. Does Oral Contraceptive-Induced Reduction in Free Testosterone Adversely Affect the Sexuality or Mood of Women? Psychoneuroendocrinology 2007, 32, 246–255. [Google Scholar] [CrossRef]
- Baker, F.; Waner, J.; Vieira, E.; Taylor, S.; Driver, H.; Mitchell, D. Sleep and 24 Hour Body Temperatures: A Comparison in Young Men, Naturally Cycling Women and Women Taking Hormonal Contraceptives. J. Physiol. 2004, 530, 565–574. [Google Scholar] [CrossRef]
- Guida, M.; Rega, A.; Vivone, I.; Saccone, G.; Sarno, L.; Di Carlo, C.; Aquino, C.I.; Troisi, J. Variations in Sleep Associated with Different Types of Hormonal Contraceptives. Gynecol. Endocrinol. 2020, 36, 166–170. [Google Scholar] [CrossRef]
- Smith, S.S. Progesterone Enhances Inhibitory Responses of Cerebellar Purkinje Cells Mediated by the GABAA Receptor Subtype. Brain Res. Bull. 1989, 23, 317–322. [Google Scholar] [CrossRef]
- Woolley, C.S. Effects of Estrogen in the CNS. Curr. Opin. Neurobiol. 1999, 9, 349–354. [Google Scholar] [CrossRef]
- Sung, E.; Han, A.; Hinrichs, T.; Vorgerd, M.; Manchado, C.; Platen, P. Effects of Follicular versus Luteal Phase-Based Strength Training in Young Women. Springerplus 2014, 3, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlington, C.L.; Ross, A.; King, J.; Smith, P.F. Menstrual Cycle Effects on Postural Stability but Not Optokinetic Function. Neurosci. Lett. 2001, 307, 147–150. [Google Scholar] [CrossRef]
- Iman Akef, K.; Haneul, L. Neuromuscular Control of Ankle-Stabilizing Muscles-Specific Effects of Sex and Menstrual Cycle. Int. J. Sports Med. 2020, 42, 270–276. [Google Scholar] [CrossRef]
- Posthuma, B.W.; Bass, M.J.; Bull, S.B.; Nisker, J.A. Detecting Changes in Functional Ability in Women with Premenstrual Syndrome. Am. J. Obs. Gynecol. 1987, 156, 275–278. [Google Scholar] [CrossRef]
- Clark, R.A.; Bartold, S.; Bryant, A.L. Tibial Acceleration Variability during Consecutive Gait Cycles Is Influenced by the Menstrual Cycle. Clin. Biomech. 2010, 25, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Sung, E.-S.; Kim, J.-H. The Influence of Ovulation on Postural Stability (Biodex Balance System) in Young Female. J. Exerc. Rehabil. 2018, 14, 638–642. [Google Scholar] [CrossRef]
- Petrofsky, J.; Lee, H. Greater Reduction of Balance as a Result of Increased Plantar Fascia Elasticity at Ovulation during the Menstrual Cycle. Tohoku J. Exp. Med. 2015, 237, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Fridén, C.; Ramsey, D.K.; Backstrom, T.; Benoit, D.L.; Saartok, T.; Lindén Hirschberg, A. Altered Postural Control during the Luteal Phase in Women with Premenstrual Symptoms. Neuroendocrinology 2005, 81, 150–157. [Google Scholar] [CrossRef]
- Mokošáková, M.; Senko, T.; Okuliarová, M.; Kršková, L.; Hlavačka, F.; Zeman, M. Effect of Oral Contraceptives Intake on Postural Stability in Young Healthy Women throughout the Menstrual Cycle. Gen. Physiol. Biophys. 2018, 37, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Petrofsky, J. Differences Between Men and Women in Balance and Tremor in Relation to Plantar Fascia Laxity During the Menstrual Cycle. J. Athl. Train. 2018, 53, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Özer Kaya, D.; Toprak Çelenay, Ş. Fluctuations of State Anxiety, Spinal Structure, and Postural Stabilityacross the Menstrual Cycle in Active Women. Turk. J. Med. Sci. 2016, 46, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Ericksen, H.; Gribble, P.A. Sex Differences, Hormone Fluctuations, Ankle Stability, and Dynamic Postural Control. J. Athl. Train. 2012, 47, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridén, C.; Hirschberg, A.L.; Saartok, T.; Bäckström, T.; Leanderson, J.; Renström, P. The Influence of Premenstrual Symptoms on Postural Balance and Kinesthesia during the Menstrual Cycle. Gynecol. Endocrinol. 2003, 17, 433–439. [Google Scholar] [CrossRef]
- Lee, H.; Yim, J. Increased Postural Sway and Changes in the Neuromuscular Activities of the Ankle Stabilizing Muscles at Ovulation in Healthy Young Women. Tohoku J. Exp. Med. 2016, 240, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, Y.Y.F.; Petrofsky, J.S.; Berk, L.; Daher, N.; Lohman, E.; Laymon, M.S.; Cavalcanti, P. Postural Sway and Rhythmic Electroencephalography Analysis of Cortical Activation during Eight Balance Training Tasks. Med. Sci. Monit. 2013, 19, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Petrofsky, J.S.; Daher, N.; Berk, L.; Laymon, M.; Khowailed, I.A. Anterior Cruciate Ligament Elasticity and Force for Flexion during the Menstrual Cycle. Med. Sci. Monit. 2013, 19, 1080–1088. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Petrofsky, J.; Daher, N.; Berk, L.; Laymon, M. Differences in Anterior Cruciate Ligament Elasticity and Force for Knee Flexion in Women: Oral Contraceptive Users versus Non-Oral Contraceptive Users. Eur. J. Appl. Physiol. 2013, 114. [Google Scholar] [CrossRef]
- Park, S.-K.; Stefanyshyn, D.J.; Ramage, B.; Hart, D.A.; Ronsky, J.L. Alterations in Knee Joint Laxity during the Menstrual Cycle in Healthy Women Leads to Increases in Joint Loads during Selected Athletic Movements. Am. J. Sports Med. 2009, 37, 1169–1177. [Google Scholar] [CrossRef]
- Miller, B.F.; Hansen, M.; Olesen, J.L.; Schwarz, P.; Babraj, J.A.; Smith, K.; Rennie, M.J.; Kjaer, M. Tendon Collagen Synthesis at Rest and after Exercise in Women. J. Appl. Physiol. 2007, 102, 541–546. [Google Scholar] [CrossRef]
- Silbernagel, K.G.; Brorsson, A.; Olsson, N.; Eriksson, B.I.; Karlsson, J.; Nilsson-Helander, K. Sex Differences in Outcome After an Acute Achilles Tendon Rupture. Orthop. J. Sports Med. 2015, 3, 2325967115586768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller Nielsen, J.; Hammar, M. Sports Injuries and Oral Contraceptive Use. Is There a Relationship? Sports Med. 1991, 12, 152–160. [Google Scholar] [CrossRef]
- Bryant, A.L.; Crossley, K.M.; Bartold, S.; Hohmann, E.; Clark, R.A. Estrogen-Induced Effects on the Neuro-Mechanics of Hopping in Humans. Eur. J. Appl. Physiol. 2011, 111, 245–252. [Google Scholar] [CrossRef]
- Möller-Nielsen, J.; Hammar, M. Women’s Soccer Injuries in Relation to the Menstrual Cycle and Oral Contraceptive Use. Med. Sci. Sports Exerc. 1989, 21, 126–129. [Google Scholar]
- Abt, J.P.; Sell, T.C.; Laudner, K.G.; McCrory, J.L.; Loucks, T.L.; Berga, S.L.; Lephart, S.M. Neuromuscular and Biomechanical Characteristics Do Not Vary across the Menstrual Cycle. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 901–907. [Google Scholar] [CrossRef]
- Lebrun, C.M.; McKenzie, D.C.; Prior, J.C.; Taunton, J.E. Effects of Menstrual Cycle Phase on Athletic Performance. Med. Sci. Sports Exerc. 1995, 27, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Bayer, U.; Hausmann, M. Menstrual Cycle-Related Changes of Functional Cerebral Asymmetries in Fine Motor Coordination. Brain Cogn. 2012, 79, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Otaka, M.; Chen, S.-M.; Zhu, Y.; Tsai, Y.-S.; Tseng, C.-Y.; Fogt, D.L.; Lim, B.-H.; Huang, C.-Y.; Kuo, C.-H. Does Ovulation Affect Performance in Tennis Players? BMJ Open Sport Exerc. Med. 2018, 4, e000305. [Google Scholar] [CrossRef]
- Davies, B.N.; Elford, J.C.; Jamieson, K.F. Variations in Performance in Simple Muscle Tests at Different Phases of the Menstrual Cycle. J. Sports Med. Phys. Fit. 1991, 31, 532–537. [Google Scholar]
- Fridén, C.; Hirschberg, A.L.; Saartok, T.; Renström, P. Knee Joint Kinaesthesia and Neuromuscular Coordination during Three Phases of the Menstrual Cycle in Moderately Active Women. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 383–389. [Google Scholar] [CrossRef]
- Ikarashi, K.; Sato, D.; Iguchi, K.; Baba, Y.; Yamashiro, K. Menstrual Cycle Modulates Motor Learning and Memory Consolidation in Humans. Brain Sci. 2020, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Niu, Y.; Li, W.; Zhang, Z.; Liu, P.; Chen, X.; Liu, H. Menstrual Cycle Phase Modulates Auditory-Motor Integration for Vocal Pitch Regulation. Front. Neurosci. 2016, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.M.W.; Lindenfeld, T.N.; Andriacchi, T.P.; Hewett, T.E.; Riccobene, J.; Myer, G.D.; Noyes, F.R. Knee and Hip Loading Patterns at Different Phases in the Menstrual Cycle: Implications for the Gender Difference in Anterior Cruciate Ligament Injury Rates. Am. J. Sports Med. 2007, 35, 793–800. [Google Scholar] [CrossRef]
- Ekenros, L.; Hirschberg, A.L.; Heijne, A.; Fridén, C. Oral Contraceptives Do Not Affect Muscle Strength and Hop Performance in Active Women. Clin. J. Sport Med. 2013, 23, 202–207. [Google Scholar] [CrossRef]
- Janse de Jonge, X.A.K. Effects of the Menstrual Cycle on Exercise Performance. Sports Med. 2003, 33, 833–851. [Google Scholar] [CrossRef]
- Petrofsky, J.; Al Malty, A.; Suh, H.J. Isometric Endurance, Body and Skin Temperature and Limb and Skin Blood Flow during the Menstrual Cycle. Med. Sci. Monit. 2007, 13, CR111–CR117. [Google Scholar]
- Pallavi, L.C.; D Souza, U.J.; Shivaprakash, G. Assessment of Musculoskeletal Strength and Levels of Fatigue during Different Phases of Menstrual Cycle in Young Adults. J. Clin. Diagn Res. 2017, 11, CC11–CC13. [Google Scholar] [CrossRef] [PubMed]
- Tenan, M.S.; Hackney, A.C.; Griffin, L. Maximal Force and Tremor Changes across the Menstrual Cycle. Eur. J. Appl. Physiol. 2016, 116, 153–160. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Wang, L. Sex Differences in Lower Limb Proprioception and Mechanical Function Among Healthy Adults. Mot. Control. 2020, 571–587. [Google Scholar] [CrossRef]
- Thompson, B.; Almarjawi, A.; Sculley, D.; Janse de Jonge, X. The Effect of the Menstrual Cycle and Oral Contraceptives on Acute Responses and Chronic Adaptations to Resistance Training: A Systematic Review of the Literature. Sports Med. 2020, 50, 171–185. [Google Scholar] [CrossRef]
- Wikström-Frisén, L.; Boraxbekk, C.J.; Henriksson-Larsén, K. Effects on Power, Strength and Lean Body Mass of Menstrual/Oral Contraceptive Cycle Based Resistance Training. J. Sports Med. Phys. Fit. 2017, 57, 43–52. [Google Scholar] [CrossRef]
- Graja, A.; Kacem, M.; Hammouda, O.; Borji, R.; Bouzid, M.A.; Souissi, N.; Rebai, H. Physical, Biochemical, and Neuromuscular Responses to Repeated Sprint Exercise in Eumenorrheic Female Handball Players: Effect of Menstrual Cycle Phases. J. Strength Cond. Res. 2020, 18. [Google Scholar] [CrossRef]
- Petrofsky, J.S.; LeDonne, D.M.; Rinehart, J.S.; Lind, A.R. Isometric Strength and Endurance during the Menstrual Cycle. Eur. J. Appl. Physiol. Occup. Physiol. 1976, 35, 1–10. [Google Scholar] [CrossRef]
- Wirth, J.C.; Lohman, T.G. The Relationship of Static Muscle Function to Use of Oral Contraceptives. Med. Sci. Sports Exerc. 1982, 14, 16–20. [Google Scholar] [CrossRef]
- Hansen, M.; Langberg, H.; Holm, L.; Miller, B.F.; Petersen, S.G.; Doessing, S.; Skovgaard, D.; Trappe, T.; Kjaer, M. Effect of Administration of Oral Contraceptives on the Synthesis and Breakdown of Myofibrillar Proteins in Young Women. Scand. J. Med. Sci. Sports 2011, 21, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.J.; Cable, N.T.; Reilly, T. Does Oral Contraceptive Use Affect Maximum Force Production in Women? Br. J. Sports Med. 2005, 39, 15–19. [Google Scholar] [CrossRef]
- Romance, R.; Vargas, S.; Espinar, S.; Petro, J.L.; Bonilla, D.A.; Schöenfeld, B.J.; Kreider, R.B.; Benítez-Porres, J. Oral Contraceptive Use Does Not Negatively Affect Body Composition and Strength Adaptations in Trained Women. Int. J. Sports Med. 2019, 40, 842–849. [Google Scholar] [CrossRef]
- Myllyaho, M.M.; Ihalainen, J.K.; Hackney, A.C.; Valtonen, M.; Nummela, A.; Vaara, E.; Häkkinen, K.; Kyröläinen, H.; Taipale, R.S. Hormonal Contraceptive Use Does Not Affect Strength, Endurance, or Body Composition Adaptations to Combined Strength and Endurance Training in Women. J. Strength Cond. Res. 2021, 35, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, G.S.; Sizer, P.S.; Merkle, J.N.; Hounshell, T.R.; Robert-McComb, J.J.; Sawyer, S.F.; Brismée, J.-M.; Roger James, C. Effect of Sex Hormones on Neuromuscular Control Patterns during Landing. J. Electromyogr. Kinesiol. 2008, 18, 68–78. [Google Scholar] [CrossRef]
- Knowles, O.E.; Aisbett, B.; Main, L.C.; Drinkwater, E.J.; Orellana, L.; Lamon, S. Resistance Training and Skeletal Muscle Protein Metabolism in Eumenorrheic Females: Implications for Researchers and Practitioners. Sports Med. 2019, 49, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Heintz, B.; Schmauder, C.; Witte, K.; Breuer, I.; Baltzer, K.; Sieberth, H.G.; Lemmer, B. Blood Pressure Rhythm and Endocrine Functions in Normotensive Women on Oral Contraceptives. J. Hypertens. 1996, 14, 333–339. [Google Scholar] [CrossRef] [PubMed]
- George, K.P.; Birch, K.M.; Jones, B.; Lea, R. Estrogen Variation and Resting Left Ventricular Structure and Function in Young Healthy Females. Med. Sci. Sports Exerc. 2000, 32, 297–303. [Google Scholar] [CrossRef]
- Lei, T.-H.; Stannard, S.R.; Perry, B.G.; Schlader, Z.J.; Cotter, J.D.; Mündel, T. Influence of Menstrual Phase and Arid vs. Humid Heat Stress on Autonomic and Behavioural Thermoregulation during Exercise in Trained but Unacclimated Women. J. Physiol. 2017, 595, 2823–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leicht, A.S.; Hirning, D.A.; Allen, G.D. Heart Rate Variability and Endogenous Sex Hormones during the Menstrual Cycle in Young Women. Exp. Physiol. 2003, 88, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Kanojia, S.; Sharma, V.K.; Gandhi, A.; Kapoor, R.; Kukreja, A.; Subramanian, S.K. Effect of Yoga on Autonomic Functions and Psychological Status during Both Phases of Menstrual Cycle in Young Healthy Females. J. Clin. Diagn. Res. 2013, 7, 2133–2139. [Google Scholar] [CrossRef]
- Claydon, V.E.; Younis, N.R.; Hainsworth, R. Phase of the Menstrual Cycle Does Not Affect Orthostatic Tolerance in Healthy Women. Clin. Auton. Res. 2006, 16, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Cooke, W.H.; Ludwig, D.A.; Hogg, P.S.; Eckberg, D.L.; Convertino, V.A. Does the Menstrual Cycle Influence the Sensitivity of Vagally Mediated Baroreflexes? Clin. Sci. 2002, 102, 639–644. [Google Scholar] [CrossRef]
- Middlekauff, H.R.; Park, J.; Gornbein, J.A. Lack of Effect of Ovarian Cycle and Oral Contraceptives on Baroreceptor and Nonbaroreceptor Control of Sympathetic Nerve Activity in Healthy Women. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2560–H2566. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, M.; Umehara, S.; Nishikawa, T. Influence of Menstrual Cycle on Baroreflex Control of Heart Rate: Comparison with Male Volunteers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1091–R1097. [Google Scholar] [CrossRef] [Green Version]
- Yildirir, A.; Kabakci, G.; Akgul, E.; Tokgozoglu, L.; Oto, A. Effects of Menstrual Cycle on Cardiac Autonomic Innervation as Assessed by Heart Rate Variability. Ann. Noninvasive Electrocardiol. 2002, 7, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, J.; Zhou, L.; Li, X. Influence of the Menstrual Cycle on Nonlinear Properties of Heart Rate Variability in Young Women. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H765–H774. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.R.; Lawrence, J.E.; Klein, J.C. Menstrual Cycle Alters Sympathetic Neural Responses to Orthostatic Stress in Young, Eumenorrheic Women. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E85–E91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Princi, T.; Parco, S.; Accardo, A.; Radillo, O.; De Seta, F.; Guaschino, S. Parametric Evaluation of Heart Rate Variability during the Menstrual Cycle in Young Women. Biomed. Sci. Instrum. 2005, 41, 340–345. [Google Scholar]
- Sato, N.; Miyake, S.; Akatsu, J.; Kumashiro, M. Power Spectral Analysis of Heart Rate Variability in Healthy Young Women during the Normal Menstrual Cycle. Psychosom. Med. 1995, 57, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Sato, N.; Miyake, S. Cardiovascular Reactivity to Mental Stress: Relationship with Menstrual Cycle and Gender. J. Physiol. Anthr. Appl. Hum. Sci. 2004, 23, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenan, M.S.; Brothers, R.M.; Tweedell, A.J.; Hackney, A.C.; Griffin, L. Changes in Resting Heart Rate Variability across the Menstrual Cycle. Psychophysiology 2014, 51, 996–1004. [Google Scholar] [CrossRef]
- Yazar, Ş.; Yazıcı, M. Impact of Menstrual Cycle on Cardiac Autonomic Function Assessed by Heart Rate Variability and Heart Rate Recovery. Med. Princ Pract. 2016, 25, 374–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, M.; Ooie, T.; Takahashi, N.; Taniguchi, Y.; Anan, F.; Yonemochi, H.; Saikawa, T. Influence of Menstrual Cycle on QT Interval Dynamics. Pacing Clin. Electrophysiol. 2006, 29, 607–613. [Google Scholar] [CrossRef]
- Tada, Y.; Yoshizaki, T.; Tomata, Y.; Yokoyama, Y.; Sunami, A.; Hida, A.; Kawano, Y. The Impact of Menstrual Cycle Phases on Cardiac Autonomic Nervous System Activity: An Observational Study Considering Lifestyle (Diet, Physical Activity, and Sleep) among Female College Students. J. Nutr. Sci. Vitam. 2017, 63, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, A.K.; Alam, T.; Jiwane, R.; Kishanrao, S.S. A Comparative Analysis of Dietary Habits on Sensory Motor Association and Heart Rate Variability during Menstrual Cycle. J. Clin. Diagn Res. 2016, 10, CC04–CC08. [Google Scholar] [CrossRef]
- Maffei, S.; Clerico, A.; Iervasi, G.; Nannipieri, M.; Del Ry, S.; Giannessi, D.; Donato, L. Circulating Levels of Cardiac Natriuretic Hormones Measured in Women during Menstrual Cycle. J. Endocrinol. Investig. 1999, 22, 1–5. [Google Scholar] [CrossRef]
- De Souza, M.J.; Maguire, M.S.; Rubin, K.R.; Maresh, C.M. Effects of Menstrual Phase and Amenorrhea on Exercise Performance in Runners. Med. Sci. Sports Exerc. 1990, 22, 575–580. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Ramos, P.S.; Vianna, L.C.; Ricardo, D.R. Effects of Ovarian Hormones and Oral Contraceptive Pills on Cardiac Vagal Withdrawal at the Onset of Dynamic Exercise. PLoS ONE 2015, 10, e0119626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba-Moreno, L.; Cupeiro, R.; Romero-Parra, N.; Janse de Jonge, X.A.K.; Peinado, A.B. Cardiorespiratory Responses to Endurance Exercise over the Menstrual Cycle and With Oral Contraceptive Use. J. Strength Cond. Res. 2019. [Google Scholar] [CrossRef]
- Nakamura, M.; Hayashi, K.; Aizawa, K.; Mesaki, N.; Kono, I. Effects of Regular Aerobic Exercise on Post-Exercise Vagal Reactivation in Young Female. Eur. J. Sport Sci. 2013, 13, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Wenner, M.M.; Prettyman, A.V.; Maser, R.E.; Farquhar, W.B. Preserved Autonomic Function in Amenorrheic Athletes. J. Appl. Physiol. 2006, 101, 590–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinberg, A.E.; Touitou, Y.; Soudant, E.; Bernard, D.; Bazin, R.; Mechkouri, M. Oral Contraceptives Alter Circadian Rhythm Parameters of Cortisol, Melatonin, Blood Pressure, Heart Rate, Skin Blood Flow, Transepidermal Water Loss, and Skin Amino Acids of Healthy Young Women. Chronobiol. Int. 1996, 13, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Ramos, P.S.; Vianna, L.C.; Ricardo, D.R. Heart Rate Variability across the Menstrual Cycle in Young Women Taking Oral Contraceptives. Psychophysiology 2015, 52, 1451–1455. [Google Scholar] [CrossRef]
- Cagnacci, A.; Zanin, R.; Napolitano, A.; Arangino, S.; Volpe, A. Modification of 24-h Ambulatory Blood Pressure and Heart Rate during Contraception with the Vaginal Ring: A Prospective Study. Contraception 2013, 88, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.E.; Hart, E.C.; Charkoudian, N.; Curry, T.B.; Carter, J.R.; Fu, Q.; Minson, C.T.; Joyner, M.J.; Barnes, J.N. Oral Contraceptive Use, Muscle Sympathetic Nerve Activity, and Systemic Hemodynamics in Young Women. Hypertension 2015, 66, 590–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giribela, C.R.G.; Melo, N.R.; Silva, R.C.G.; Hong, V.M.; Guerra, G.M.; Baracat, E.C.; Consolim-Colombo, F.M. A Combined Oral Contraceptive Containing Drospirenone Changes Neither Endothelial Function nor Hemodynamic Parameters in Healthy Young Women: A Prospective Clinical Trial. Contraception 2012, 86, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Nisenbaum, M.G.; de Melo, N.R.; Giribela, C.R.G.; de Morais, T.L.; Guerra, G.M.; de Angelis, K.; Mostarda, C.; Baracat, E.C.; Consolim-Colombo, F.M. Effects of a Contraceptive Containing Drospirenone and Ethinyl Estradiol on Blood Pressure and Autonomic Tone: A Prospective Controlled Clinical Trial. Eur. J. Obs. Gynecol. Reprod. Biol. 2014, 175, 62–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilczak, A.; Marciniak, K.; Kłapciński, M.; Rydlewska, A.; Danel, D.; Jankowska, E.A. Relations between Combined Oral Contraceptive Therapy and Indices of Autonomic Balance (Baroreflex Sensitivity and Heart Rate Variability) in Young Healthy Women. Ginekol. Pol. 2013, 84, 915–921. [Google Scholar] [CrossRef]
- Schueller, P.O.; Feuring, M.; Sharkova, Y.; Grimm, W.; Christ, M. Effects of Synthetic Progestagens on Autonomic Tone, Neurohormones and C-Reactive Protein Levels in Young Healthy Females in Reproductive Age. Int. J. Cardiol. 2006, 111, 42–48. [Google Scholar] [CrossRef]
- Lehtovirta, P.; Kuikka, J.; Pyörälä, T. Hemodynamic Effects of Oral Contraceptives during Exercise. Int. J. Gynaecol. Obs. 1977, 15, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Bauza, D.E.; Silveyra, P. Sex Differences in Exercise-Induced Bronchoconstriction in Athletes: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 7270. [Google Scholar] [CrossRef]
- Goodland, R.L.; Reynolds, J.G.; Mccoord, A.B.; Pommerenke, W.T. Respiratory and Electrolyte Effects Induced by Estrogen and Progesterone. Fertil. Steril. 1953, 4, 300–317. [Google Scholar] [CrossRef]
- Regensteiner, J.G.; McCullough, R.G.; McCullough, R.E.; Pickett, C.K.; Moore, L.G. Combined Effects of Female Hormones and Exercise on Hypoxic Ventilatory Response. Respir Physiol. 1990, 82, 107–114. [Google Scholar] [CrossRef]
- Bayliss, D.A.; Millhorn, D.E. Central Neural Mechanisms of Progesterone Action: Application to the Respiratory System. J. Appl. Physiol. 1992, 73, 393–404. [Google Scholar] [CrossRef]
- Behan, M.; Zabka, A.G.; Thomas, C.F.; Mitchell, G.S. Sex Steroid Hormones and the Neural Control of Breathing. Respir Physiol. Neurobiol. 2003, 136, 249–263. [Google Scholar] [CrossRef]
- Skatrud, J.B.; Dempsey, J.A.; Kaiser, D.G. Ventilatory Response to Medroxyprogesterone Acetate in Normal Subjects: Time Course and Mechanism. J. Appl. Physiol. Respir Environ. Exerc. Physiol. 1978, 44, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Jurkowski, J.E.; Jones, N.L.; Toews, C.J.; Sutton, J.R. Effects of Menstrual Cycle on Blood Lactate, O2 Delivery, and Performance during Exercise. J. Appl. Physiol. Respir Environ. Exerc. Physiol. 1981, 51, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Schoene, R.B.; Robertson, H.T.; Pierson, D.J.; Peterson, A.P. Respiratory Drives and Exercise in Menstrual Cycles of Athletic and Nonathletic Women. J. Appl. Physiol. Respir Environ. Exerc. Physiol. 1981, 50, 1300–1305. [Google Scholar] [CrossRef]
- Slatkovska, L.; Jensen, D.; Davies, G.A.L.; Wolfe, L.A. Phasic Menstrual Cycle Effects on the Control of Breathing in Healthy Women. Respir Physiol. Neurobiol. 2006, 154, 379–388. [Google Scholar] [CrossRef]
- Smekal, G.; von Duvillard, S.P.; Frigo, P.; Tegelhofer, T.; Pokan, R.; Hofmann, P.; Tschan, H.; Baron, R.; Wonisch, M.; Renezeder, K.; et al. Menstrual Cycle: No Effect on Exercise Cardiorespiratory Variables or Blood Lactate Concentration. Med. Sci. Sports Exerc. 2007, 39, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.J.; Krahenbuhl, G.S. Menstrual Cycle Phase and Running Economy. Med. Sci. Sports Exerc. 1997, 29, 1609–1618. [Google Scholar] [CrossRef]
- Beidleman, B.A.; Rock, P.B.; Muza, S.R.; Fulco, C.S.; Forte, V.A.; Cymerman, A. Exercise VE and Physical Performance at Altitude Are Not Affected by Menstrual Cycle Phase. J. Appl. Physiol. 1999, 86, 1519–1526. [Google Scholar] [CrossRef] [Green Version]
- Bemben, D.A.; Salm, P.C.; Salm, A.J. Ventilatory and Blood Lactate Responses to Maximal Treadmill Exercise during the Menstrual Cycle. J. Sports Med. Phys. Fit. 1995, 35, 257–262. [Google Scholar]
- Macnutt, M.J.; De Souza, M.J.; Tomczak, S.E.; Homer, J.L.; Sheel, A.W. Resting and Exercise Ventilatory Chemosensitivity across the Menstrual Cycle. J. Appl. Physiol. 2012, 112, 737–747. [Google Scholar] [CrossRef]
- Dombovy, M.L.; Bonekat, H.W.; Williams, T.J.; Staats, B.A. Exercise Performance and Ventilatory Response in the Menstrual Cycle. Med. Sci. Sports Exerc. 1987, 19, 111–117. [Google Scholar] [CrossRef]
- Brutsaert, T.D.; Spielvogel, H.; Caceres, E.; Araoz, M.; Chatterton, R.T.; Vitzthum, V.J. Effect of Menstrual Cycle Phase on Exercise Performance of High-Altitude Native Women at 3600 m. J. Exp. Biol. 2002, 205, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Byrne-Quinn, E.; Weil, J.V.; Sodal, I.E.; Filley, G.F.; Grover, R.F. Ventilatory Control in the Athlete. J. Appl. Physiol. 1971, 30, 91–98. [Google Scholar] [CrossRef]
- Saunders, N.A.; Leeder, S.R.; Rebuck, A.S. Ventilatory Response to Carbon Dioxide in Young Athletes: A Family Study. Am. Rev. Respir Dis. 1976, 113, 497–502. [Google Scholar] [CrossRef]
- Dutton, K.; Blanksby, B.A.; Morton, A.R. CO2 Sensitivity Changes during the Menstrual Cycle. J. Appl. Physiol. 1989, 67, 517–522. [Google Scholar] [CrossRef]
- Takase, K.; Nishiyasu, T.; Asano, K. Modulating Effects of the Menstrual Cycle on Cardiorespiratory Responses to Exercise under Acute Hypobaric Hypoxia. Jpn. J. Physiol. 2002, 52, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.J.; Sparks, K.E.; Zwillich, C.W.; Weil, J.V. Low Exercise Ventilation in Endurance Athletes. Med. Sci. Sports 1979, 11, 181–185. [Google Scholar] [PubMed]
- Muza, S.R.; Rock, P.B.; Fulco, C.S.; Zamudio, S.; Braun, B.; Cymerman, A.; Butterfield, G.E.; Moore, L.G. Women at Altitude: Ventilatory Acclimatization at 4300 m. J. Appl. Physiol. 2001, 91, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.-P.; Lhuissier, F.; Jean, D. Ventilatory Response to Hypoxia and Tolerance to High Altitude in Women: Influence of Menstrual Cycle, Oral Contraception, and Menopause. High. Alt. Med. Biol. 2020, 21, 12–19. [Google Scholar] [CrossRef]
- Burrows, M.; Bird, S.R.; Bishop, N. The Menstrual Cycle and Its Effect on the Immune Status of Female Endurance Runners. J. Sports Sci. 2002, 20, 339–344. [Google Scholar] [CrossRef]
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Fagoaga, O.R.; Henson, D.A.; Shannon, M.; Hjertman, J.M.; Schmitt, R.L.; Bolton, M.R.; Austin, M.D.; Schilling, B.K.; et al. Immune Function in Female Elite Rowers and Non-Athletes. Br. J. Sports Med. 2000, 34, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Suzuki, N.; Nakamura, M.; Aizawa, K.; Imai, T.; Suzuki, S.; Eda, N.; Hanaoka, Y.; Nakao, K.; Suzuki, N.; et al. Mucosal Immune Function Comparison between Amenorrheic and Eumenorrheic Distance Runners. J. Strength Cond. Res. 2012, 26, 1402–1406. [Google Scholar] [CrossRef]
- Philpott, C.M.; El-Alami, M.; Murty, G.E. The Effect of the Steroid Sex Hormones on the Nasal Airway during the Normal Menstrual Cycle. Clin. Otolaryngol. Allied Sci. 2004, 29, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Drosdzol, A.; Skrzypulec, V.; Wilk, K.; Rachel, M. The Influence of Bronchial Asthma on Menstrual Cycle. J. Physiol. Pharm. 2007, 58 (Suppl. 5), 165–173. [Google Scholar]
- Oguzulgen, I.K.; Turktas, H.; Erbas, D. Airway Inflammation in Premenstrual Asthma. J. Asthma 2002, 39, 517–522. [Google Scholar] [CrossRef]
- Stanford, K.I.; Mickleborough, T.D.; Ray, S.; Lindley, M.R.; Koceja, D.M.; Stager, J.M. Influence of Menstrual Cycle Phase on Pulmonary Function in Asthmatic Athletes. Eur. J. Appl. Physiol. 2006, 96, 703–710. [Google Scholar] [CrossRef]
- Kirsch, E.A.; Yuhanna, I.S.; Chen, Z.; German, Z.; Sherman, T.S.; Shaul, P.W. Estrogen Acutely Stimulates Endothelial Nitric Oxide Synthase in H441 Human Airway Epithelial Cells. Am. J. Respir Cell Mol. Biol. 1999, 20, 658–666. [Google Scholar] [CrossRef]
- Juniper, E.F.; Kline, P.A.; Roberts, R.S.; Hargreave, F.E.; Daniel, E.E. Airway Responsiveness to Methacholine during the Natural Menstrual Cycle and the Effect of Oral Contraceptives. Am. Rev. Respir Dis. 1987, 135, 1039–1042. [Google Scholar] [CrossRef]
- Pauli, B.D.; Reid, R.L.; Munt, P.W.; Wigle, R.D.; Forkert, L. Influence of the Menstrual Cycle on Airway Function in Asthmatic and Normal Subjects. Am. Rev. Respir Dis. 1989, 140, 358–362. [Google Scholar] [CrossRef]
- Shames, R.S.; Heilbron, D.C.; Janson, S.L.; Kishiyama, J.L.; Au, D.S.; Adelman, D.C. Clinical Differences among Women with and without Self-Reported Perimenstrual Asthma. Ann. Allergy Asthma Immunol. 1998, 81, 65–72. [Google Scholar] [CrossRef]
- Weinmann, G.G.; Zacur, H.; Fish, J.E. Absence of Changes in Airway Responsiveness during the Menstrual Cycle. J. Allergy Clin. Immunol. 1987, 79, 634–638. [Google Scholar] [CrossRef]
- Kluft, C.; Leuven, J.A.G.; Helmerhorst, F.M.; Krans, H.M.J. Pro-Inflammatory Effects of Oestrogens during Use of Oral Contraceptives and Hormone Replacement Treatment. Vasc. Pharm. 2002, 39, 149–154. [Google Scholar] [CrossRef]
- Packer, N.; Hoffman-Goetz, L.; Ward, G. Does Physical Activity Affect Quality of Life, Disease Symptoms and Immune Measures in Patients with Inflammatory Bowel Disease? A Systematic Review. J. Sports Med. Phys. Fit. 2010, 50, 1–18. [Google Scholar]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic Review: Exercise-Induced Gastrointestinal Syndrome-Implications for Health and Intestinal Disease. Aliment. Pharm. 2017, 46, 246–265. [Google Scholar] [CrossRef] [Green Version]
- Moses, F.M. Exercise-Associated Intestinal Ischemia. Curr. Sports Med. Rep. 2005, 4, 91–95. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal Complaints during Exercise: Prevalence, Etiology, and Nutritional Recommendations. Sports Med. 2014, 44 (Suppl. 1), S79–S85. [Google Scholar] [CrossRef] [Green Version]
- Ho, G.W.K. Lower Gastrointestinal Distress in Endurance Athletes. Curr. Sports Med. Rep. 2009, 8, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Waterman, J.J.; Kapur, R. Upper Gastrointestinal Issues in Athletes. Curr. Sports Med. Rep. 2012, 11, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Diduch, B.K. Gastrointestinal Conditions in the Female Athlete. Clin. Sports Med. 2017, 36, 655–669. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Barber, M.D.; Graff, L.A.; Shen, B. Symptomatology of Irritable Bowel Syndrome and Inflammatory Bowel Disease during the Menstrual Cycle. Gastroenterol. Rep. 2015, 3, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Heitkemper, M.M.; Shaver, J.F.; Mitchell, E.S. Gastrointestinal Symptoms and Bowel Patterns across the Menstrual Cycle in Dysmenorrhea. Nurs. Res. 1988, 37, 108–113. [Google Scholar] [CrossRef]
- Moore, J.; Barlow, D.; Jewell, D.; Kennedy, S. Do Gastrointestinal Symptoms Vary with the Menstrual Cycle? Br. J. Obs. Gynaecol. 1998, 105, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Judkins, T.C.; Dennis-Wall, J.C.; Sims, S.M.; Colee, J.; Langkamp-Henken, B. Stool Frequency and Form and Gastrointestinal Symptoms Differ by Day of the Menstrual Cycle in Healthy Adult Women Taking Oral Contraceptives: A Prospective Observational Study. BMC Womens Health 2020, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.A.; Houghton, L.A.; Whorwell, P.J.; Currer, B. Does the Menstrual Cycle Affect Anorectal Physiology? Dig. Dis. Sci. 1994, 39, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Lea, R.; Jackson, N.; Whorwell, P.J. The Menstrual Cycle Affects Rectal Sensitivity in Patients with Irritable Bowel Syndrome but Not Healthy Volunteers. Gut 2002, 50, 471–474. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The Microbiome of Professional Athletes Differs from That of More Sedentary Subjects in Composition and Particularly at the Functional Metabolic Level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The Athletic Gut Microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- Bo, K.; Fernandes, A.C.N.L.; Duarte, T.B.; Brito, L.G.O.; Ferreira, C.H.J. Is Pelvic Floor Muscle Training Effective for Symptoms of Overactive Bladder in Women? A Systematic Review. Physiotherapy 2020, 106, 65–76. [Google Scholar] [CrossRef]
- Cerruto, M.A.; Balzarro, M.; Rubilotta, E.; Processali, T.; Latini, M.T.; Porcaro, A.B.; Scancarello, C.; Cantaluppi, S.; Di Dedda, M.C.; Antonelli, A.; et al. Lower Urinary Tract and Gastrointestinal Dysfunction in Sportswomen: A Systematic Review and Meta-Analysis of Observational Studies. Minerva Urol. Nefrol. 2020, 72, 698–711. [Google Scholar] [CrossRef]
- de Mattos Lourenco, T.R.; Matsuoka, P.K.; Baracat, E.C.; Haddad, J.M. Urinary Incontinence in Female Athletes: A Systematic Review. Int. Urogynecol. J. 2018, 29, 1757–1763. [Google Scholar] [CrossRef]
- Sorrigueta-Hernández, A.; Padilla-Fernandez, B.-Y.; Marquez-Sanchez, M.-T.; Flores-Fraile, M.-C.; Flores-Fraile, J.; Moreno-Pascual, C.; Lorenzo-Gomez, A.; Garcia-Cenador, M.-B.; Lorenzo-Gomez, M.-F. Benefits of Physiotherapy on Urinary Incontinence in High-Performance Female Athletes. Meta-Analysis. J. Clin. Med. 2020, 9, 3240. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.V.; Colla, C.; Sbruzzi, G.; Mallmann, A.; Paiva, L.L. Prevalence of Urinary Incontinence in Female Athletes: A Systematic Review with Meta-Analysis. Int. Urogynecol. J. 2018, 29, 1717–1725. [Google Scholar] [CrossRef]
- Hagovska, M.; Švihra, J.; Buková, A.; Dračková, D.; Švihrová, V. Prevalence and Risk of Sport Types to Stress Urinary Incontinence in Sportswomen: A Cross-Sectional Study. Neurourol. Urodyn. 2018, 37, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Simeone, C.; Moroni, A.; Pettenò, A.; Antonelli, A.; Zani, D.; Orizio, C.; Cosciani Cunico, S. Occurrence Rates and Predictors of Lower Urinary Tract Symptoms and Incontinence in Female Athletes. Urologia 2010, 77, 139–146. [Google Scholar] [CrossRef]
- Gram, M.C.D.; Bø, K. High Level Rhythmic Gymnasts and Urinary Incontinence: Prevalence, Risk Factors, and Influence on Performance. Scand. J. Med. Sci. Sports 2020, 30, 159–165. [Google Scholar] [CrossRef]
- Whitney, K.E.; Holtzman, B.; Cook, D.; Bauer, S.; Maffazioli, G.D.N.; Parziale, A.L.; Ackerman, K.E. Low Energy Availability and Impact Sport Participation as Risk Factors for Urinary Incontinence in Female Athletes. J. Pediatr. Urol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Boileau, M.; Fuchs, E.; Barry, J.M.; Hodges, C.V. Stress Hematuria: Athletic Pseudonephritis in Marathoners. Urology 1980, 15, 471–474. [Google Scholar] [CrossRef]
- Bellinghieri, G.; Savica, V.; Santoro, D. Renal Alterations during Exercise. J. Ren. Nutr. 2008, 18, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Gilli, P.; De Paoli Vitali, E.; Tataranni, G.; Farinelli, A. Exercise-Induced Urinary Abnormalities in Long-Distance Runners. Int. J. Sports Med. 1984, 5, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. Exercise Proteinuria and Hematuria: Current Knowledge and Future Directions. J. Sports Med. Phys. Fit. 2016, 56, 1060–1076. [Google Scholar]
- Enea, C.; Boisseau, N.; Ottavy, M.; Mulliez, J.; Millet, C.; Ingrand, I.; Diaz, V.; Dugué, B. Effects of Menstrual Cycle, Oral Contraception, and Training on Exercise-Induced Changes in Circulating DHEA-Sulphate and Testosterone in Young Women. Eur. J. Appl. Physiol. 2009, 106, 365–373. [Google Scholar] [CrossRef]
- Vibarel-Rebot, N.; Rieth, N.; Lasne, F.; Jaffré, C.; Collomp, K. Oral Contraceptive Use and Saliva Diurnal Pattern of Metabolic Steroid Hormones in Young Healthy Women. Contraception 2015, 91, 245–247. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.B.; Lehman, C.; Koltun, K.; Smith-Ryan, A.; Hackney, A.C. Response of Testosterone to Prolonged Aerobic Exercise during Different Phases of the Menstrual Cycle. Eur. J. Appl. Physiol. 2013, 113, 2419–2424. [Google Scholar] [CrossRef]
- Keizer, H.A.; Beckers, E.; de Haan, J.; Janssen, G.M.; Kuipers, H.; van Kranenburg, G.; Geurten, P. Exercise-Induced Changes in the Percentage of Free Testosterone and Estradiol in Trained and Untrained Women. Int. J. Sports Med. 1987, 8 (Suppl. 3), 151–153. [Google Scholar] [CrossRef]
- Lane, A.R.; O’Leary, C.B.; Hackney, A.C. Menstrual Cycle Phase Effects Free Testosterone Responses to Prolonged Aerobic Exercise. Acta Physiol. Hung. 2015, 102, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Crewther, B.T.; Cook, C.J. A Longitudinal Analysis of Salivary Testosterone Concentrations and Competitiveness in Elite and Non-Elite Women Athletes. Physiol. Behav. 2018, 188, 157–161. [Google Scholar] [CrossRef]
- Cook, C.J.; Crewther, B.T.; Smith, A.A. Comparison of Baseline Free Testosterone and Cortisol Concentrations between Elite and Non-Elite Female Athletes. Am. J. Hum. Biol. 2012, 24, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.P.; Weeke, J. Fasting Serum Growth Hormone Levels and Growth Hormone Responses to Exercise during Normal Menstrual Cycles and Cycles of Oral Contraceptives. Scand. J. Clin. Lab. Investig. 1974, 34, 199–205. [Google Scholar] [CrossRef]
- Hornum, M.; Cooper, D.M.; Brasel, J.A.; Bueno, A.; Sietsema, K.E. Exercise-Induced Changes in Circulating Growth Factors with Cyclic Variation in Plasma Estradiol in Women. J. Appl. Physiol. 1997, 82, 1946–1951. [Google Scholar] [CrossRef]
- Ovesen, P.; Vahl, N.; Fisker, S.; Veldhuis, J.D.; Christiansen, J.S.; Jørgensen, J.O. Increased Pulsatile, but Not Basal, Growth Hormone Secretion Rates and Plasma Insulin-like Growth Factor I Levels during the Periovulatory Interval in Normal Women. J. Clin. Endocrinol. Metab. 1998, 83, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, H.K.; Shalet, S.M. GH Responsiveness Varies during the Menstrual Cycle. Eur. J. Endocrinol. 2005, 153, 775–779. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.S.; Lee, J.D.; Soong, Y.K. Serum Levels of Insulin-like Growth Factor I and Insulin-like Growth Factor-Binding Protein-1 and -3 in Women with Regular Menstrual Cycles. Fertil. Steril. 1995, 63, 1204–1209. [Google Scholar] [CrossRef]
- Cano Sokoloff, N.; Misra, M.; Ackerman, K.E. Exercise, Training, and the Hypothalamic-Pituitary-Gonadal Axis in Men and Women. Front. Horm. Res. 2016, 47, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Yen, S.S. Nutritional and Endocrine-Metabolic Aberrations in Amenorrheic Athletes. J. Clin. Endocrinol. Metab. 1996, 81, 4301–4309. [Google Scholar] [CrossRef] [PubMed]
- Rickenlund, A.; Thorén, M.; Nybacka, A.; Frystyk, J.; Hirschberg, A.L. Effects of Oral Contraceptives on Diurnal Profiles of Insulin, Insulin-like Growth Factor Binding Protein-1, Growth Hormone and Cortisol in Endurance Athletes with Menstrual Disturbance. Hum. Reprod. 2010, 25, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McComb, J.J.R.; Qian, X.-P.; Veldhuis, J.D.; J McGlone, J.; Norman, R.L. Neuroendocrine Responses to Psychological Stress in Eumenorrheic and Oligomenorrheic Women. Stress 2006, 9, 41–51. [Google Scholar] [CrossRef]
- Timmons, B.W.; Hamadeh, M.J.; Devries, M.C.; Tarnopolsky, M.A. Influence of Gender, Menstrual Phase, and Oral Contraceptive Use on Immunological Changes in Response to Prolonged Cycling. J. Appl. Physiol. 2005, 99, 979–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crewther, B.T.; Hamilton, D.; Casto, K.; Kilduff, L.P.; Cook, C.J. Effects of Oral Contraceptive Use on the Salivary Testosterone and Cortisol Responses to Training Sessions and Competitions in Elite Women Athletes. Physiol. Behav. 2015, 147, 84–90. [Google Scholar] [CrossRef]
- Mackay, K.; González, C.; Zbinden-Foncea, H.; Peñailillo, L. Effects of Oral Contraceptive Use on Female Sexual Salivary Hormones and Indirect Markers of Muscle Damage Following Eccentric Cycling in Women. Eur. J. Appl. Physiol. 2019, 119, 2733–2744. [Google Scholar] [CrossRef]
- Edwards, D.A.; O’Neal, J.L. Oral Contraceptives Decrease Saliva Testosterone but Do Not Affect the Rise in Testosterone Associated with Athletic Competition. Horm. Behav. 2009, 56, 195–198. [Google Scholar] [CrossRef]
- Bonen, A.; Haynes, F.W.; Graham, T.E. Substrate and Hormonal Responses to Exercise in Women Using Oral Contraceptives. J. Appl. Physiol. 1991, 70, 1917–1927. [Google Scholar] [CrossRef]
- Altemus, M.; Roca, C.; Galliven, E.; Romanos, C.; Deuster, P. Increased Vasopressin and Adrenocorticotropin Responses to Stress in the Midluteal Phase of the Menstrual Cycle. J. Clin. Endocrinol. Metab. 2001, 86, 2525–2530. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, N.; Enea, C.; Diaz, V.; Dugué, B.; Corcuff, J.B.; Duclos, M. Oral Contraception but Not Menstrual Cycle Phase Is Associated with Increased Free Cortisol Levels and Low Hypothalamo-Pituitary-Adrenal Axis Reactivity. J. Endocrinol. Investig. 2013, 36, 955–964. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Boileau, R.A.; Bahr, J.M.; Misner, J.E.; Nelson, R.A. Cortisol Levels during Prolonged Exercise: The Influence of Menstrual Phase and Menstrual Status. Int. J. Sports Med. 1992, 13, 332–336. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Francois, M.; Webb, N.D.; Worley, J.R.; Rogers, S.N.; Norman, R.L.; Shah, U.; Castracane, V.D.; Daniel Castracane, V. No Effect of Menstrual Cycle Phase on Glucose and Glucoregulatory Endocrine Responses to Prolonged Exercise. Eur. J. Appl. Physiol. 2013, 113, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Bouma, E.M.C.; Riese, H.; Ormel, J.; Verhulst, F.C.; Oldehinkel, A.J. Adolescents’ Cortisol Responses to Awakening and Social Stress; Effects of Gender, Menstrual Phase and Oral Contraceptives. The TRAILS Study. Psychoneuroendocrinology 2009, 34, 884–893. [Google Scholar] [CrossRef] [Green Version]
- Kayacan, Y.; Makaracı, Y.; Ozgocer, T.; Ucar, C.; Yıldız, S. Cortisol Awakening Response and Heart Rate Variability in the Menstrual Cycle of Sportswomen. Res. Q. Exerc. Sport 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Huang, Y.; Zhou, R. Premenstrual Syndrome Is Associated with Altered Cortisol Awakening Response. Stress 2019, 22, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Ozgocer, T.; Ucar, C.; Yildiz, S. Cortisol Awakening Response Is Blunted and Pain Perception Is Increased during Menses in Cyclic Women. Psychoneuroendocrinology 2017, 77, 158–164. [Google Scholar] [CrossRef]
- Sawin, C.T.; Hershman, J.M.; Boyd, A.E.; Longcope, C.; Bacharach, P. The Relationship of Changes in Serum Estradiol and Progesterone during the Menstrual Cycle to the Thyrotropin and Prolactin Responses to Thyrotropin-Releasing Hormone. J. Clin. Endocrinol. Metab. 1978, 47, 1296–1302. [Google Scholar] [CrossRef]
- Weeke, J.; Hansen, A.P. Serum Tsh and Serum T3 Levels during Normal Menstrual Cycles and during Cycles on Oral Contraceptives. Acta Endocrinol. 1975, 79, 431–438. [Google Scholar] [CrossRef]
- De Souza, M.J.; Maresh, C.M.; Maguire, M.S.; Kraemer, W.J.; Flora-Ginter, G.; Goetz, K.L. Menstrual Status and Plasma Vasopressin, Renin Activity, and Aldosterone Exercise Responses. J. Appl. Physiol. 1989, 67, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Harber, V.J.; Sutton, J.R.; MacDougall, J.D.; Woolever, C.A.; Bhavnani, B.R. Plasma Concentrations of Beta-Endorphin in Trained Eumenorrheic and Amenorrheic Women. Fertil. Steril. 1997, 67, 648–653. [Google Scholar] [CrossRef]
- Ding, J.H.; Sheckter, C.B.; Drinkwater, B.L.; Soules, M.R.; Bremner, W.J. High Serum Cortisol Levels in Exercise-Associated Amenorrhea. Ann. Intern. Med. 1988, 108, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, C.; Hirschberg, A.L.; Carlström, K.; von Schoultz, B. Altered Adrenal Steroid Metabolism Underlying Hypercortisolism in Female Endurance Athletes. Fertil. Steril. 1995, 63, 1190–1194. [Google Scholar] [CrossRef]
- De Souza, M.J.; Maguire, M.S.; Maresh, C.M.; Kraemer, W.J.; Rubin, K.R.; Loucks, A.B. Adrenal Activation and the Prolactin Response to Exercise in Eumenorrheic and Amenorrheic Runners. J. Appl. Physiol. 1991, 70, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, K.E.; Patel, K.T.; Guereca, G.; Pierce, L.; Herzog, D.B.; Misra, M. Cortisol Secretory Parameters in Young Exercisers in Relation to LH Secretion and Bone Parameters. Clin. Endocrinol. 2013, 78, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Schaal, K.; Van Loan, M.D.; Casazza, G.A. Reduced Catecholamine Response to Exercise in Amenorrheic Athletes. Med. Sci. Sports Exerc. 2011, 43, 34–43. [Google Scholar] [CrossRef]
- Loucks, A.B.; Horvath, S.M. Exercise-Induced Stress Responses of Amenorrheic and Eumenorrheic Runners. J. Clin. Endocrinol. Metab. 1984, 59, 1109–1120. [Google Scholar] [CrossRef]
- De Souza, M.J.; Luciano, A.A.; Arce, J.C.; Demers, L.M.; Loucks, A.B. Clinical Tests Explain Blunted Cortisol Responsiveness but Not Mild Hypercortisolism in Amenorrheic Runners. J. Appl. Physiol. 1994, 76, 1302–1309. [Google Scholar] [CrossRef]
- Hohtari, H.; Elovainio, R.; Salminen, K.; Laatikainen, T. Plasma Corticotropin-Releasing Hormone, Corticotropin, and Endorphins at Rest and during Exercise in Eumenorrheic and Amenorrheic Athletes. Fertil. Steril. 1988, 50, 233–238. [Google Scholar] [CrossRef]
- Thong, F.S.; McLean, C.; Graham, T.E. Plasma Leptin in Female Athletes: Relationship with Body Fat, Reproductive, Nutritional, and Endocrine Factors. J. Appl. Physiol. 2000, 88, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.E.; Richards, S.R.; Kim, M.H.; Malarkey, W.B. Twenty Four-Hour Prolactin Profiles and Prolactin Responses to Dopamine in Long Distance Running Women. J. Clin. Endocrinol. Metab. 1984, 59, 631–635. [Google Scholar] [CrossRef]
- Meyer, W.R.; Muoio, D.; Hackney, T.C. Effect of Sex Steroids on Beta-Endorphin Levels at Rest and during Submaximal Treadmill Exercise in Anovulatory and Ovulatory Runners. Fertil. Steril. 1999, 71, 1085–1091. [Google Scholar] [CrossRef]
- Hohtari, H.; Salminen-Lappalainen, K.; Laatikainen, T. Response of Plasma Endorphins, Corticotropin, Cortisol, and Luteinizing Hormone in the Corticotropin-Releasing Hormone Stimulation Test in Eumenorrheic and Amenorrheic Athletes. Fertil. Steril. 1991, 55, 276–280. [Google Scholar] [CrossRef]
- Hinton, P.S.; Rector, R.S.; Peppers, J.E.; Imhoff, R.D.; Hillman, L.S. Serum Markers of Inflammation and Endothelial Function Are Elevated by Hormonal Contraceptive Use but Not by Exercise-Associated Menstrual Disorders in Physically Active Young Women. J. Sports Sci. Med. 2006, 5, 235–242. [Google Scholar] [PubMed]
- Larsen, B.; Cox, A.; Colbey, C.; Drew, M.; McGuire, H.; Fazekas de St Groth, B.; Hughes, D.; Vlahovich, N.; Waddington, G.; Burke, L.; et al. Inflammation and Oral Contraceptive Use in Female Athletes Before the Rio Olympic Games. Front. Physiol. 2020, 11, 497. [Google Scholar] [CrossRef]
- Larsen, B.; Cox, A.J.; Quinn, K.; Fisher, R.; Minahan, C. Immune Response in Women during Exercise in the Heat: A Spotlight on Oral Contraception. J. Sports Sci. Med. 2018, 17, 229–236. [Google Scholar]
- Jankowski, C.M.; Ben-Ezra, V.; Gozansky, W.S.; Scheaffer, S.E. Effects of Oral Contraceptives on Glucoregulatory Responses to Exercise. Metabolism 2004, 53, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyse, T.; Bosch, A.N. The Effect of the Menstrual Cycle on Exercise Metabolism: Implications for Exercise Performance in Eumenorrhoeic Women. Sports Med. 2010, 40, 207–227. [Google Scholar] [CrossRef]
- Campbell, S.E.; Angus, D.J.; Febbraio, M.A. Glucose Kinetics and Exercise Performance during Phases of the Menstrual Cycle: Effect of Glucose Ingestion. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E817–E825. [Google Scholar] [CrossRef]
- Devries, M.C.; Hamadeh, M.J.; Phillips, S.M.; Tarnopolsky, M.A. Menstrual Cycle Phase and Sex Influence Muscle Glycogen Utilization and Glucose Turnover during Moderate-Intensity Endurance Exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1120–R1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, S.P.; Zacher, C.M.; Mittleman, K.D. Effect of Menstrual Cycle Phase on Carbohydrate Supplementation during Prolonged Exercise to Fatigue. J. Appl. Physiol. 2000, 88, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Horton, T.J.; Miller, E.K.; Bourret, K. No Effect of Menstrual Cycle Phase on Glycerol or Palmitate Kinetics during 90 Min of Moderate Exercise. J. Appl. Physiol. 2006, 100, 917–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaiksaar, S.; Jürimäe, J.; Mäestu, J.; Purge, P.; Kalytka, S.; Shakhlina, L.; Jürimäe, T. No Effect of Menstrual Cycle Phase on Fuel Oxidation during Exercise in Rowers. Eur. J. Appl. Physiol. 2011, 111, 1027–1034. [Google Scholar] [CrossRef]
- Ortega-Santos, C.P.; Barba-Moreno, L.; Cupeiro, R.; Peinado, A.B. Substrate Oxidation in Female Adults during Endurance Exercise throughout Menstrual Cycle Phases: IronFEMME Pilot Study. J. Hum. Sport Exerc. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, E.; Glaister, M. The Effect of the Menstrual Cycle on Running Economy. J. Sports Med. Phys. Fit. 2020, 60, 610–617. [Google Scholar]
- Dokumacı, B.; Hazır, T. Effects of the Menstrual Cycle on Running Economy: Oxygen Cost Versus Caloric Cost. Res. Q. Exerc. Sport 2019, 90, 318–326. [Google Scholar] [CrossRef]
- Romero-Parra, N.; Barba-Moreno, L.; Rael, B.; Alfaro-Magallanes, V.M.; Cupeiro, R.; Díaz, Á.E.; Calderón, F.J.; Peinado, A.B. Influence of the Menstrual Cycle on Blood Markers of Muscle Damage and Inflammation Following Eccentric Exercise. Int. J. Environ. Res. Public Health 2020, 17, 1618. [Google Scholar] [CrossRef] [Green Version]
- Sipavičienė, S.; Daniusevičiutė, L.; Klizienė, I.; Kamandulis, S.; Skurvydas, A. Effects of Estrogen Fluctuation during the Menstrual Cycle on the Response to Stretch-Shortening Exercise in Females. BioMed Res. Int. 2013, 2013, 243572. [Google Scholar] [CrossRef] [PubMed]
- Gillum, T.; Kuennen, M.; Miller, T.; Riley, L. The Effects of Exercise, Sex, and Menstrual Phase on Salivary Antimicrobial Proteins. Exerc. Immunol. Rev. 2014, 20, 23–38. [Google Scholar] [PubMed]
- Rickenlund, A.; Eriksson, M.J.; Schenck-Gustafsson, K.; Hirschberg, A.L. Amenorrhea in Female Athletes Is Associated with Endothelial Dysfunction and Unfavorable Lipid Profile. J. Clin. Endocrinol. Metab. 2005, 90, 1354–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayres, S.; Baer, J.; Subbiah, M.T. Exercised-Induced Increase in Lipid Peroxidation Parameters in Amenorrheic Female Athletes. Fertil. Steril. 1998, 69, 73–77. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Ji, L.L. Antioxidant Enzyme Activity during Prolonged Exercise in Amenorrheic and Eumenorrheic Athletes. Metabolism 1991, 40, 88–92. [Google Scholar] [CrossRef]
- Suh, S.-H.; Casazza, G.A.; Horning, M.A.; Miller, B.F.; Brooks, G.A. Effects of Oral Contraceptives on Glucose Flux and Substrate Oxidation Rates during Rest and Exercise. J. Appl. Physiol. 2003, 94, 285–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isacco, L.; Duché, P.; Boisseau, N. Influence of Hormonal Status on Substrate Utilization at Rest and during Exercise in the Female Population. Sports Med. 2012, 42, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Isacco, L.; Thivel, D.; Pelle, A.M.; Zouhal, H.; Duclos, M.; Duche, P.; Boisseau, N. Oral Contraception and Energy Intake in Women: Impact on Substrate Oxidation during Exercise. Appl. Physiol. Nutr. Metab. 2012, 37, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Cauci, S.; Buligan, C.; Marangone, M.; Francescato, M.P. Oxidative Stress in Female Athletes Using Combined Oral Contraceptives. Sports Med. Open 2016, 2, 40. [Google Scholar] [CrossRef] [Green Version]
- Hicks, K.M.; Onambélé-Pearson, G.; Winwood, K.; Morse, C.I. Oral Contraceptive Pill Use and the Susceptibility to Markers of Exercise-Induced Muscle Damage. Eur. J. Appl. Physiol. 2017, 117, 1393–1402. [Google Scholar] [CrossRef]
- Romero-Parra, N.; Rael, B.; Alfaro-Magallanes, V.M.; Janse de Jonge, X.; Cupeiro, R.; Peinado, A.B.; IronFEMME Study Group. The Effect of the Oral Contraceptive Cycle Phase on Exercise-Induced Muscle Damage after Eccentric Exercise in Resistance-Trained Women. J. Strength Cond. Res. 2021, 35, 353–359. [Google Scholar] [CrossRef]
- Lei, T.-H.; Cotter, J.D.; Schlader, Z.J.; Stannard, S.R.; Perry, B.G.; Barnes, M.J.; Mündel, T. On Exercise Thermoregulation in Females: Interaction of Endogenous and Exogenous Ovarian Hormones. J. Physiol. 2019, 597, 71–88. [Google Scholar] [CrossRef] [Green Version]
- Available online: Https://Wada-Ama.Org/En/Athlete-Biological-Passport (accessed on 29 June 2021).
- Sottas, P.-E.; Robinson, N.; Rabin, O.; Saugy, M. The Athlete Biological Passport. Clin. Chem. 2011, 57, 969–976. [Google Scholar] [CrossRef]
- Sottas, P.-E.; Vernec, A. Current Implementation and Future of the Athlete Biological Passport. Bioanalysis 2012, 4, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Confounding Factors and Genetic Polymorphism in the Evaluation of Individual Steroid Profiling—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/24764553/ (accessed on 26 April 2021).
- Mareck-Engelke, U.; Geyer, H.; Donike, M. Stability of steroid profiles (4): The circadian rhythm of urinary ratios and excretion rates of endogenous steroids in female and its menstrual dependency. In Recent Advances in Doping Analysis; Sport und Buch Strauss: Köln, Germany, 1995; pp. 135–155. [Google Scholar]
- Mareck-Engelke, U.; Flenker, U.; Donike, M. Stability of steroid profiles (5): The annual rhythm of urinary ratios and excretion rates of endogenous steroids in female and its menstrual dependency. In Recent Advances in Doping Analysis; Sport und Buch Strauss: Köln, Germany, 1996; pp. 177–190. [Google Scholar]
- Mullen, J.; Thoerngren, J.-O.; Schulze, J.; Ericsson, M.; Gårevik, N.; Lehtihet, M.; Ekström, L. Urinary Steroid Profile in Females -the Impact of Menstrual Cycle and Emergency Contraceptives. Drug Test. Anal. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Suominen, T.; Bergström, H.; Ericsson, M.; Bergman, L.B.; Ekström, L. Urinary Steroid Profile in Relation to the Menstrual Cycle. Drug Test. Anal. 2021, 13, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Mullen, J.; Bækken, L.V.; Törmäkangas, T.; Ekström, L.; Ericsson, M.; Hullstein, I.R.; Schulze, J.J. Inter-Individual Variation of the Urinary Steroid Profiles in Swedish and Norwegian Athletes. Drug Test. Anal. 2020, 12, 720–730. [Google Scholar] [CrossRef]
- Elings Knutsson, J.; Andersson, A.; Baekken, L.V.; Pohanka, A.; Ekström, L.; Hirschberg, A.L. Disposition of Urinary and Serum Steroid Metabolites in Response to Testosterone Administration in Healthy Women. J. Clin. Endocrinol. Metab. 2021, 106, 697–707. [Google Scholar] [CrossRef]
- Ekström, L.; Knutsson, J.E.; Mullen, J.; Ericsson, M.; Hirschberg, A.L. Impact of Hormonal Contraceptives on Urinary Steroid Profile in Relation to Serum Hormone Changes and CYP17A1 Polymorphism. Drug Test. Anal. 2019, 11, 1284–1289. [Google Scholar] [CrossRef]
- Schulze, J.J.; Mullen, J.E.; Berglund Lindgren, E.; Ericsson, M.; Ekström, L.; Hirschberg, A.L. The Impact of Genetics and Hormonal Contraceptives on the Steroid Profile in Female Athletes. Front. Endocrinol. 2014, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Buisson, C.; Frelat, C.; Privat, K.; Martinat, N.; Audran, M.; Collomp, K. Metabolic and Isotopic Signature of Short-Term DHEA Administration in Women: Comparison with Findings in Men. Drug Test. Anal. 2018, 10, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Flenker, U.; Mareck, U.; Platen, P.; Piper, T.; Schmechel, A. The detection of the misuse of testosterone gel. In Recent Advances in Doping Analysis; Sport und Buch Strauss: Köln, Germany, 2007; pp. 133–142. [Google Scholar]
- Sensitivity of Doping Biomarkers after Administration of a Single Dose Testosterone Gel—Mullen—2018—Drug Testing and Analysis—Wiley Online Library. Available online: https://0-analyticalsciencejournals-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/10.1002/dta.2341 (accessed on 26 April 2021).
- Börjesson, A.; Lehtihet, M.; Andersson, A.; Dahl, M.-L.; Vicente, V.; Ericsson, M.; Ekström, L. Studies of Athlete Biological Passport Biomarkers and Clinical Parameters in Male and Female Users of Anabolic Androgenic Steroids and Other Doping Agents. Drug Test. Anal. 2020, 12, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Detection of Testosterone Doping in Female Athletes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31454165/ (accessed on 26 April 2021).
- Xing, Y.; Liu, X.; Yan, M.; Chen, T.; Lu, F.; Xu, B.; Gong, Y.; Chu, F.; Lei, H. Impact of Nonsteroidal Aromatase Inhibitors on Steroid Profile in a Chinese Population. Medicine 2017, 96, e7411. [Google Scholar] [CrossRef]
- Bornø, A.; Aachmann-Andersen, N.J.; Munch-Andersen, T.; Hulston, C.J.; Lundby, C. Screening for Recombinant Human Erythropoietin Using [Hb], Reticulocytes, the OFF(Hr Score), OFF(z Score) and Hb(z Score): Status of the Blood Passport. Eur. J. Appl. Physiol. 2010, 109, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Sottas, P.; Robinson, N.; Giraud, S.; Taroni, F.; Kamber, M.; Mangin, P.; Saugy, M. Statistical Classification of Abnormal Blood Profiles in Athletes. Int. J. Biostat. 2006, 2, 1011. [Google Scholar] [CrossRef]
- Mullen, J.; Baekken, L.; Bergström, H.; Björkhem Bergman, L.; Ericsson, M.; Ekström, L. Fluctuations in Hematological Athlete Biological Passport Biomarkers in Relation to the Menstrual Cycle. Drug Test. Anal. 2020, 12, 1229–1240. [Google Scholar] [CrossRef]
- The Intra-Individual Stability of GH Biomarkers IGF-I and P-III-NP in Relation to GHRH Administration, Menstrual Cycle, and Hematological Parameters—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33125822/ (accessed on 26 April 2021).
- Vermersch, P. Describing the Practice of Introspection. J. Conscious. Stud. 2009, 16, 20–57. [Google Scholar]
- Robbins, P.; Aydede, M. (Eds.) The Cambridge Handbook of Situated Cognition; Cambridge Handbooks in Psychology; Cambridge University Press: Cambridge, UK, 2008; ISBN 978-0-521-84832-9. [Google Scholar]
- Briki, W.; Hartigh, R.J.R.D.; Hauw, D.; Gernigon, C. A Qualitative Exploration of the Psychological Contents and Dynamics of Momentum in Sport. Int. J. Sport Psychol. 2012, 43, 365–384. [Google Scholar] [CrossRef]
- Hauw, D.; Durand, M. Situated Analysis of Elite Trampolinists’ Problems in Competition Using Retrospective Interviews. J. Sports Sci. 2007, 25, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Villemain, A.; Hauw, D. A Situated Analysis of Football Goalkeepers’ Experiences in Critical Game Situations. Percept. Mot. Ski. 2014, 119, 811–824. [Google Scholar] [CrossRef]
- Krabak, B.J.; Waite, B.; Schiff, M.A. Study of Injury and Illness Rates in Multiday Ultramarathon Runners. Med. Sci. Sports Exerc. 2011, 43, 2314–2320. [Google Scholar] [CrossRef]
- Baldassarre, R.; Bonifazi, M.; Zamparo, P.; Piacentini, M.F. Characteristics and Challenges of Open-Water Swimming Performance: A Review. Int. J. Sports Physiol. Perform. 2017, 12, 1275–1284. [Google Scholar] [CrossRef]
- Giorgis-Allemand, L.; Thalabard, J.C.; Rosetta, L.; Siroux, V.; Bouyer, J.; Slama, R. Can Atmospheric Pollutants Influence Menstrual Cycle Function? Environ. Pollut. 2020, 257, 113605. [Google Scholar] [CrossRef] [PubMed]
- Merklinger-Gruchala, A.; Jasienska, G.; Kapiszewska, M. Effect of Air Pollution on Menstrual Cycle Length-A Prognostic Factor of Women’s Reproductive Health. Int. J. Environ. Res. Public Health 2017, 14, 816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, A.B.; Whitworth, K.W.; Haug, L.S.; Sabaredzovic, A.; Impinen, A.; Papadopoulou, E.; Longnecker, M.P. Menstrual Cycle Characteristics as Determinants of Plasma Concentrations of Perfluoroalkyl Substances (PFASs) in the Norwegian Mother and Child Cohort (MoBa Study). Environ. Res. 2018, 166, 78–85. [Google Scholar] [CrossRef] [PubMed]
| Variables | NMC Women | COC Women |
|---|---|---|
| Postural control | No consensus | Too few studies |
| Motor control | No consensus | Too few studies |
| Strength and resistance training | No consensusConsensus? | No consensus |
| Variables | NMC Women | AM Women | COC Women |
|---|---|---|---|
| Cardiac function and hemodynamics | Rest: No consensus | Too few studies
| Rest: No consensus |
| Exercise: No consensus | Exercise: Too few studies | ||
| Autonomic control | Consensus | Too few studies
| Too few studies
|
| NMC Women | AM Women | COC Women | |
|---|---|---|---|
| TES | Rest: No consensus | Too few studies
| Rest: Consensus
|
| GH | Consensus | No consensus
| Consensus |
| IGF-1 | No consensus | Too few studies
| Too few studies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castanier, C.; Bougault, V.; Teulier, C.; Jaffré, C.; Schiano-Lomoriello, S.; Vibarel-Rebot, N.; Villemain, A.; Rieth, N.; Le-Scanff, C.; Buisson, C.; et al. The Specificities of Elite Female Athletes: A Multidisciplinary Approach. Life 2021, 11, 622. https://0-doi-org.brum.beds.ac.uk/10.3390/life11070622
Castanier C, Bougault V, Teulier C, Jaffré C, Schiano-Lomoriello S, Vibarel-Rebot N, Villemain A, Rieth N, Le-Scanff C, Buisson C, et al. The Specificities of Elite Female Athletes: A Multidisciplinary Approach. Life. 2021; 11(7):622. https://0-doi-org.brum.beds.ac.uk/10.3390/life11070622
Chicago/Turabian StyleCastanier, Carole, Valérie Bougault, Caroline Teulier, Christelle Jaffré, Sandrine Schiano-Lomoriello, Nancy Vibarel-Rebot, Aude Villemain, Nathalie Rieth, Christine Le-Scanff, Corinne Buisson, and et al. 2021. "The Specificities of Elite Female Athletes: A Multidisciplinary Approach" Life 11, no. 7: 622. https://0-doi-org.brum.beds.ac.uk/10.3390/life11070622