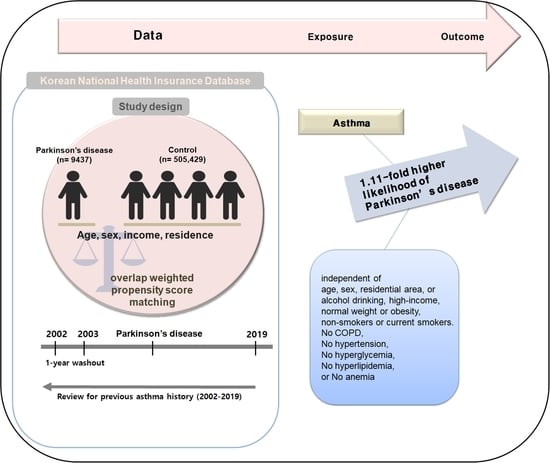
Possible Incidental Parkinson’s Disease following Asthma: A Nested Case–Control Study in Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Parkinson’s Disease (Outcome)
2.3. Asthma (Exposure)
2.4. Participant Selection
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Association of Asthma with Parkinson’s Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Rijk, M.C.; Launer, L.J.; Berger, K.; Breteler, M.M.; Dartigues, J.F.; Baldereschi, M.; Fratiglioni, L.; Lobo, A.; Martinez-Lage, J.; Trenkwalder, C.; et al. Prevalence of Parkinson’s disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000, 54, S21–S23. [Google Scholar] [PubMed]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, D.H.; Kwon, D.Y.; Choi, M.; Kim, S.; Jung, J.H.; Han, K.; Park, Y.G. Trends in the incidence and prevalence of Parkinson’s disease in Korea: A nationwide, population-based study. BMC Geriatr. 2019, 19, 320. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.R.; Bower, J.H.; Ahlskog, J.E.; Rocca, W.A. Time Trends in the Incidence of Parkinson Disease. JAMA Neurol. 2016, 73, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.M.; Wu, R.M.; Lin, J.W.; Liu, Y.C.; Chang, C.H.; Lin, C.H. Time trends in the prevalence and incidence of Parkinson’s disease in Taiwan: A nationwide, population-based study. J. Med. Assoc. 2016, 115, 531–538. [Google Scholar] [CrossRef]
- Horsfall, L.; Petersen, I.; Walters, K.; Schrag, A. Time trends in incidence of Parkinson’s disease diagnosis in UK primary care. J. Neurol. 2013, 260, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, S.K.; Koudstaal, P.J.; Stricker, B.H.; Hofman, A.; Ikram, M.A. Trends in the Incidence of Parkinson Disease in the General Population: The Rotterdam Study. Am. J. Epidemiol. 2016, 183, 1018–1026. [Google Scholar] [CrossRef]
- Sears, M.R. Trends in the prevalence of asthma. Chest 2014, 145, 219–225. [Google Scholar] [CrossRef]
- Forno, E.; Celedon, J.C. Asthma and ethnic minorities: Socioeconomic status and beyond. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 154–160. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Prim. 2015, 1, 15025. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Sohn, K.H.; Shin, J.E.; Park, M.; Lim, J.; Lee, J.Y.; Yang, M.S. Changing patterns of adult asthma incidence: Results from the National Health Insurance Service-National Sample Cohort (NHIS-NSC) database in Korea. Sci. Rep. 2018, 8, 15052. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Pereira, A.M.; Morais-Almeida, M. Asthma costs and social impact. Asthma Res. Pract. 2017, 3, 1. [Google Scholar] [CrossRef]
- Gumber, A.; Ramaswamy, B.; Thongchundee, O. Effects of Parkinson’s on employment, cost of care, and quality of life of people with condition and family caregivers in the UK: A systematic literature review. Patient Relat. Outcome Meas. 2019, 10, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Wu, Y.H.; Tsai, S.J.; Bai, Y.M.; Hsu, J.W.; Huang, K.L.; Su, T.P.; Li, C.T.; Tsai, C.F.; Yang, A.C.; et al. Risk of developing Parkinson’s disease among patients with asthma: A nationwide longitudinal study. Allergy 2015, 70, 1605–1612. [Google Scholar] [CrossRef]
- Yeh, J.J.; Wei, Y.F.; Lin, C.L.; Hsu, W.H. Effect of the asthma-chronic obstructive pulmonary disease syndrome on the stroke, Parkinson’s disease, and dementia: A national cohort study. Oncotarget 2018, 9, 12418–12431. [Google Scholar] [CrossRef]
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharm. 2014, 88, 640–651. [Google Scholar] [CrossRef]
- Svenningsen, S.; Nair, P. Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Front. Med. 2017, 4, 158. [Google Scholar] [CrossRef]
- Christiansen, S.C.; Schatz, M.; Yang, S.J.; Ngor, E.; Chen, W.; Zuraw, B.L. Hypertension and Asthma: A Comorbid Relationship. J. Allergy Clin. Immunol. Pract. 2016, 4, 76–81. [Google Scholar] [CrossRef]
- Perez, M.K.; Piedimonte, G. Metabolic asthma: Is there a link between obesity, diabetes, and asthma? Immunol. Allergy Clin. N. Am. 2014, 34, 777–784. [Google Scholar] [CrossRef]
- Wang, L.; Gao, S.; Yu, M.; Sheng, Z.; Tan, W. Association of asthma with coronary heart disease: A meta analysis of 11 trials. PLoS ONE 2017, 12, e0179335. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Tsai, S.J.; Perng, C.L.; Kuo, B.I.; Yang, A.C. Risk of Parkinson disease after depression: A nationwide population-based study. Neurology 2013, 81, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.H.; Maraganore, D.M.; Peterson, B.J.; Ahlskog, J.E.; Rocca, W.A. Immunologic diseases, anti-inflammatory drugs, and Parkinson disease: A case-control study. Neurology 2006, 67, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, D.; Jiang, S.; Mao, J.; Yang, X. NF-kappaB is negatively associated with Nurr1 to reduce the inflammatory response in Parkinson’s disease. Mol. Med. Rep. 2021, 23, 396. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Uh, I.; Kim, K.S.; Kim, K.H.; Park, J.; Kim, Y.; Jung, J.H.; Jung, H.J.; Jang, H.J. Anti-Inflammatory Effects of Ginsenoside Rg3 via NF-kappaB Pathway in A549 Cells and Human Asthmatic Lung Tissue. J. Immunol. Res. 2016, 2016, 7521601. [Google Scholar] [CrossRef]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Williams, G.P.; Schonhoff, A.M.; Jurkuvenaite, A.; Gallups, N.J.; Standaert, D.G.; Harms, A.S. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 2021, 144, 2047–2059. [Google Scholar] [CrossRef]
- Fellner, L.; Irschick, R.; Schanda, K.; Reindl, M.; Klimaschewski, L.; Poewe, W.; Wenning, G.K.; Stefanova, N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 2013, 61, 349–360. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, J.H.; Kim, J.H.; Park, H.R.; Kim, N.Y.; Hong, S.; Choi, H.G. Incident Rheumatoid Arthritis Following Statin Use: From the View of a National Cohort Study in Korea. J. Pers. Med. 2022, 12, 559. [Google Scholar] [CrossRef]
- Choi, H.G.; Kang, H.S.; Lim, H.; Kim, J.H.; Kim, J.H.; Cho, S.J.; Nam, E.S.; Min, K.W.; Park, H.Y.; Kim, N.Y.; et al. Changes in the Incidence Rates of Gastrointestinal Diseases Due to the COVID-19 Pandemic in South Korea: A Long-Term Perspective. J. Pers. Med. 2022, 12, 1144. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional Association Between GERD and Asthma: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1005–1013.e9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Oh, D.J.; Park, B.; Choi, H.G. Bell’s palsy and obesity, alcohol consumption and smoking: A nested case-control study using a national health screening cohort. Sci. Rep. 2020, 10, 4248. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.H.; Kim, Y.; Kim, K.; Oh, Y.M.; Yoo, K.H.; Rhee, C.K.; Yoon, H.K.; Kim, Y.S.; Park, Y.B.; et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: A national cross-sectional cohort study. BMC Pulm. Med. 2013, 13, 51. [Google Scholar] [CrossRef]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Li, F.; Morgan, K.L.; Zaslavsky, A.M. Balancing Covariates via Propensity Score Weighting. J. Am. Stat. Assoc. 2018, 113, 390–400. [Google Scholar] [CrossRef]
- Nadeem, H.; Zhou, B.; Goldman, D.; Romley, J. Association between use of ss2-adrenergic receptor agonists and incidence of Parkinson’s disease: Retrospective cohort analysis. PLoS ONE 2022, 17, e0276368. [Google Scholar] [CrossRef]
- Mittal, S.; Bjornevik, K.; Im, D.S.; Flierl, A.; Dong, X.; Locascio, J.J.; Abo, K.M.; Long, E.; Jin, M.; Xu, B.; et al. beta2-Adrenoreceptor is a regulator of the alpha-synuclein gene driving risk of Parkinson’s disease. Science 2017, 357, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sadatsafavi, M.; Tavakoli, H.; Samii, A.; Etminan, M. Effects of beta2-Adrenergic Agonists on Risk of Parkinson’s Disease in COPD: A Population-Based Study. Pharmacotherapy 2020, 40, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Yssel, J.D.; McNamara, C.; Harkin, A. Pharmacological targeting of beta(2) -adrenoceptors is neuroprotective in the LPS inflammatory rat model of Parkinson’s disease. Br. J. Pharm. 2020, 177, 282–297. [Google Scholar] [CrossRef]
- Choi, D.; Lee, G.; Kim, K.H.; Bae, H. Particulate Matter Exacerbates the Death of Dopaminergic Neurons in Parkinson’s Disease through an Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 6487. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, I.Z. Free Radicals and Oxidative Stress: Signaling Mechanisms, Redox Basis for Human Diseases, and Cell Cycle Regulation. Curr. Mol. Med. 2023, 23, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, D.; Feng, Y.; Wu, W.; Gao, J.; Chang, C.; Chen, S.; Zhen, G. Identification of Key Signaling Pathways and Genes in Eosinophilic Asthma and Neutrophilic Asthma by Weighted Gene Co-Expression Network Analysis. Front. Mol. Biosci. 2022, 9, 805570. [Google Scholar] [CrossRef] [PubMed]
- Golovina, E.; Fadason, T.; Jaros, R.K.; Kumar, H.; John, J.; Burrowes, K.; Tawhai, M.; O’Sullivan, J.M. De novo discovery of traits co-occurring with chronic obstructive pulmonary disease. Life Sci. Alliance 2023, 6, e202201609. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Zhang, X.; Yuan, Y.L.; Chen, Z.H.; Chen-Yu Hsu, A.; Oliver, B.G.; Xie, M.; Qin, L.; Li, W.M.; et al. Dyslipidemia Is Associated with Worse Asthma Clinical Outcomes: A Prospective Cohort Study. J. Allergy Clin. Immunol. Pract. 2022. [Google Scholar] [CrossRef]
- Li, L.Y.; Liu, S.F.; Zhuang, J.L.; Li, M.M.; Huang, Z.P.; Chen, Y.H.; Chen, X.R.; Chen, C.N.; Lin, S.; Ye, L.C. Recent research progress on metabolic syndrome and risk of Parkinson’s disease. Rev. Neurosci. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, I.B.; Kim, Y.H.; Kwon, M.J.; Kim, J.H.; Choi, H.G. Association between statin use and Parkinson’s disease in Korean patients with hyperlipidemia. Park. Relat. Disord. 2022, 97, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.H.; Li, Z.F.; An, Z.Y.; Huang, S.C.; Hao, M.D.; Zhang, W.X. Meta-Analysis of the Association Between Asthma and the Risk of Stroke. Front. Neurol. 2022, 13, 900438. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, L.; Zhang, Y.; Xie, A. Association Between Stroke and Parkinson’s Disease: A Meta-analysis. J. Mol. Neurosci. 2020, 70, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Ogbu, C.E.; Ravilla, J.; Okoli, M.L.; Ahaiwe, O.; Ogbu, S.C.; Kim, E.S.; Kirby, R.S. Association of Depression, Poor Mental Health Status and Asthma Control Patterns in US Adults Using a Data-Reductive Latent Class Method. Cureus 2023, 15, e33966. [Google Scholar] [CrossRef]


| Characteristics | Before Overlap Weighting Adjustment | After Overlap Weighting Adjustment | ||||
|---|---|---|---|---|---|---|
| Parkinson’s Disease | Control | Standardized Difference | Parkinson’s Disease | Control | Standardized Difference | |
| Age (n, %) | 0.00 | 0.00 | ||||
| 40–44 | 8 (0.09) | 32 (0.09) | 6 (0.09) | 6 (0.09) | ||
| 45–49 | 75 (0.83) | 300 (0.83) | 59 (0.83) | 59 (0.83) | ||
| 50–54 | 243 (2.69) | 972 (2.69) | 191 (2.68) | 191 (2.68) | ||
| 55–59 | 514 (5.69) | 2056 (5.69) | 404 (5.68) | 404 (5.68) | ||
| 60–64 | 926 (10.26) | 3704 (10.26) | 726 (10.20) | 726 (10.20) | ||
| 65–69 | 1388 (15.37) | 5552 (15.37) | 1093 (15.36) | 1093 (15.36) | ||
| 70–74 | 2012 (22.28) | 8048 (22.28) | 1583 (22.25) | 1583 (22.25) | ||
| 75–79 | 2158 (23.90) | 8632 (23.90) | 1702 (23.93) | 1702 (23.93) | ||
| 80–84 | 1299 (14.39) | 5196 (14.39) | 1027 (14.44) | 1027 (14.44) | ||
| 85+ | 406 (4.50) | 1624 (4.50) | 322 (4.53) | 322 (4.53) | ||
| Sex (n, %) | 0.00 | 0.00 | ||||
| Male | 4313 (47.77) | 17,252 (47.77) | 3397 (47.77) | 3397 (47.77) | ||
| Female | 4716 (52.23) | 18,864 (52.23) | 3715 (52.23) | 3715 (52.23) | ||
| Income (n, %) | 0.00 | 0.00 | ||||
| 1 (lowest) | 1663 (18.42) | 6652 (18.42) | 1308 (18.38) | 1308 (18.38) | ||
| 2 | 985 (10.91) | 3940 (10.91) | 777 (10.92) | 777 (10.92) | ||
| 3 | 1206 (13.36) | 4824 (13.36) | 950 (13.36) | 950 (13.36) | ||
| 4 | 1739 (19.26) | 6956 (19.26) | 1370 (19.26) | 1370 (19.26) | ||
| 5 (highest) | 3436 (38.06) | 13,744 (38.06) | 2708 (38.07) | 2708 (38.07) | ||
| Region of residence (n, %) | 0.00 | 0.00 | ||||
| Urban | 3395 (37.60) | 13,580 (37.60) | 2672 (37.57) | 2672 (37.57) | ||
| Rural | 5634 (62.40) | 22,536 (62.40) | 4440 (62.43) | 4440 (62.43) | ||
| Obesity † (n, %) | 0.02 | 0.00 | ||||
| Underweight | 333 (3.69) | 1321 (3.66) | 262 (3.68) | 262 (3.68) | ||
| Normal | 3183 (35.25) | 12,994 (35.98) | 2519 (35.41) | 2519 (35.41) | ||
| Overweight | 2371 (26.26) | 9445 (26.15) | 1869 (26.28) | 1869 (26.28) | ||
| Obese I | 2834 (31.39) | 11,205 (31.03) | 2224 (31.28) | 2224 (31.28) | ||
| Obese II | 308 (3.41) | 1151 (3.19) | 238 (3.35) | 238 (3.35) | ||
| Smoking status (n, %) | 0.09 | 0.00 | ||||
| Non-smoker | 7005 (77.58) | 26,777 (74.14) | 5471 (76.92) | 5471 (76.92) | ||
| Past smoker | 1202 (13.31) | 5186 (14.36) | 964 (13.55) | 964 (13.55) | ||
| Current smoker | 822 (9.10) | 4153 (11.50) | 678 (9.53) | 678 (9.53) | ||
| Alcohol consumption (n, %) | 0.10 | 0.00 | ||||
| <1 time a week | 6544 (72.48) | 24,535 (67.93) | 5090 (71.57) | 5090 (71.57) | ||
| ≥1 time a week | 2485 (27.52) | 11,581 (32.07) | 2022 (28.43) | 2022 (28.43) | ||
| SBP (Mean, SD) | 129.86 (17.41) | 129.96 (16.90) | 0.01 | 129.88 (15.44) | 129.88 (7.52) | 0.00 |
| DBP (Mean, SD) | 78.33 (10.78) | 78.26 (10.51) | 0.01 | 78.31 (9.55) | 78.31 (4.69) | 0.00 |
| FBG (Mean, SD) | 106.82 (36.23) | 103.59 (29.56) | 0.10 | 105.86 (29.91) | 105.86 (15.27) | 0.00 |
| Total cholesterol (Mean, SD) | 193.89 (43.68) | 195.53 (40.55) | 0.04 | 194.24 (39.21) | 194.24 (17.77) | 0.00 |
| Hemoglobin (Mean, SD) | 13.45 (1.49) | 13.52 (1.49) | 0.05 | 13.47 (1.32) | 13.47 (0.67) | 0.00 |
| CCI score (Mean, SD) | 1.73 (1.90) | 1.24 (1.81) | 0.27 | 1.62 (1.61) | 1.62 (0.95) | 0.00 |
| COPD (n, %) | 1198 (13.27) | 4235 (11.73) | 0.05 | 920 (12.94) | 920 (12.94) | 0.00 |
| Asthma (n, %) | 2624 (29.06) | 9697 (26.85) | 0.05 | 2062 (28.99) | 1930 (27.13) | 0.04 |
| Characteristics | N of Parkinson’s Disease | N of Control | Odds Ratios for Parkinson’s Disease (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude † | p-Value | Overlap-Weighted Model † | p-Value | |
| Total participants (n = 45,145) | ||||||
| Non-asthma | 6405/9029 (70.9) | 26,419/36,116 (73.2) | 1 | 1 | ||
| Asthma | 2624/9029 (29.1) | 9697/36,116 (26.8) | 1.12 (1.06–1.17) | <0.001 * | 1.11 (1.06–1.16) | <0.001 * |
| Age < 75 years old (n = 25,830) | ||||||
| Non-asthma | 3876/5166 (75.0) | 16,034/20,664 (77.6) | 1 | 1 | ||
| Asthma | 1290/5166 (25.0) | 4630/20,664 (22.4) | 1.15 (1.07–1.24) | <0.001 * | 1.15 (1.08–1.22) | <0.001 * |
| Age ≥ 75 years old (n = 19,315) | ||||||
| Non-asthma | 2529/3863 (65.5) | 10,385/15,452 (67.2) | 1 | 1 | ||
| Asthma | 1334/3863 (34.5) | 5067/15,452 (32.8) | 1.08 (1.00–1.16) | 0.04 * | 1.07 (1.00–1.14) | 0.041 * |
| Male (n = 21,565) | ||||||
| Non-asthma | 3183/4313 (73.8) | 13,110/17,252 (76.0) | 1 | 1 | ||
| Asthma | 1130/4313 (26.2) | 4142/17,252 (24.0) | 1.12 (1.04–1.21) | 0.003 * | 1.12 (1.05–1.19) | 0.001 * |
| Female (n = 23,580) | ||||||
| Non-asthma | 3222/4716 (68.3) | 13,309/18,864 (70.6) | 1 | 1 | ||
| Asthma | 1494/4716 (31.7) | 5555/18,864 (29.4) | 1.11 (1.04–1.19) | 0.003 * | 1.09 (1.03–1.16) | 0.002 * |
| Low income group (n = 19,270) | ||||||
| Non-asthma | 2758/3854 (71.6) | 11,282/15,416 (73.2) | 1 | 1 | ||
| Asthma | 1096/3854 (28.4) | 4134/15,416 (26.8) | 1.08 (1.00–1.17) | 0.043 * | 1.06 (0.99–1.13) | 0.101 |
| High income group (n = 25,875) | ||||||
| Non-asthma | 3647/5175 (70.5) | 15,137/20,700 (73.1) | 1 | 1 | ||
| Asthma | 1528/5175 (29.5) | 5563/20,700 (26.9) | 1.14 (1.07–1.22) | <0.001 * | 1.15 (1.08–1.21) | <0.001 * |
| Urban resident (n = 16,975) | ||||||
| Non-asthma | 2430/3395 (71.6) | 10,017/13,580 (73.8) | 1 | 1 | ||
| Asthma | 965/3395 (28.4) | 3563/13,580 (26.2) | 1.12 (1.03–1.21) | 0.01 * | 1.12 (1.04–1.20) | 0.003 * |
| Rural resident (n = 28,170) | ||||||
| Non-asthma | 3975/5634 (70.6) | 16,402/22,536 (72.8) | 1 | 1 | ||
| Asthma | 1659/5634 (29.4) | 6134/22,536 (27.2) | 1.12 (1.05–1.19) | <0.001 * | 1.10 (1.05–1.17) | <0.001 * |
| Characteristics | N of Parkinson’s Disease | N of Control | Odds Ratios for Parkinson’s Disease (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude † | p-Value | Overlap-Weighted Model † | p-Value | |
| Obesity | ||||||
| Underweight (n = 1654) | 88/333 (26.4) | 379/1321 (28.7) | 0.89 (0.68–1.17) | 0.412 | 0.94 (0.74–1.20) | 0.623 |
| Normal (n = 16,177) | 904/3183 (28.4) | 3246/12,994 (25.0) | 1.19 (1.09–1.30) | <0.001 * | 1.20 (1.12–1.29) | <0.001 * |
| Overweight (n = 11,816) | 637/2371 (26.9) | 2471/9445 (26.2) | 1.04 (0.94–1.15) | 0.484 | 1.00 (0.92–1.09) | 0.995 |
| Obese (n = 15,498) | 995/3142 (31.7) | 3601/12,356 (29.1) | 1.13 (1.04–1.23) | 0.006 * | 1.12 (1.04–1.20) | 0.003 * |
| Smoking status | ||||||
| Non-smoker (n = 33,782) | 2062/7005 (29.4) | 7370/26,777 (27.5) | 1.10 (1.04–1.16) | 0.001 * | 1.11 (1.05–1.16) | <0.001 * |
| Past smoker (n = 6388) | 330/1202 (27.5) | 1398/5186 (27.0) | 1.03 (0.89–1.18) | 0.726 | 1.02 (0.90–1.15) | 0.793 |
| Current smoker (n = 4975) | 232/822 (28.2) | 929/4153 (22.4) | 1.36 (1.15–1.62) | <0.001 * | 1.25 (1.09–1.44) | 0.002 * |
| Alcohol consumption | ||||||
| <1 time a week (n = 31,079) | 1924/6544 (29.4) | 6725/24,535 (27.4) | 1.10 (1.04–1.17) | 0.001 * | 1.11 (1.05–1.17) | <0.001 * |
| ≥1 time a week (n = 14,066) | 700/2485 (28.2) | 2972/11,581 (25.7) | 1.14 (1.03–1.25) | 0.010 * | 1.10 (1.02–1.19) | 0.020 * |
| Blood pressure (mmHg) | ||||||
| SBP < 140 and DBP < 90 (n = 31,370) | 1874/6223 (30.1) | 6826/25,147 (27.1) | 1.16 (1.09–1.23) | <0.001 * | 1.14 (1.09–1.20) | <0.001 * |
| SBP ≥ 140 or DBP ≥ 90 (n = 13,775) | 750/2806 (26.7) | 2871/10,969 (26.2) | 1.03 (0.94–1.13) | 0.550 | 1.03 (0.95–1.12) | 0.453 |
| Fasting blood glucose | ||||||
| <100 mg/dL (n = 25,211) | 1422/4758 (29.9) | 5451/20,453 (26.7) | 1.17 (1.09–1.26) | <0.001 * | 1.16 (1.10–1.23) | <0.001 * |
| ≥100 mg/dL (n = 19,934) | 1202/4271 (28.1) | 4246/15,663 (27.1) | 1.05 (0.98–1.14) | 0.179 | 1.05 (0.98–1.12) | 0.175 |
| Total cholesterol | ||||||
| <240 mg/dL (n = 39,364) | 2290/7862 (29.1) | 8498/31,502 (27.0) | 1.11 (1.05–1.18) | <0.001 * | 1.11 (1.06–1.16) | <0.001 * |
| 240 mg/dL (n = 5781) | 334/1167 (28.6) | 1199/4614 (26.0) | 1.14 (0.99–1.32) | 0.069 | 1.11 (0.98–1.25) | 0.100 |
| Hemoglobin ≥ (g/dL) | ||||||
| ≥12 for men and ≥10 for women (n = 43,653) | 2521/8695 (29.0) | 9363/34,958 (26.8) | 1.12 (1.06–1.18) | <0.001 * | 1.11 (1.06–1.16) | <0.001 * |
| <12 for men and <10 for women (n = 1492) | 103/334 (30.8) | 334/1158 (28.8) | 1.10 (0.84–1.43) | 0.480 | 1.06 (0.83–1.36) | 0.629 |
| CCI scores | ||||||
| 0 (n = 21,447) | 820/2950 (27.8) | 4592/18,497 (24.8) | 1.17 (1.07–1.27) | <0.001 * | 1.18 (1.11–1.26) | <0.001 * |
| 1 (n = 8638) | 568/2177 (26.1) | 1937/6461 (30.0) | 0.82 (0.74–0.92) | <0.001 * | 0.86 (0.77–0.95) | 0.002 * |
| ≥2 (n = 15,060) | 1236/3902 (31.7) | 3168/11,158 (28.4) | 1.17 (1.08–1.27) | <0.001 * | 1.20 (1.11–1.29) | <0.001 * |
| COPD history | ||||||
| No (n = 39,712) | 1880/7831 (24.0) | 7088/31,881 (22.2) | 1.11 (1.04–1.17) | <0.001 * | 1.12 (1.07–1.18) | <0.001 * |
| Yes (n = 5433) | 744/1198 (62.1) | 2609/4235 (61.6) | 1.02 (0.89–1.17) | 0.755 | 1.04 (0.93–1.16) | 0.475 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.J.; Kim, J.-H.; Kang, H.S.; Lim, H.; Kim, M.-J.; Kim, N.Y.; Kim, S.H.; Choi, H.G.; Kim, E.S. Possible Incidental Parkinson’s Disease following Asthma: A Nested Case–Control Study in Korea. J. Pers. Med. 2023, 13, 718. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm13050718
Kwon MJ, Kim J-H, Kang HS, Lim H, Kim M-J, Kim NY, Kim SH, Choi HG, Kim ES. Possible Incidental Parkinson’s Disease following Asthma: A Nested Case–Control Study in Korea. Journal of Personalized Medicine. 2023; 13(5):718. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm13050718
Chicago/Turabian StyleKwon, Mi Jung, Joo-Hee Kim, Ho Suk Kang, Hyun Lim, Min-Jeong Kim, Nan Young Kim, Se Hoon Kim, Hyo Geun Choi, and Eun Soo Kim. 2023. "Possible Incidental Parkinson’s Disease following Asthma: A Nested Case–Control Study in Korea" Journal of Personalized Medicine 13, no. 5: 718. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm13050718