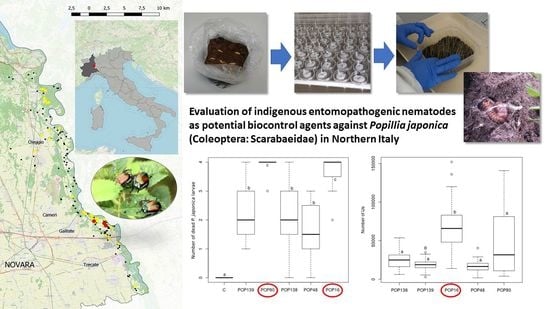
Evaluation of Indigenous Entomopathogenic Nematodes as Potential Biocontrol Agents against Popillia japonica (Coleoptera: Scarabaeidae) in Northern Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Field Sampling and Isolation of Nematodes
2.2. Nematode Identification
2.3. Laboratory Virulence Assays
2.4. Semi-Field Virulence Assay
2.5. Statistical Analysis
3. Results
3.1. Field Sampling and Isolation of Nematodes
3.2. Nematode Identification
3.3. Laboratory Virulence Assays
3.4. Semi-Field Virulence Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potter, D.A.; Held, D.W. Biology and Management of the Japanese Beetle. Annu. Rev. Entomol. 2002, 47, 175–205. [Google Scholar] [CrossRef] [Green Version]
- Fleming, W.E. Biology of the Japanese Beetle (Technical Bulletin 1449); US Department of Agriculture: Washington, DC, USA, 1972.
- USDA/APHIS. Managing the Japanese Beetle: A Homeowner’s Handbook; US Department of Agriculture: Washington, DC, USA, 2000.
- Kistner-Thomas, E.J. The Potential Global Distribution and Voltinism of the Japanese Beetle (Coleoptera: Scarabaeidae) Under Current and Future Climates. J. Insect Sci. 2019, 19. [Google Scholar] [CrossRef]
- Pavesi, M. Popillia japonica specie aliena invasiva segnalata in Lombardia. L’informatore Agrario 2014, 32, 53–55. [Google Scholar]
- EPPO/OEPP. Update on the Situation of Popillia japonica in Italy; EPPO Reporting Service: Paris, France, 2020. [Google Scholar]
- Marianelli, L.; Paoli, F.; Sabbatini Peverieri, G.; Benvenuti, C.; Barzanti, G.P.; Bosio, G.; Venanzio, D.; Giacometto, E.; Roversi, P.F. Long-lasting insecticide-treated nets: A new integrated pest management approach for Popillia japonica (Coleoptera: Scarabaeidae). Integr. Environ. Assess. 2019, 15, 259–265. [Google Scholar] [CrossRef]
- Redmond, C.T.; Potter, D.A. Incidence of Turf-Damaging White Grubs (Coleoptera: Scarabaeidae) and Associated Pathogens and Parasitoids on Kentucky Golf Courses. Environ. Entomol. 2010, 39, 1838–1847. [Google Scholar] [CrossRef] [Green Version]
- Behle, R.W.; Richmond, D.S.; Jackson, M.A.; Dunlap, C.A. Evaluation of Metarhizium brunneum F52 (Hypocreales: Clavicipitaceae) for Control of Japanese Beetle Larvae in Turfgrass. J. Econ. Entomol. 2015, 108, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Helmberger, M.S.; Thaler, J.S.; Shields, E.J.; Wickings, K.G. Entomopathogenic nematode performance against Popillia japonica (Coleoptera: Scarabaeidae) in school athletic turf: Effects of traffic and soil properties. Biol. Control 2018, 126, 177–184. [Google Scholar] [CrossRef]
- Klein, M.G.; Kaya, H.K. Efficacy against soil inhabiting pests. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 195–231. [Google Scholar]
- Koppenhöfer, A.M.; Fuzy, E.M.; Crocker, R.L.; Gelernter, W.D.; Polavarapu, S. Pathogenicity of Heterorhabditis bacteriophora, Steinernema glaseri, and S. scarabaei (Rhabditida: Heterorhabditidae, Steinernematidae) against 12 White Grub Species (Coleoptera: Scarabaeidae). Biocontrol Sci. Technol. 2004, 14, 87–92. [Google Scholar] [CrossRef]
- Marianelli, L.; Paoli, F.; Torrini, G.; Mazza, G.; Benvenuti, C.; Binazzi, F.; Sabbatini Peverieri, G.; Bosio, G.; Venanzio, D.; Giacometto, E.; et al. Entomopathogenic nematodes as potential biological control agents of Popillia japonica (Coleoptera, Scarabaeidae) in Piedmont Region (Italy). J. Appl. Entomol. 2018, 142, 311–318. [Google Scholar] [CrossRef]
- Hominick, W.M.; Reid, A.P.; Bohan, D.A.; Briscoe, B.R. Entomopathogenic Nematodes: Biodiversity, Geographical Distribution and the Convention on Biological Diversity. Biocontrol Sci. Technol. 1996, 6, 317–332. [Google Scholar] [CrossRef] [Green Version]
- Mráček, Z.; Bečvář, S.; Kindlmann, P.; Jersáková, J. Habitat preference for entomopathogenic nematodes, their insect hosts and new faunistic records for the Czech Republic. Biol. Control 2005, 34, 27–37. [Google Scholar] [CrossRef]
- Atwa, A. Entomopathogenic nematodes as biopesticides. In Basic and Applied Aspects of Biopesticides; Springer: Berlin/Heidelberg, Germany, 2014; pp. 69–98. [Google Scholar]
- Boschetti, M.; Boschetti, L.; Oliveri, S.; Casati, L.; Canova, I. Tree species mapping with Airborne hyper-spectral MIVIS data: The Ticino Park study case. Int. J. Remote Sens. 2007, 28, 1251–1261. [Google Scholar] [CrossRef]
- Paoli, F.; Marianelli, L.; Binazzi, F.; Mazza, G.; Benvenuti, C.; Sabbatini Peverieri, G.; Bosio, G.; Venanzio, D.; Giacometto, E.; Klein, M. Effectiveness of different doses of Heterorhabditis bacteriophora against Popillia japonica 3rd instars: Laboratory evaluation and field application. Redia 2017, 100, 135–138. [Google Scholar]
- Mazza, G.; Paoli, F.; Strangi, A.; Torrini, G.; Marianelli, L.; Peverieri, G.S.; Binazzi, F.; Bosio, G.; Sacchi, S.; Benvenuti, C.; et al. Hexamermis popilliae n. sp. (Nematoda: Mermithidae) parasitizing the Japanese beetle Popillia japonica Newman (Coleoptera: Scarabaeidae) in Italy. Syst. Parasitol. 2017, 94, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Bedding, R.A.; Akhurst, R.J. A simple technique for the detection o f insect parasitic rhabditid nematodes in soil. Nematologica 1975, 21, 109. [Google Scholar] [CrossRef]
- Kaya, H.K.; Stock, P.S. Chapter VI—Techniques in insect nematology A2—Lacey, Lawrence A. In Manual of Techniques in Insect Pathology; Academic Press: London, UK, 1997; pp. 281–324. [Google Scholar] [CrossRef]
- Brenna, S.; D’Alessio, M.; Solaro, S. Carta dei suoli della Lombardia—Scala 1: 250.000; Regione Lombardia–ERSAF: Milano, Italy, 2004. [Google Scholar]
- IPLA, Regione Piemonte. Carta dei Suoli del Piemonte—Scala 1: 250.000; Selca: Firenze, Italy, 2007. [Google Scholar]
- Nguyen, K.B. Methodology, morphology and identification. In Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts; Brill: Leiden, The Netherlands, 2010; pp. 59–119. [Google Scholar]
- Paoli, F.; Marianelli, L.; Torrini, G.; Mazza, G.; Benvenuti, C.; Bosio, G.; Venanzio, D.; Tarasco, E.; Klein, M.; Roversi, P.F. Differential susceptibility of Popillia japonica 3rd instars to Heterorhabditis bacteriophora (Italian strain) at three different seasons. Biocontrol Sci. Technol. 2017, 27, 439–444. [Google Scholar] [CrossRef]
- Benvenuti, C.; Cutini, A. Possibilità di impiego del Sistema TRIME®-FM per la stima del contenuto idrico dei terreni. Ann. Ist. Sper. Selvic. 1997, 28, 47–52. [Google Scholar]
- Marcus, R.; Eaves, D. Statistical and computational analysis of bioassay data. In Bioassays of Entomopathogenic Microbes and Nematodes; Navon, A., Ascher, K.R.S., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 249–293. [Google Scholar]
- Team, R.C. R Version 3.6.3; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Ehlers, R.-U.; Deseö, K.V.; Stackebrandt, E. Identification of Steinernema spp. (Nematoda) and their symbiotic bacteria Xenorhabdus spp. from Italian and German soils. Nematologica 1991, 37, 360. [Google Scholar] [CrossRef]
- Tarasco, E.; Clausi, M.; Rappazzo, G.; Panzavolta, T.; Curto, G.; Sorino, R.; Oreste, M.; Longo, A.; Leone, D.; Tiberi, R. Biodiversity of entomopathogenic nematodes in Italy. J. Helminthol. 2015, 89, 359. [Google Scholar] [CrossRef]
- Tarasco, E.; Triggiani, O. Survey of Steinernema and Heterorhabditis (Rhabditida: Nematoda) in Southern Italian soils. Entomologica 1997, 31, 117–123. [Google Scholar]
- Triggiani, O.; Tarasco, E. Indagini sui nematodi entomopatogeni (Rhabditida: Steinernematidae e Heterorhabditidae) in pinete e querceti dell’Italia meridionale. Entomologica 2000, 34, 23–32. [Google Scholar] [CrossRef]
- Mráček, Z.; Bečvář, S.; Kindlmann, P. Survey of entomopathogenic nematodes from the families Steinernematidae and Heterorhabditidae (Nematoda: Rhabditida) in the Czech Republic. Folia Parasitol. 1999, 46, 145–148. [Google Scholar]
- Bruck, D.J. Natural Occurrence of Entomopathogens in Pacific Northwest Nursery Soils and Their Virulence to the Black Vine Weevil, Otiorhynchus sulcatus (F.) (Coleoptera: Curculionidae). Environ. Entomol. 2004, 33, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Razia, M.; Padmanaban, R.; Karthik Raja, R.; Chellapandi, P.; Sivaramakrishnan, K. Monitoring entomopathogenic nematodes as ecological indicators in the cultivated lands of Karur District, Tamil Nadu: A Survey Report. Electron. J. Biol. 2011, 7, 16–19. [Google Scholar]
- del Pino, F.G.; Palomo, A. Natural Occurrence of Entomopathogenic Nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Spanish Soils. J. Invertebr. Pathol. 1996, 68, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Berry, R.E. Natural Distribution of Entomopathogenic Nematodes (Rhabditida: Heterorhabditidae and Steinernematidae) in Oregon Soils. Environ. Entomol. 1995, 24, 159–163. [Google Scholar] [CrossRef]
- Stock, P.S.; Pryor, B.M.; Kaya, H.K. Distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in natural habitats in California, USA. Biodivers. Conserv. 1999, 8, 535–549. [Google Scholar] [CrossRef]
- Hominick, W.M. Biogeography. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 115–143. [Google Scholar]
- Deseö, K.V.; Fantoni, P.; Lazzari, G.L. Presenza di nematodi entomopatogeni (Steinernema spp., Heterorhabditis spp.) nei terreni agricoli in Italia. Atti Giornate Fitopatol. 1988, 2, 269–280. [Google Scholar]
- Torrini, G.; Landi, S.; Benvenuti, C.; De Luca, F.; Fanelli, E.; Troccoli, A.; Tarasco, E.; Bazzoffi, P. Morphological and molecular characterization of a Steinernema carpocapsae (Nematoda Steinernematidae) strain isolated in Veneto region (Italy). Redia 2014, 97, 89–94. [Google Scholar]
- Torrini, G.; Mazza, G.; Carletti, B.; Benvenuti, C.; Roversi, P.F.; Fanelli, E.; De Luca, F.; Troccoli, A.; Tarasco, E. Oscheius onirici sp. n. (Nematoda: Rhabditidae): A new entomopathogenic nematode from an Italian cave. Zootaxa 2015, 3937, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Stuart, R.J.; Gaugler, R. Patchiness in Populations of Entomopathogenic Nematodes. J. Invertebr. Pathol. 1994, 64, 39–45. [Google Scholar] [CrossRef]
- Campbell, J.F.; Lewis, E.; Yoder, F.; Gaugler, R. Entomopathogenic Nematode (Heterorhabditidae and Steinernematidae) Seasonal Population Dynamics and Impact on Insect Populations in Turfgrass. Biol. Control 1995, 5, 598–606. [Google Scholar] [CrossRef]
- Půža, V.; Mráček, Z. Seasonal dynamics of entomopathogenic nematodes of the genera Steinernema and Heterorhabditis as a response to abiotic factors and abundance of insect hosts. J. Invertebr. Pathol. 2005, 89, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Jaffuel, G.; Mäder, P.; Blanco-Perez, R.; Chiriboga, X.; Fliessbach, A.; Turlings, T.C.J.; Campos-Herrera, R. Prevalence and activity of entomopathogenic nematodes and their antagonists in soils that are subject to different agricultural practices. Agric. Ecosyst. Environ. 2016, 230, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Campos-Herrera, R.; Blanco-Pérez, R.; Bueno-Pallero, F.Á.; Duarte, A.; Nolasco, G.; Sommer, R.J.; Rodríguez Martín, J.A. Vegetation drives assemblages of entomopathogenic nematodes and other soil organisms: Evidence from the Algarve, Portugal. Soil Biol. Biochem. 2019, 128, 150–163. [Google Scholar] [CrossRef]
- Kondo, E. Studies on the infectivity and propagation of entomogenous nematodes, Steinernema spp.,(Rhabditida: Steinernematidae), in the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Bull. Fac. Agric. Saga Univ. 1989, 67, 1–88. [Google Scholar]
- Koppenhöfer, A.M.; Kaya, H.K.; Shanmugam, S.; Wood, G.L. Interspecific Competition between Steinernematid Nematodes within an Insect Host. J. Invertebr. Pathol. 1995, 66, 99–103. [Google Scholar] [CrossRef]
- Neumann, G.; Shields, E.J. Interspecific Interactions Among Three Entomopathogenic Nematodes, Steinernema carpocapsae Weiser, S. feltiae Filipjev, and Heterorhabditis bacteriophora Poinar, with Different Foraging Strategies for Hosts in Multipiece Sand Columns. Environ. Entomol. 2006, 35, 1578–1583. [Google Scholar] [CrossRef]
- Půža, V.; Mráček, Z. Mixed infection of Galleria mellonella with two entomopathogenic nematode (Nematoda: Rhabditida) species: Steinernema affine benefits from the presence of Steinernema kraussei. J. Invertebr. Pathol. 2009, 102, 40–43. [Google Scholar] [CrossRef]
- Lewis, E.E.; Campbell, J.; Griffin, C.; Kaya, H.K.; Peters, A. Behavioral ecology of entomopathogenic nematodes. Biol. Control 2006, 38, 66–79. [Google Scholar] [CrossRef] [Green Version]
- Blackshaw, R.P. A survey of insect parasitic nematodes in Northern Ireland. Ann. Appl. Biol. 1988, 113, 561–565. [Google Scholar] [CrossRef]
- Kung, S.P.; Gaugler, R.; Kaya, H.K. Influence of Soil pH and Oxygen on Persistence of Steinernema spp. J. Nematol. 1990, 22, 440–445. [Google Scholar] [PubMed]
- Hazir, S.; Keskin, N.; Stock, P.S.; Kaya, H.K.; Özcan, S. Diversity and distribution of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Turkey. Biodivers. Conserv. 2003, 12, 375–386. [Google Scholar] [CrossRef]
- Mwaniki, S.W.; Nderitu, J.H.; Olubayo, F.; Kimenju, J.W.; Nguyen, K. Factors influencing the occurrence of entomopathogenic nematodes in the Central Rift Valley Region of Kenya. Afr. J. Ecol. 2008, 46, 79–84. [Google Scholar] [CrossRef]
- Miduturi, J.; Waeyenberge, L.; Moens, M. Natural distribution of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) in Belgian soils. Russ. J. Nematol. 1997, 5, 55–66. [Google Scholar]
- Khatri-Chhetri, H.B.; Waeyenberge, L.; Manandhar, H.K.; Moens, M. Natural occurrence and distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in Nepal. J. Invertebr. Pathol. 2010, 103, 74–78. [Google Scholar] [CrossRef]
- Mráček, Z.; Webster, J.M. Survey of Heterorhabditidae and Steinernematidae (Rhabditida, Nematoda) in Western Canada. J. Nematol. 1993, 25, 710–717. [Google Scholar]
- Campos-Herrera, R.; Escuer, M.; Labrador, S.; Robertson, L.; Barrios, L.; Gutiérrez, C. Distribution of the entomopathogenic nematodes from La Rioja (Northern Spain). J. Invertebr. Pathol. 2007, 95, 125–139. [Google Scholar] [CrossRef]
- Benseddik, Y.; Boutaleb Joutei, A.; Blenzar, A.; Ezrari, S.; Molina, C.M.; Radouane, N.; Mokrini, F.; Tahiri, A.; Lahlali, R.; Dababat, A.A. Occurrence and distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in Morocco. Biocontrol Sci. Technol. 2020, 30, 1060–1072. [Google Scholar] [CrossRef]
- Barker, C.W.; Barker, G.M. Generalist entomopathogens as biological indicators of deforestation and agricultural land use impacts on Waikato soils. N. Z. J. Ecol. 1998, 22, 189–196. [Google Scholar]
- Mekete, T.; Gaugler, R.; Nguyen, K.; Mandefro, W.; Tessera, M. Biogeography of entomopathogenic nematodes in Ethiopia. Nematropica 2005, 35, 31–36. [Google Scholar]
- Valadas, V.; Laranjo, M.; Mota, M.; Oliveira, S. A survey of entomopathogenic nematode species in continental Portugal. J. Helminthol. 2014, 88, 327–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhurst, R.J.; Bedding, R.A. Natural occurrence of insect pathogenic nematodes (Steinernematidae and Heterorhabditidae) in soil in Australia. Aust. J. Entomol. 1986, 25, 241–244. [Google Scholar] [CrossRef]
- Mráček, Z.; Sturhan, D. Epizootic of the entomopathogenic nematode Steinernema intermedium (Poinar) in an aggregation of the bibionid fly, Bibio marci L. J. Invertebr. Pathol. 2000, 76, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Menta, C.; Conti, F.D.; Pinto, S.; Bodini, A. Soil Biological Quality index (QBS-ar): 15 years of application at global scale. Ecol. Indic. 2018, 85, 773–780. [Google Scholar] [CrossRef]
- Simões, N.; Caldas, C.; Rosa, J.S.; Bonifassi, E.; Laumond, C. Pathogenicity Caused by High Virulent and Low Virulent Strains of Steinernema carpocapsae to Galleria mellonella. J. Invertebr. Pathol. 2000, 75, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Tarasco, E. Infectivity comparison among eight Steinernema feltiae (Filipjev, 1934) (Rhabditida: Steinernematidae) isolated from southern Italian soils. Entomologica 1997, 31, 171–179. [Google Scholar]



| EPN Species | Soil Sample | Habitat | Soil Type | pH | Geographical Coordinates WGS 84 |
|---|---|---|---|---|---|
| Steinernema carpocapsae | POP 4 | Perennial meadows | Sandy loam | 5.03 | 45°29′37″ N; 8°44′24″ E |
| POP 5 | Perennial meadows | Sandy loam | 5.31 | 45°29′59″ N; 8°43′40″ E | |
| POP 6 | Perennial meadows | Sandy loam | 5.15 | 45°30′2″ N; 8°43′34″ E | |
| POP 8 | Perennial meadows | Sandy loam | 5.50 | 45°18′7″ N; 8°44′26″ E | |
| POP 12 | Perennial meadows | Sandy loam | 5.24 | 45°29′18″ N; 8°44′43″ E | |
| POP 14 | Perennial meadows | Sandy loam | 5.20 | 45°29′7″ N; 8°45′39″ E | |
| POP 28 | Cropland | Sandy loam | 4.96 | 45°27′36″ N; 8°47′15″ E | |
| POP 34 | Perennial meadows | Sandy loam | 5.32 | 45°24′9″ N; 8°49′3″ E | |
| POP 38 | Perennial meadows | Sandy loam | 5.55 | 45°28′55″ N; 8°46′27″ E | |
| POP 44 | Perennial meadows | Silty loam | 5.05 | 45°35′8″ N; 8°38′2″ E | |
| POP 46 | Perennial meadows | Sandy loam | 5.33 | 45°40′10″ N; 8°38′55″ E | |
| POP 54 | Perennial meadows | Sandy loam | 5.31 | 45°36′25″ N; 8°38′48″ E | |
| POP 55 | Perennial meadows | Sandy loam | 5.65 | 45°36′48″ N; 8°39′36″ E | |
| POP 59 | Perennial meadows | Sandy loam | 5.61 | 45°36′7″ N; 8°39′40″ E | |
| POP 69 | Perennial meadows | Sandy loam | 5.10 | 45°29′39″ N; 8°44′56″ E | |
| POP 70 | Uncultivated field | Sandy loam | 5.08 | 45°29′26″ N; 8°44′54″ E | |
| POP 71 | Uncultivated field | Sandy loam | 5.17 | 45°29′27″ N; 8°44′44″ E | |
| POP 74 | Perennial meadows | Sandy loam | 5.51 | 45°29′25″ N; 8°44′34″ E | |
| POP 139 | Woodland | Sandy loam | 4.73 | 45°29′51″ N; 8°44′20″ E | |
| Steinernema feltiae | POP 48 | Woodland | Sandy loam | 4.65 | 45°40′10″ N; 8°38′48″ E |
| POP 73 | Perennial meadows | Sandy loam | 5.26 | 45°29′30″ N; 8°44′39″ E | |
| POP 78 | Perennial meadows | Sandy loam | 5.78 | 45°29′15″ N; 8°45′37″ E | |
| POP 79 | Perennial meadows | Sandy loam | 5.61 | 45°29′14″ N; 8°45′27″ E | |
| POP 91 | Woodland | Sandy loam | 4.19 | 45°37′0″ N; 8°40′45″ E | |
| POP 100 | Woodland | Sandy loam | 4.08 | 45°39′5″ N; 8°39′35″ E | |
| POP 101 | Woodland | Sandy loam | 3.95 | 45°39′3″ N; 8°39′22″ E | |
| POP 102 | Woodland | Sandy loam | 5.29 | 45°39′12″ N; 8°39′17″ E | |
| POP 103 | Woodland | Sandy loam | 4.11 | 45°39′26″ N; 8°39′5″ E | |
| POP 105 | Woodland | Sandy loam | 4.25 | 45°40′4″ N; 8°39′11″ E | |
| POP 127 | Woodland | Sandy loam | 3.71 | 45°31′24″ N; 8°42′12″ E | |
| POP 135 | Woodland | Sandy loam | 4.54 | 45°30′9″ N; 8°43′25″ E | |
| POP 142 | Woodland | Sandy loam | 4.74 | 45°29′34″ N; 8°45′41″ E | |
| POP 144 | Woodland | Sandy loam | 4.12 | 45°29′11″ N; 8°45′51″ E | |
| POP 152 | Woodland | Sandy loam | 3.95 | 45°28′14″ N; 8°45′52″ E | |
| POP 153 | Woodland | Sandy loam | 3.74 | 45°27′59″ N; 8°46′14″ E | |
| Heterorhabditis bacteriophora | POP 9 | Perennial meadows | Sandy loam | 5.16 | 45°29′26″ N; 8°44′33″ E |
| POP 16 | Uncultivated field | Sandy loam | 5.01 | 45°29′4″ N; 8°45′45″ E | |
| H. bacteriophora + S. carpocapsae | POP 80 | Perennial meadows | Sandy loam | 5.09 | 45°29′15″ N; 8°45′40″ E |
| S. carpocapsae + S. feltiae | POP 138 | Woodland | Sandy loam | 4.16 | 45°29′55″ N; 8°44′16″ E |
| EPN Species | Soil Sample | Mortality (%) | LT50 | Days of Emergence (Mean) | Emergence (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| May | September | May | September | May | September | May | September | ||
| Control | 0.0 | 0.0 | |||||||
| Steinernema carpocapsae | POP 4 | 16.7 | 50.0 | - | 8.0 | - | 13 | - | 33.3 |
| POP 5 | 16.7 | 50.0 | - | 7.0 | 14 | 13 | 16.7 | 50.0 | |
| POP 6 | 16.7 | 83.3 | - | 4.0 | - | 9 | - | 50.0 | |
| POP 8 | 33.3 | 16.7 | - | - | 8 | 15 | 16.7 | 16.7 | |
| POP 12 | 0.0 | 33.3 | - | - | - | 15 | - | 16.7 | |
| POP 14 | 0.0 | 0.0 | - | - | - | - | - | - | |
| POP 28 | 33.3 | 66.7 | - | 8.0 | 15 | 15 | 16.7 | 50.0 | |
| POP 34 | 33.3 | 33.3 | - | - | 10 | 13 | 33.3 | 16.7 | |
| POP 38 | 33.3 | 16.7 | - | - | 13 | 19 | 16.7 | ||
| POP 44 | 0.0 | 66.7 | - | 10.0 | - | 13 | - | 50.0 | |
| POP 46 | 0.0 | 16.7 | - | - | - | 10 | - | 16.7 | |
| POP 54 | 0.0 | 0.0 | - | - | - | - | - | - | |
| POP 55 | 50.0 | 50.0 | 9.0 | 8.0 | 6 | 9 | 50.0 | 16.7 | |
| POP 59 | 50.0 | 66.7 | 6.0 | 8.0 | 19 | 11 | 50.0 | 50.0 | |
| POP 69 | 33.3 | 33.3 | - | - | - | - | - | - | |
| POP 70 | 0.0 | 16.7 | - | - | - | - | - | - | |
| POP 71 | 16.7 | 50.0 | - | 10.0 | - | 11 | - | 50.0 | |
| POP 74 | 0.0 | 66.7 | - | 11.0 | - | 17 | - | 33.3 | |
| POP 139 | 16.7 | 100.0 | - | 6.0 | - | 10 | - | 100.0 | |
| Steinernema feltiae | POP 48 | 33.3 | 83.3 | - | 2.6 | 11 | 14 | 33.3 | 50.0 |
| POP 73 | 33.3 | 83.3 | - | 3.0 | 22 | 12 | 33.3 | 83.3 | |
| POP 78 | 16.7 | 83.3 | - | 10.0 | 11 | 12 | 16.7 | 83.3 | |
| POP 79 | 16.7 | 0.0 | - | - | - | - | - | - | |
| POP 91 | 16.7 | 83.3 | - | 11.5 | 15 | 9 | 16.7 | 83.3 | |
| POP 100 | 33.3 | 66.7 | - | 5.0 | 19 | 15 | 33.3 | 50.0 | |
| POP 101 | 0.0 | 33.3 | - | - | - | 13 | - | 33.3 | |
| POP 102 | 16.7 | 66.7 | - | 8.0 | 9 | 11 | 16.7 | 66.7 | |
| POP 103 | 33.3 | 83.3 | - | 8.0 | 11 | 11 | 16.7 | 83.3 | |
| POP 105 | 33.3 | 33.3 | - | - | - | 11 | - | 33.3 | |
| POP 127 | 33.3 | 66.7 | - | 5.0 | 12 | 9 | 16.7 | 33.3 | |
| POP 135 | 16.7 | 16.7 | - | - | - | 8 | - | 16.7 | |
| POP 142 | 0.0 | 66.7 | - | 12.0 | - | 9 | - | 66.7 | |
| POP 144 | 50.0 | 66.7 | 13 | 12.0 | 12 | 9 | 33.3 | 66.7 | |
| POP 152 | 0.0 | 83.3 | - | 10.0 | - | 10 | - | 83.3 | |
| POP 153 | 16.7 | 50.0 | - | 10.0 | - | 7 | - | 50.0 | |
| Heterorhabditis bacteriophora | POP 9 | 100.0 | 100.0 | 7.5 | 8.0 | 14 | 13 | 83.3 | 100.0 |
| POP 16 | 83.3 | 100.0 | 6.0 | 4.7 | 12 | 11 | 83.3 | 100.0 | |
| H. bacteriophora + S. carpocapsae | POP 80 | 83.3 | 100.0 | 5.5 | 2.8 | 16 | 13 | 66.7 | 83.3 |
| S. carpocapsae + S. feltiae | POP 138 | 33.3 | 83.3 | - | 3.0 | 8 | 9 | 33.3 | 83.3 |
| Sample | Nematode | Mortality (%) | Progeny (± Standard Error) | ||
|---|---|---|---|---|---|
| Control | 0 | - | |||
| POP16 | H.b. | 91.7 | 68,339 | ± | 4639.71 |
| POP48 | S.f. | 39.6 | 17,710 | ± | 2046.30 |
| POP80 | S.c. + H.b. | 97.9 | 44,964 | ± | 5983.27 |
| POP138 | S.c. + S.f. | 54.2 | 25,729 | ± | 2590.45 |
| POP139 | S c. | 54.2 | 20,173 | ± | 1847.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrini, G.; Paoli, F.; Mazza, G.; Simoncini, S.; Benvenuti, C.; Strangi, A.; Tarasco, E.; Barzanti, G.P.; Bosio, G.; Cutino, I.; et al. Evaluation of Indigenous Entomopathogenic Nematodes as Potential Biocontrol Agents against Popillia japonica (Coleoptera: Scarabaeidae) in Northern Italy. Insects 2020, 11, 804. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110804
Torrini G, Paoli F, Mazza G, Simoncini S, Benvenuti C, Strangi A, Tarasco E, Barzanti GP, Bosio G, Cutino I, et al. Evaluation of Indigenous Entomopathogenic Nematodes as Potential Biocontrol Agents against Popillia japonica (Coleoptera: Scarabaeidae) in Northern Italy. Insects. 2020; 11(11):804. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110804
Chicago/Turabian StyleTorrini, Giulia, Francesco Paoli, Giuseppe Mazza, Stefania Simoncini, Claudia Benvenuti, Agostino Strangi, Eustachio Tarasco, Gian Paolo Barzanti, Giovanni Bosio, Ilaria Cutino, and et al. 2020. "Evaluation of Indigenous Entomopathogenic Nematodes as Potential Biocontrol Agents against Popillia japonica (Coleoptera: Scarabaeidae) in Northern Italy" Insects 11, no. 11: 804. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110804