Sublethal Effects of Imidacloprid on Fecundity, Apoptosis and Virus Transmission in the Small Brown Planthopper Laodelphax striatellus
Abstract
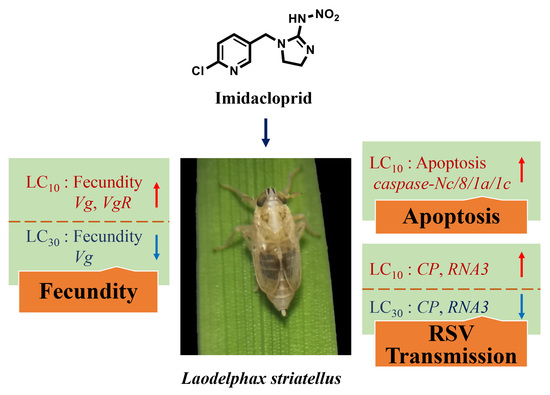
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Bioassays
2.3. Effects of Imidacloprid on Fecundity in SBPH
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Effects of Imidacloprid on Vertical Transmission of RSV by SBPH
2.6. TUNEL Assay
2.7. Statistical Analysis
3. Results
3.1. Effects of Imidacloprid on the Fecundity of SBPH
3.2. Effects of Imidacloprid on Apoptosis in Ovaries
3.3. Effects of Imidacloprid on the Expression Levels of Four Caspase Genes in SBPH
3.4. Effects of Imidacloprid on RSV Transmission by SBPH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, J.H.; Li, S.; Hong, J.; Ji, Y.H.; Zhou, Y.J. Investigation on subcellular localization of Rice stripe virus in its vector small brown planthopper by electron microscopy. Virol. J. 2013, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, D.Y.; Hu, J.; Zhang, K.; Kang, L.; Chen, Y.; Huang, L.J.; Zhang, L.; Xiang, Y.; Song, Q.S.; et al. The α-tubulin of Laodelphax striatellus mediates the passage of rice stripe virus (RSV) and enhances horizontal transmission. PLoS Pathog. 2020, 16, e1008710. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, S.; Tao, X.; Zhou, X. Rice stripe virus: Exploring molecular weapons in the arsenal of a negative-sense RNA virus. Annu. Rev. Phytopathol. 2021, 59, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Liu, W.W.; Zhang, F.J.; Chen, X.Y.; Li, L.; Liu, Q.F.; Zhou, Y.J.; Wei, T.Y.; Fang, R.X.; Wang, X.F. Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 2014, 10, e1003949. [Google Scholar] [CrossRef]
- Jia, D.S.; Chen, Q.; Mao, Q.Z.; Zhang, X.F.; Wu, W.; Chen, H.Y.; Yu, X.Z.; Wang, Z.Q.; Wei, T.Y. Vector mediated transmission of persistently transmitted plant viruses. Curr. Opin. Virol. 2018, 28, 127–132. [Google Scholar] [CrossRef]
- Huo, Y.; Yu, Y.L.; Chen, L.Y.; Li, Q.; Zhang, M.T.; Song, Z.Y.; Chen, X.Y.; Fang, R.X.; Zhang, L.L. Insect tissue-specific vitellogenin facilitates transmission of plant virus. PLoS Pathog. 2018, 14, e1006909. [Google Scholar] [CrossRef] [Green Version]
- Huo, Y.; Yu, Y.L.; Liu, Q.; Liu, D.; Zhang, M.T.; Liang, J.N.; Chen, X.Y.; Zhang, L.L.; Fang, R.X. Rice stripe virus hitchhikes the vector insect vitellogenin ligand-receptor pathway for ovary entry. Phil. Trans. R. Soc. B 2019, 374, 20180312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zhang, W.; Zhang, S.; Wu, S.F.; Ban, L.F.; Su, J.Y.; Gao, C.F. Susceptibility of Sogatella furcifera and Laodelphax striatellus (Hemiptera: Delphacidae) to six insecticides in China. J. Econ. Entomol. 2014, 107, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Ge, L.Q.; Liu, F.; Song, Q.S.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2020, 65, 409–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Yang, G.Q.; Gao, X.; Zhang, N.N.; Chen, D.Y.; Liu, F.; Xu, J.X.; Wu, J.C. Pesticide-induced changes in fecundity and rice stripe virus transmission ability in Laodelphax striatellus (Homoptera: Delphacidae). J. Asia-Pac. Entomol. 2017, 20, 830–834. [Google Scholar] [CrossRef]
- Bantz, A.; Camon, J.; Froger, J.A.; Goven, D.; Raymond, V. Exposure to sublethal doses of insecticide and their effects on insects at cellular and physiological levels. Curr. Opin. Insect Sci. 2018, 30, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Ali, E.; Li, W.; He, B.; Gong, P.; Xu, P.; Li, J.; Wan, H. Sublethal effects of sulfoxaflor on the development and reproduction of the brown planthopper, Nilaparvata lugens (Stål). Crop. Prot. 2019, 118, 6–14. [Google Scholar] [CrossRef]
- Xu, P.; Shu, R.; Gong, P.; Li, W.; Wan, H.; Li, J. Sublethal and transgenerational effects of triflumezopyrim on the biological traits of the brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Crop. Prot. 2019, 117, 63–68. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, H.; Li, J.; Wang, C.; Li, C.; Gao, Y.L. Sublethal effects of imidacloprid on the population development of western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae). Insects 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, S.; Lin, Z.; Zhang, Y.; Zhang, W.; Alansary, N.; Mishra, S.; Bhatt, P.; Chen, S. Insights into the toxicity and degradation mechanisms of imidacloprid via physicochemical and microbial approaches. Toxics 2020, 8, 65. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.; Mao, K.; Yang, P.; Li, D.; Ali, E.; Wan, H.; Li, J. Neonicotinoid insecticide resistance in the field populations of Sogatella furcifera (Horváth) in Central China from 2011 to 2015. J. Asia-Pac. Entomol. 2017, 20, 955–958. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.W.; Liu, F.H.; Pang, R.P.; Tian, H.G.; Liu, T.X. Effect of sublethal doses of imidacloprid on the biological performance of aphid endoparasitoid Aphidius gifuensis (Hymenoptera: Aphidiidae) and influence on its related gene expression. Front. Physiol. 2018, 9, 1729. [Google Scholar] [CrossRef]
- Li, W.Q.; Lu, Z.B.; Li, L.L.; Yu, Y.; Dong, S.; Men, X.Y.; Ye, B.H. Sublethal effects of imidacloprid on the performance of the bird cherry-oat aphid Rhopalosiphum padi. PLoS ONE 2018, 13, e0204097. [Google Scholar] [CrossRef]
- He, C.; Liang, J.J.; Liu, S.N.; Wang, S.L.; Wu, Q.J.; Xie, W.; Zhang, Y.J. Changes in the expression of four ABC transporter genes in response to imidacloprid in Bemisia tabaci Q (Hemiptera: Aleyrodidae). Pestic. Biochem. Physiol. 2019, 153, 136–143. [Google Scholar] [CrossRef]
- He, Y.X.; Zhao, J.W.; Zheng, Y.; Weng, Q.Y.; Biondi, A.; Desneux, N.; Wu, K.M. Assessment of potential sublethal effects of various insecticides on key biological traits of the tobacco whitefly, Bemisia tabaci. Int. J. Biol. Sci. 2013, 9, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Liu, L.L.; Yang, H.; Wang, Z.; Long, G.Y.; Jin, D.C. Sublethal effects of imidacloprid on the development, reproduction, and susceptibility of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J. Asia-Pac. Entomol. 2017, 20, 996–1000. [Google Scholar] [CrossRef]
- Dai, C.; Ricupero, M.; Wang, Z.; Desneux, N.; Biondi, A.; Lu, Y. Transgenerational effects of a neonicotinoid and a novel sulfoximine insecticide on the harlequin ladybird. Insects 2021, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Skouras, P.J.; Darras, A.I.; Mprokaki, M.; Demopoulos, V.; Margaritopoulos, J.T.; Delis, C.; Stathas, G.J. Toxicity, Sublethal and low dose effects of imidacloprid and deltamethrin on the aphidophagous predator Ceratomegilla undecimnotata (Coleoptera: Coccinellidae). Insects 2021, 12, 696. [Google Scholar] [CrossRef]
- Qu, Y.Y.; Xiao, D.; Li, J.Y.; Chen, Z.; Biondi, A.; Desneux, N.; Gao, X.W.; Song, D.L. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology 2015, 24, 479–487. [Google Scholar] [CrossRef]
- Yu, Y.S.; Shen, G.Q.; Zhu, H.L.; Lu, Y.T. Imidacloprid-induced hormesis on the fecundity and juvenile hormone levels of the green peach aphid Myzus persicae (Sulzer). Pestic. Biochem. Physiol. 2010, 98, 238–242. [Google Scholar] [CrossRef]
- Lu, W.W.; Xu, Q.J.; Zhu, J.; Liu, C.; Ge, L.Q.; Yang, G.Q.; Liu, F. Inductions of reproduction and population growth in the generalist predator Cyrtorhinus lividipennis (Hemiptera: Miridae) exposed to sub-lethal concentrations of insecticides. Pest. Manag. Sci. 2017, 73, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 2009, 55, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, Z.Q.; Ou-Yang, Y.Y.; Huang, G.H. Identification of four caspase genes from Spodoptera exigua (Lepidoptera: Noctuidae) and their regulations toward different apoptotic stimulations. Insect Sci. 2019, 27, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.; Kumar, S. Death to flies: Drosophila as a model system to study programmed cell death. J. Immunol. Methods 2002, 265, 21–38. [Google Scholar] [CrossRef]
- Ruttanaphan, T.; de Sousa, G.; Pengsook, A.; Pluempanupat, W.; Huditz, H.; Bullangpoti, V.; Le Goff, G. A novel insecticidal molecule extracted from Alpinia galanga with potential to control the pest insect Spodoptera frugiperda. Insects 2020, 11, 686. [Google Scholar] [CrossRef]
- Tang, X.T.; Ibanez, F.; Tamborindeguy, C. Concanavalin A toxicity towards potato psyllid and apoptosis induction in midgut cells. Insects 2020, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.M.; Granville, D.J.; Lowenberger, C. The insect caspases. Apoptosis 2009, 14, 247–256. [Google Scholar] [CrossRef]
- Lord, C.E.; Gunawardena, A.H. Programmed cell death in C. elegans, mammals and plants. Eur. J. Cell Biol. 2012, 91, 603–613. [Google Scholar] [CrossRef]
- Ju, J.F.; Hoffmann, A.A.; Zhang, Y.K.; Duan, X.Z.; Guo, Y.; Gong, J.T.; Zhu, W.C.; Hong, X.Y. Wolbachia-induced loss of male fertility is likely related to branch chain amino acid biosynthesis and iLvE in Laodelphax striatellus. Insect Mol. Biol. 2017, 85, 11–20. [Google Scholar] [CrossRef]
- Farder-Gomes, C.F.; Fernandes, K.M.; Bernardes, R.C.; Bastos, D.S.S.; Martins, G.F.; Serrao, J.E. Acute exposure to fipronil induces oxidative stress, apoptosis and impairs epithelial homeostasis in the midgut of the stingless bee Partamona helleri Friese (Hymenoptera: Apidae). Sci. Total Environ. 2021, 774, 145679. [Google Scholar] [CrossRef]
- Gregorc, A.; Ellis, J.D. Cell death localization in situ in laboratory reared honey bee (Apis mellifera L.) larvae treated with pesticides. Pestic. Biochem. Physiol. 2011, 99, 200–207. [Google Scholar] [CrossRef]
- Farder-Gomes, C.F.; Fernandes, K.M.; Bernardes, R.C.; Bastos, D.S.S.; de Oliveira, L.L.; Martins, G.F.; Serrao, J.E. Harmful effects of fipronil exposure on the behavior and brain of the stingless bee Partamona helleri Friese (Hymenoptera: Meliponini). Sci. Total Environ. 2021, 794, 148678. [Google Scholar] [CrossRef]
- Almeida Rossi, C.; Roat, T.C.; Tavares, D.A.; Cintra-Socolowski, P.; Malaspina, O. Effects of sublethal doses of imidacloprid in malpighian tubules of africanized Apis mellifera (Hymenoptera, Apidae). Microsc. Res. Tech. 2013, 76, 552–558. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, T.; Wang, Q.; Dai, P.; Xu, S.F.; Jia, H.; Wang, X. Programmed cell death in the honey bee (Apis mellifera) (Hymenoptera: Apidae) worker brain induced by imidacloprid. J. Econ. Entomol. 2015, 108, 1486–1494. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Gonçalves, W.G.; Freire, A.F.; Zanuncio, J.C.; Bozdoğan, H.; Serrão, J.E. Toxicity and cytotoxicity of the insecticide imidacloprid in the midgut of the predatory bug, Podisus nigrispinus. Ecotoxicol. Environ. Saf. 2019, 167, 69–75. [Google Scholar] [CrossRef]
- Castellanos, N.L.; Ferreira-Filho, N.A.; Rodrigues, H.D.; Martínez, L.C.; Serrão, J.E.; Oliveira, E.E. Imidacloprid-mediated alterations on the salivary glands of the Neotropical brown stink bug, Euschistus heros. Ecotoxicology 2021, 30, 678–688. [Google Scholar] [CrossRef]
- Mpakou, V.E.; Velentzas, A.D.; Velentzas, P.D.; Margaritis, L.H.; Stravopodis, D.J.; Papassideri, I.S. Programmed cell death of the ovarian nurse cells during oogenesis of the ladybird beetle Adalia bipunctata (Coleoptera: Coccinellidae). Dev. Growth Differ. 2011, 53, 804–815. [Google Scholar] [CrossRef]
- Guo, Y.; Hoffmann, A.A.; Xu, X.Q.; Zhang, X.; Huang, H.J.; Ju, J.F.; Gong, J.T.; Hong, X.Y. Wolbachia-induced apoptosis associated with increased fecundity in Laodelphax striatellus (Hemiptera: Delphacidae). Insect Mol. Biol. 2018, 27, 796–807. [Google Scholar] [CrossRef]
- Wang, X.R.; Wang, C.; Ban, F.X.; Ghanim, M.; Pan, L.L.; Qian, L.X.; Liu, Y.Q.; Wang, X.W.; Liu, S.S. Apoptosis in a whitefly vector activated by a begomovirus enhances viral transmission. mSystems 2020, 5, e00433-20. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, H.; Yuan, P.; Zhang, X.; Chen, Q.; Jiang, X.; Zhou, Y. Development of a simplified RT-PCR without RNA isolation for rapid detection of RNA viruses in a single small brown planthopper (Laodelphax striatellus Fallén). Virol. J. 2017, 14, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wu, W.; Liu, H.Q.; Dong, Y.; Zhang, Y.; Wong, S.; Wang, C.C.; Zhou, Y.J.; Xu, Q.F. Determination of Suitable RT-qPCR reference genes for studies of gene functions in Laodelphax striatellus (Fallén). Genes 2019, 10, 887. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Jiang, Y.; Zhang, N.N.; Liu, F.; Yang, G.Q. Triazophos-induced vertical transmission of rice stripe virus is associated with host vitellogenin in the small brown planthopper Laodelphax striatellus. Pest. Manag. Sci. 2020, 76, 1949–1957. [Google Scholar] [CrossRef]
- Landmann, F.; Foster, J.M.; Slatko, B.; Sullivan, W. Asymmetric Wolbachia segregation during early Brugia malayi embryogenesis determines its distribution in adult host tissues. PLoS Negl. Trop. Dis. 2010, 4, e758. [Google Scholar] [CrossRef] [Green Version]
- Biondi, A.; Zappalà, L.; Stark, J.D.; Desneux, N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 2013, 8, e76548. [Google Scholar] [CrossRef] [Green Version]
- Guedes, R.N.C.; Cutler, G.C. Insecticide-induced hormesis and arthropod pest management. Pest. Manag. Sci. 2014, 70, 690–697. [Google Scholar] [CrossRef] [Green Version]
- Rix, R.R.; Ayyanath, M.M.; Cutler, G.C. Sublethal concentrations of imidacloprid increase reproduction, alter expression of detoxification genes, and prime Myzus persicae for subsequent stress. J. Pest. Sci. 2016, 89, 581–589. [Google Scholar] [CrossRef]
- Rix, R.R.; Cutler, G.C. Low doses of a neonicotinoid stimulate reproduction in a beneficial predatory insect. J. Econ. Entomol. 2020, 113, 2179–2186. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Tang, Y.Y.; Liu, J.Q.; Chen, Y.Y.; Lv, Z.X.; Liu, F. Juvenile hormone-mediated reproduction induced by a sublethal concentration of imidacloprid in Cyrtorhinus lividipennis (Hemiptera: Miridae). J. Asia-Pac. Entomol. 2020, 23, 98–106. [Google Scholar] [CrossRef]
- Wang, A.H.; Wu, J.C.; Yu, Y.S.; Liu, J.L.; Yue, J.F.; Wang, M.Y. Selective insecticide-induced stimulation on fecundity and biochemical changes in Tryporyza incertulas (Lepidoptera: Pyralidae). J. Econ. Entomol. 2005, 98, 1144–1149. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, C.Q.; Zhang, Y.N.; Liu, Y.; Gu, Z.Y. Lethal and sublethal effects of sulfoxaflor on the small brown planthopper Laodelphax striatellus. J. Asia-Pac. Entomol. 2016, 19, 683–689. [Google Scholar] [CrossRef]
- Ge, L.Q.; Wu, J.C.; Zhao, K.F.; Chen, Y.; Yang, G.Q. Induction of Nlvg and suppression of Nljhe gene expression in Nilaparvata lugens (Stål) (Hemiptera: Delphacidae) adult females and males exposed to two insecticides. Pestic. Biochem. Physiol. 2010, 98, 269–278. [Google Scholar] [CrossRef]
- Liu, B.; Preisser, E.L.; Yang, Z.; Jiao, X.; Zhang, Y. Sulfoxaflor alters Bemisia tabaci MED (Hemiptera: Aleyrodidae) preference, feeding, and TYLCV Transmission. J. Econ. Entomol. 2021, 114, 1568–1574. [Google Scholar] [CrossRef]
- Roditakis, E.; Stavrakaki, M.; Grispou, M.; Achimastou, A.; Van Waetermeulen, X.; Nauen, R.; Tsagkarakou, A. Flupyradifurone effectively manages whitefly Bemisia tabaci MED (Hemiptera: Aleyrodidae) and tomato yellow leaf curl virus in tomato. Pest. Manag. Sci. 2017, 73, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Preisser, E.L.; Jiao, X.; Xu, W.; Zhang, Y. Lethal and sublethal effects of flupyradifurone on Bemisia tabaci MED (Hemiptera: Aleyrodidae) feeding behavior and TYLCV transmission in tomato. J. Econ. Entomol. 2021, 114, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doublet, V.; Labarussias, M.; de Miranda, J.R.; Moritz, R.F.; Paxton, R.J. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2015, 174, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Coulon, M.; Schurr, F.; Martel, A.C.; Cougoule, N.; Be’gaud, A.; Mangoni, P.; Di Prisco, G.; Dalmon, A.; Alaux, C.; Ribière-Chabert, M.; et al. Influence of chronic exposure to thiamethoxam and chronic bee paralysis virus on winter honey bees. PLoS ONE 2019, 14, e0220703. [Google Scholar] [CrossRef] [Green Version]
- Diao, Q.; Li, B.; Zhao, H.; Wu, Y.; Guo, R.; Dai, P.; Chen, D.; Wang, Q.; Hou, C. Enhancement of chronic bee paralysis virus levels in honeybees acute exposed to imidacloprid: A Chinese case study. Sci. Total Environ. 2018, 630, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Q.S.; Xu, Z.T.; Liu, R.Y.; Cui, F. Distinct replication and gene expression strategies of the Rice Stripe virus in vector insects and host plants. J. Gen. Virol. 2019, 100, 877–888. [Google Scholar] [CrossRef]





| Insecticide | N | Regression Equation | Dose, 95% Confidence Limits (mg/L) | χ2 (df) | p | ||
|---|---|---|---|---|---|---|---|
| LC10 | LC20 | LC30 | |||||
| imidacloprid | 540 | Y = 1.5256 + 2.2602X | 9.34 (7.32–11.90) | 14.62 (12.02–17.77) | 20.19 (16.95–24.06) | 0.770 (3) | 0.857 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xu, G.; Jiang, Y.; Ma, C.; Yang, G. Sublethal Effects of Imidacloprid on Fecundity, Apoptosis and Virus Transmission in the Small Brown Planthopper Laodelphax striatellus. Insects 2021, 12, 1131. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12121131
Zhang Y, Xu G, Jiang Y, Ma C, Yang G. Sublethal Effects of Imidacloprid on Fecundity, Apoptosis and Virus Transmission in the Small Brown Planthopper Laodelphax striatellus. Insects. 2021; 12(12):1131. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12121131
Chicago/Turabian StyleZhang, Yuanyuan, Gang Xu, Yu Jiang, Chao Ma, and Guoqing Yang. 2021. "Sublethal Effects of Imidacloprid on Fecundity, Apoptosis and Virus Transmission in the Small Brown Planthopper Laodelphax striatellus" Insects 12, no. 12: 1131. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12121131