Chemical Composition of an Aphid Antifeedant Extract from an Endophytic Fungus, Trichoderma sp. EFI671
Abstract
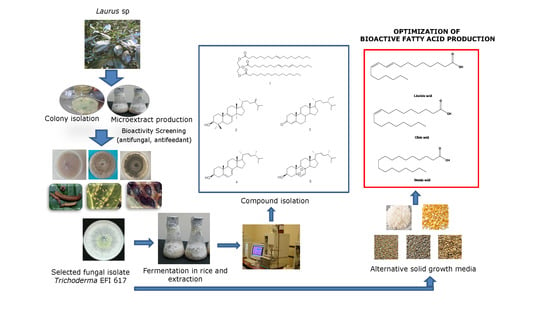
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Isolation of Endophytic Fungus Trichoderma sp. EFI 671
2.3. Molecular Characterization of Trichoderma sp. EFI 671
2.4. Large-Scale Cultivation for Compound Isolation
2.5. Optimization of Media
2.6. Isolation and Identification of Bioactive Compounds
2.7. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.8. Antifungal Bioassay
2.9. Insect Bioassay
2.10. Nematode Bioassay
2.11. Phytotoxicity Tests
3. Results
3.1. Identification of Endophytic Fungi
3.2. Bioactivity Screening of Endophytic Fungi
3.3. Chemical Characterization of the Extract
3.4. Optimization of Media
3.5. Phytotoxicity of Bioactive Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S.; Kaushik, N. Metabolites of endophytic fungi as novel source of biofungicide: A review. Phytochem. Rev. 2012, 11, 507–522. [Google Scholar] [CrossRef]
- Nicoletti, R.; Fiorentino, A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture 2015, 5, 918–970. [Google Scholar] [CrossRef] [Green Version]
- Pores-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef]
- Jalgaonwala, R.E.; Mohite, B.V.; Mahajan, R.T. A review: Natural products from plant associated endophytic fungi. J. Microbiol. Biotech. Res. 2011, 1, 21–32. [Google Scholar]
- Kaul, S.; Ahmed, M.; Sharma, T.; Dhar, M.K. Unlocking the yriad Bbenefits of endophytes: An overview. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Upadhyay, R.S., Dubey, N.K., Raghuwanshi, R., Eds.; Springer: New Delhi, India, 2014; pp. 41–57. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Mishra, A.; Sharma, V.K.; Gond, S.K.; Verma, S.K.; Kumar, A.; Kumar, J.; Singh, D.K.; Goutam, J. Diversity and -biopotential of endophytic fungal flora isolated from eight medicinal plants of Uttar Pradesh, India. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Upadhyay, R.S., Dubey, N.K., Raghuwanshi, R., Eds.; Springer: New Delhi, India, 2014; pp. 23–39. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L. Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their ccurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef] [Green Version]
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef]
- Kusari, S.; Lamshöft, M.; Zühlke, S.; Spiteller, M. An Endophytic fungus from Hypericum perforatum that produces hypericin. J. Nat. Prod. 2008, 71, 159–162. [Google Scholar] [CrossRef]
- Kusari, S.; Verma, V.C.; Lamshoeft, M.; Spiteller, M. An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J. Microbiol. Biotechnol. 2012, 28, 1287–1294. [Google Scholar] [CrossRef]
- Puri, S.C.; Nazir, A.; Chawla, R.; Arora, R.; Riyaz-ul-Hasan, S.; Amna, T.; Ahmed, B.; Verma, V.; Singh, S.; Sagar, R.; et al. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryl tetralin lignans. J. Biotechnol. 2006, 122, 494–510. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D.; Grothaus, P.; Bignami, G. The search for a taxol-producing microorganism among the endophytic fungi of the Pacific yew, Taxus brevifolia. J. Nat. Prod. 1995, 58, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Andrés, M.F.; Díaz, C.E.; Giménez, C.; Cabrera, R.; González-Coloma, A. Endophytic fungi as novel sources of biopesticides: The Macaronesian Laurel forest, a case study. Phytochem. Rev. 2017, 16, 1009–1022. [Google Scholar] [CrossRef]
- González-Coloma, A.; Díaz, C.E.; Andrés, M.F.; Fraga, M. Biocidal Products and Use Thereof for Controlling Phytopathogens and Pest Organism that Harm Plants. PCT Patent WO 2016/034751 A1, 27 March 2016. Available online: http://hdl.handle.net/10261/135793 (accessed on 12 March 2020).
- Kumar, S.; Kaushik, N.; Proksch, P. Identification of antifungal principle in the solvent extract of an endophytic fungus Chaetomium globosum from Withania somnifera. SpringerPlus 2013, 2, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Kaushik, N.; Edrada-Ebel, R.; Ebel, R.; Proksch, P. Isolation, characterization, and bioactivity of endophytic fungi of Tylophora indica. World J. Microbiol. Biotechnol. 2011, 27, 571–577. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, N. Batch culture fermentation of endophytic fungi and extraction of their metabolites. Bio-Protocol 2013, 3, e926. [Google Scholar] [CrossRef]
- Chowdhary, K.; Kaushik, N. Fungal endophyte diversity and bioactivity in the Indian medicinal plant Ocimum sanctum Linn. PLoS ONE 2015, 10, e0141444. [Google Scholar] [CrossRef]
- Poitout, S.; Bues, S. Elevage de plusieurs espèces de Lepidopteres Noctuidae sur milleu artificiel simplifié. Ann. Zool. Ecol. Anim. 1970, 2, 79–91. [Google Scholar]
- Julio, L.F.; Burillo, J.; Giménez, C.; Cabrera, R.; Díaz, C.E.; Sanz, J.; González-Coloma, A. Chemical and biocidal characterization of two cultivated Artemisia absinthium populations with different domestication levels. Crops Prod. 2015, 76, 787–792. [Google Scholar] [CrossRef]
- Andrés, M.F.; González-Coloma, A.; Sanz, J.; Burillo, J.; Sainz, P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2012, 11, 371–390. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Mills, G.L.; Nair, M.G. Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J. Agric. Food Chem. 2002, 50, 7581–7585. [Google Scholar] [CrossRef]
- Hui, S.P.; Murai, T.; Yoshimura, T.; Chiba, H.; Kurosawa, T. Simple chemical syntheses of TAG monohydroperoxides. Lipids 2003, 38, 1287–1292. [Google Scholar] [CrossRef]
- Shirane, N.; Takenaka, H.; Ueda, K.; Hashimoto, Y.; Katoh, K.; Ishii, H. Sterol analysis of DMI-resistant and -sensitive strains of Venturia inaequalis. Phytochemistry 1996, 41, 1301–1308. [Google Scholar] [CrossRef]
- Ferreira, R.J.; Kincses, A.; Gajdács, M.; Spengler, G.; dos Santos, D.J.V.A.; Molnár, J.; Ferreira, M.-J.U. Terpenoids from Euphorbia pedroi as multidrug-resistance reversers. J. Nat. Prod. 2018, 81, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Krzyczkowski, W.; Malinowska, E.; Suchocki, P.; Kleps, J.; Olejnik, M.; Herold, F. Isolation and quantitative determination of ergosterol peroxide in various edible mushroom species. Food Chem. 2009, 113, 351–355. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.H.; Lu, G.D. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef] [PubMed]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Casquero, P.A.; Suárez-Villanueva, V.; Carro-Huerga, G.; Álvarez-García, S.; Mayo-Prieto, S.; Lorenzana, A.; Cardoza, R.E.; Gutiérrez, S. Effect of trichodiene production by Trichoderma harzianum on Acanthoscelides obtectus. J. Stored Prod. Res. 2018, 77, 231–239. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Mayo, S.; González-López, Ó.; Reinoso, B.; Gutierrez, S.; Casquero, P.A. Inhibitory activity of Beauveria bassiana and Trichoderma spp. on the insect pests Xylotrechus arvicola (Coleoptera: Cerambycidae) and Acanthoscelides obtectus (Coleoptera: Chrisomelidae: Bruchinae). Environ. Monit. Assess 2016, 189, 12. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, H.; Ge, X.; Wu, F. Effects of vanillin on the community structures and abundances of Fusarium and Trichoderma spp. in cucumber seedling rhizosphere. J. Plant Interact. 2018, 13, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Amin, N.; Daha, L.; Agus, N. The study on the role of entomopathogenic fungal endophytes in controling the cocoa pod borer (Conopomorpha cramerella (snellen)) (Lepidoptera: Gracillariidae) on cocoa plant. J. Entomol. 2014, 11, 42–152. [Google Scholar] [CrossRef] [Green Version]
- Gange, A.C.; Eschen, R.; Wearn, J.A.; Thawer, A.; Sutton, B.C. Differential effects of foliar endophytic fungi on insect herbivores attacking a herbaceous plant. Oecologia 2012, 168, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Porras-Troncoso, M.D.; Olmedo-Monfil, V.; Herrera-Estrella, A. Trichoderma species: Versatile plant symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.F.; Li, G.H.; Zhang, K.Q. Non-volatile metabolites from Trichoderma spp. Metabolites 2019, 9, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma. Chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Ma, L.; Liu, W.; Huang, Y.; Rong, X. Two acid sorbicillin analogues from saline lands-derived fungus Trichoderma sp. J. Antibiot. 2011, 64, 645–647. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Sati, O.P.; Walia, S. Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat. Prod. Res. 2015, 29, 914–920. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Chen, G.-Y.; Li, X.-Z.; Hu, M.; Wang, B.-Y.; Ruan, B.-H.; Zhou, H.; Zhao, L.-X.; Zhou, J.; Ding, Z.-T.; et al. Phytotoxic, antibacterial, and antioxidant activities of mycotoxins and other metabolites from Trichoderma sp. Nat. Prod. Res. 2017, 31, 2745–2752. [Google Scholar] [CrossRef]
- Ganassi, S.; Grazioso, P.; De Cristofaro, A.; Fiorentini, F.; Sabatini, M.A.; Evidente, A.; Altomare, C. Long chain alcohols produced by Trichoderma citrinoviride have phagodeterrent activity against the bird cherry-oat aphid Rhopalosiphum padi. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Ishangulyyeva, G.; Najar, A.; Curtis, J.M.; Erbilgin, N. Fatty acid composition of novel host jack pine do not prevent host acceptance and colonization by the invasive mountain pine beetle and its symbiotic fungus. PLoS ONE 2016, 11, e0162046. [Google Scholar] [CrossRef]
- Ramsewak, R.S.; Nair, M.G.; Murugesan, S.; Mattson, W.J.; Zasada, J. Insecticidal fatty acids and triglycerides from Dirca palustris. J. Agric. Food Chem. 2001, 49, 5852–5856. [Google Scholar] [CrossRef] [PubMed]
- Suqi, L.; Caceres, L.; Schieck, K.; Booker, C.J.; McGarvey, B.M.; Yeung, K.K.C.; Pariente, S.; Briens, C.; Berruti, F.; Scott, I.M. Insecticidal activity of bio-oil from the pyrolysis of straw from Brassica spp. J. Agric. Food Chem. 2014, 62, 3610–3618. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.; Díaz, M.; González-Coloma, A.; González, A.; Alonso-Paz, E.; Bassagoda, M.J.; Rossini, C. Clytostoma callistegioides (Bignoniaceae) wax extract with activity on aphid settling. Phytochemistry 2010, 71, 2052–2057. [Google Scholar] [CrossRef]
- Santana, O.; Reina, M.; Fraga, B.M.; Sanz, J.; González-Coloma, A. Antifeedant activity of fatty acid esters and phytosterols from Echium wildpretii. Chem. Biodivers. 2012, 9, 567–576. [Google Scholar] [CrossRef]
- Díaz Napal, G.; Carpinella, M.C.; Palacios, S.M. Insecticidal properties of a highly potent wax isolated from Dolichandra cynanchoides Cham. Molecules 2016, 21, 1039. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ma, H.; Xie, H.; Xuan, N.; Guo, X.; Fan, Z.; Rajashekar, B.; Arnaud, P.; Offmann, B.; Picimbon, J.-F. Biotype characterization, developmental profiling, insecticide response and binding property of Bemisia tabaci chemosensory proteins: Role of CSP in insect defense. PLoS ONE 2016, 11, e0154706. [Google Scholar] [CrossRef]
- Bos, J.I.B.; Prince, D.; Pitino, M.; Maffei, M.E.; Win, J.; Hogenhout, S.A. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 2010, 6, e1001216. [Google Scholar] [CrossRef]
- Morales-Sánchez, V.; Andrés, M.F.; Díaz, C.E.; González-Coloma, A. Factors afecting the metabolite production in endophytes. Curr. Med. Chem. 2019, 26, 1–19. [Google Scholar] [CrossRef]
- Xu, F.; Wang, S.; Li, Y.; Zheng, M.; Xi, X.; Cao, H.; Cui, X.; Guo, H.; Han, C. Yield enhancement strategies of rare pharmaceutical metabolites from endophytes. Biotechnol. Lett. 2018, 40, 797–807. [Google Scholar] [CrossRef]
- Macías, F.A.; Chinchilla, N.; Varela, R.M.; Molinillo, J.M.G. Bioactive steroids from Oryza sativa L. Steroids 2006, 71, 603–608. [Google Scholar] [CrossRef]


| Extract | Fusarium graminearum | Rhizoctonia solani | Sclerotinia sclerotiorum | Botrytis cinerea | Meloidogyne javanica |
|---|---|---|---|---|---|
| % Inhibition | % Mortality | ||||
| EtOAc | 03.95 ± 8.46 | 43.40 ± 05.33 | 65.4 ± 03.50 | 09.8 ± 05.26 | 6.21 ± 1.29 |
| MeOH | 10.12 ± 8.95 | 45.30 ± 05.38 | 50.5 ± 11.38 | 22.2 ± 12.90 | 2.73 ± 0.60 |
| Hex | 08.56 ± 6.10 | 28.05 ± 18.04 | 05.6 ± 15.50 | 30.8 ± 06.53 | 7.50 ± 1.21 |
| Extract | Myzus persicae | Rhopalosiphum padi | Spodoptera littoralis |
|---|---|---|---|
| %SI a (100 µg/cm2) | %FI a (100 µg/cm2) | ||
| EtOAc | 82.52 ± 6.19 * | 72.63 ± 5.99 * | 17.29 ± 07.80 |
| MeOH | 76.89 ± 7.97 * | 54.25 ± 6.71 | 11.66 ± 07.30 |
| Hex | 87.42 ± 5.32 * | 39.79 ± 7.80 | 30.01 ± 15.94 |
| HexN | 76.63 ± 7.30 * | ||
| HexA | 97.10 ± 1.30 * | ||
| Retention Time (min) | Compound | Hex | HexA | 1A | 1M |
|---|---|---|---|---|---|
| (% Abundance) | |||||
| 23.57 | Hexadecanoic acid methyl ester | 0.78 | - | - | 19.72 |
| (methyl palmitate) | |||||
| 24.19 | Hexadecanoic acid | 15.89 | 26.70 | 24.70 | - |
| (palmitic acid) | |||||
| 26.41 | Octadecadienoic acid methyl ester | 2.89 | - | - | 36.35 |
| (methyl linoleate) | |||||
| 26.50 | Octadecenoic acid methyl ester | 0.67 | - | - | 39.25 |
| (methyl oleate) | |||||
| 26.90 | Octadecanoic acid methyl ester | - | - | - | 4.68 |
| (methyl stearate) | |||||
| 27.03 | Octadecadienoic acid | 42.60 | 43.05 | 20.76 | - |
| (linoleic acid) | |||||
| 27.12 | Octadecenoic acid | 32.30 | 27.47 | 46.15 | - |
| (oleic acid) | |||||
| 27.45 | Octadecanoic acid | 3.90 | 2.78 | 8.39 | - |
| (stearic acid) | |||||
| Extract/Compound | Myzus persicae %SI a (50 µg/cm2) | EC50 b (µg/cm2) |
|---|---|---|
| 1 | 35.86 ± 8.4 | |
| 1A | 85.56 ± 5.3 * | 6.87 (4.34–23.8) |
| 1M | 72.67 ± 6.9 * | 1.03 (0.18–5.64) |
| 2 | 15.35 ± 6.94 | |
| 3 | 18.33 ± 7.09 | |
| 4 | 17.08 ± 6.7 | |
| 5 | 38.76 ± 7.5 |
| Media | Extract Yield (g/mL)/(g/g) | Spore Count/mL |
|---|---|---|
| PDB | 00.02 | 5.04 × 106 |
| SDB | 00.07 | 8.96 × 106 |
| Sorghum | 09.84 | 7.92 × 108 |
| Barley | 03.56 | 3.95 × 108 |
| Corn | 13.16 | 4.27 × 108 |
| Pearl millet | 22.64 | 9.29 × 107 |
| Rice | 06.48 | 2.88 × 108 |
| Extract | Myzus persicae %SI a (100 µg/cm2) | EC50 b (µg/cm2) | Rhopalosiphum padi %SI a (100 µg/cm2) |
|---|---|---|---|
| Rice | 87.16 ± 3.22 * | 33.54 (26.12–42.30) | 64.49 ± 5.58 |
| Corn | 88.6 ± 5.22 * | 14.63 (08.98–23.82) | 30.43 ± 7.23 |
| Sorghum | 90.19 ± 2.28 * | 1.39 (0.01–2.00) | 62.62 ± 5.76 |
| Barley | 85.38 ± 5.32 * | 23.10 (14.07–37.58 | 54.59 ± 7.53 |
| Pearl millet | 70.31 ± 5.45 * | 38.57 (26.46–54.36) | 40.62 ± 8.75 |
| PDB | 41.08 ± 8.71 | ~100 c | 64.17 ± 6.78 |
| SDB | 51.08 ± 7.64 | ~100 c | na |
| Growth Medium | % Abundance | M. persicae EC50 (µg/cm2) | |||||
|---|---|---|---|---|---|---|---|
| Methyl Palmitate | Palmitic Acid | Methyl Linoleate | Linoleic Acid | Oleic Acid | Methyl Oleate | ||
| Rice | - | 25.87 | 30.12 | 28.19 | 3.01 | - | 33.54 |
| Corn | 0.84 | 14.23 | 41.67 | 29.45 | 3.85 | 2.12 | 14.63 |
| Sorghum | - | 11.34 | 34.19 | 36.28 | 5.50 | - | 1.39 |
| Barley | - | 21.50 | 29.49 | 23.30 | 3.09 | - | 23.10 |
| Pearl millet | 1.13 | 17.77 | 26.89 | 35.16 | 11.74 | 2.29 | 38.57 |
| PDB | - | 4.47 | 0.86 | - | - | - | 100.0 |
| SDB | - | 4.49 | 0.96 | 0.83 | 0.18 | - | 100.0 |
| CC (p) | −0.272 (0.555) | −0.599 (0.155) | −0.969 * (0.0003) | −0.932 * (0.002) | −0.497 (0.255) | −0.298 (0.516) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushik, N.; Díaz, C.E.; Chhipa, H.; Julio, L.F.; Andrés, M.F.; González-Coloma, A. Chemical Composition of an Aphid Antifeedant Extract from an Endophytic Fungus, Trichoderma sp. EFI671. Microorganisms 2020, 8, 420. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8030420
Kaushik N, Díaz CE, Chhipa H, Julio LF, Andrés MF, González-Coloma A. Chemical Composition of an Aphid Antifeedant Extract from an Endophytic Fungus, Trichoderma sp. EFI671. Microorganisms. 2020; 8(3):420. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8030420
Chicago/Turabian StyleKaushik, Nutan, Carmen E. Díaz, Hemraj Chhipa, L. Fernando Julio, M. Fe Andrés, and Azucena González-Coloma. 2020. "Chemical Composition of an Aphid Antifeedant Extract from an Endophytic Fungus, Trichoderma sp. EFI671" Microorganisms 8, no. 3: 420. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8030420