Biotransformation of Flaxseed Oil Cake into Bioactive Camembert-Analogue Using Lactic Acid Bacteria, Penicillium camemberti and Geotrichum candidum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
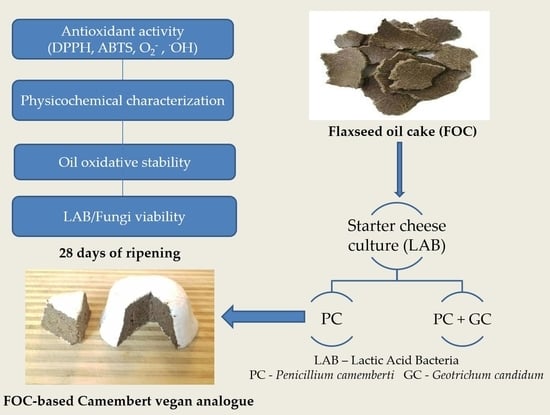
2.2. Products Preparation, Fermentation and Ripening
2.3. Microbiological Analyses
2.4. Extracts Preparation and Determination of Their Total Polyphenolic Content (TPC), Total Flavonoid Content (TFC) and Reducing Sugars Content (RCS)
2.5. Determination of Reducing Power and Radical Scavenging Activity
2.6. Determination of Total Solids Content (TSC), Protein Content (PC), Total Free Amino Acids Level (TFAAL), Ash Content (AC), pH, and Titrable Acidity (TA)
2.7. Determination of Oil Content (OC) and Oxidative Stability
2.8. Texture Profile Analyses
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Lactic Acid Bacteria and Fungi Survivability during the Ripening
3.2. The Changes of Total Solids Content, Ash Content, pH, Titrable Acidity, Protein Content and Free Amino Acids Level
3.3. The Changes of Total Phenolic, Total Flavonoid and Reducing Sugars Contents
3.4. The Changes of Antioxidant and Radical Scavenging Activities
3.5. The Changes of Oil Content and Oxidative Stability
3.6. The Textural Changes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ranadheera, C.; Vidanarachchi, J.; Rocha, R.; Cruz, A.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Tabanelli, G.; Pasini, F.; Riciputi, Y.; Vannini, L.; Gozzi, G.; Balestra, F.; Caboni, M.F.; Gardini, F.; Montanari, C. Fermented Nut-Based Vegan Food: Characterization of a Home made Product and Scale-Up to an Industrial Pilot-Scale Production. J. Food Sci. 2018, 83, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.J.; Frutos, M.J. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Sendra, E. Dairy Fat and Cardiovascular Health. Foods 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Nazhand, A.; Souto, E.B.; Lucarini, M.; Souto, S.B.; Durazzo, A.; Santini, A. Ready to Use Therapeutical Beverages: Focus on Functional Beverages Containing Probiotics, Prebiotics and Synbiotics. Beverages 2020, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Dueñas, M.; García-Estévez, I. Agricultural and Food Waste: Analysis, Characterization and Extraction of Bioactive Compounds and Their Possible Utilization. Foods 2020, 9, 817. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Marinelli, V.; Saccotelli, M.A.; Del Nobile, M.A.; Conte, A. Fruit and vegetable by-products to fortify spreadable cheese. Antioxidants 2018, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Michalak, M.; Skrzypczak, K.; Nastaj, M.; Terpiłowski, K.; Skrzypek, T.; Waśko, A.; Polak-Berecka, M. Possibility of Using Fermented Curly Kale Juice to Manufacture Feta-Type Cheese. Appl. Sci. 2020, 10, 4020. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.-M.; Biliaderis, C.G. Fermented Cereal-based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef]
- Gille, D.; Schmid, A.; Walther, B.; Vergères, G. Fermented food and non-communicable chronic diseases: A review. Nutrients 2018, 10, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekhit, A.E.D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Aseervatham, G.S.B.; Sivasudha, T.; Jeyadevi, R.; Arul Ananth, D. Environmental factors and unhealthy lifestyle influence oxidative stress in humans-an overview. Environ. Sci. Pollut. Res. Int. 2013, 20, 4356–4369. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Al, K.F.; Craven, L.J.; Seney, S.; Coons, M.; McCormick, H.; Reid, G.; O’connor, C.; Burton, J.P. Nutritional, microbial, and allergenic changes during the fermentation of cashew ‘cheese’ product using a quinoa-based rejuvelac starter culture. Nutrients 2020, 12, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Gamboa, C.; Hicks-Pérez, L.; Gutiérrez-Méndez, N.; Heredia, N.; García, S.; Nevárez-Moorillón, G.V. Microbiological changes during ripening of Chihuahua cheese manufactured with raw milk and its seasonal variations. Foods 2018, 7, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cankurt, H. The effects of adding different stabilizers in brine on the physicochemical, sensory, microbiological and textural properties of white cheese. Foods 2019, 8, 133. [Google Scholar] [CrossRef] [Green Version]
- Paszczyk, B.; Polak-śliwińska, M.; Łuczyńska, J. Fatty acids profile, trans isomers, and lipid quality indices in smoked and unsmoked cheeses and cheese-like products. Int. J. Environ. Res. Public Health 2020, 17, 71. [Google Scholar] [CrossRef] [Green Version]
- Diarra, K.; Nong, Z.G.; Jie, C. Peanut milk and peanut milk based products production: A review. Crit. Rev. Food Sci. Nutr. 2005, 45, 405–423. [Google Scholar] [CrossRef]
- Uraz, T.; Özer, B.H. Starter Cultures: Molds Employed in Food Processing. Encycl. Food Microbiol. Second Ed. 2014, 3, 522–528. [Google Scholar]
- Zulkurnain, M.; Goh, M.H.; Karim, A.A.; Liong, M.T. Development of a soy-based cream cheese. J. Texture Stud. 2008, 39, 635–654. [Google Scholar] [CrossRef]
- Tuntragul, S.; Surapat, S.; Hongsprabhas, P. Influence of rice bran oil and rice flours on physicochemical properties of a mozzarella cheese analog. Kasetsart J. Nat. Sci. 2010, 44, 924–934. [Google Scholar]
- Mohamed, A.G.; Shalaby, S.M. Texture, chemical properties and sensory evaluation of a spreadable processed cheese analogue made with apricot pulp (Prunus armeniaca L.). Int. J. Dairy Sci. 2016, 11, 61–68. [Google Scholar] [CrossRef]
- Aini, N.; Prihananto, V.; Sustriawan, B.; Romadhon, D.; Ramadhan, R.N. The formulation of cheese analogue from sweet corn extract. Int. J. Food Sci. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, B.D.; Baptista, D.P.; Cavalheiro, F.G.; Negrão, F.; Eberlin, M.N.; Gigante, M.L. Peptide profile of Camembert-type cheese: Effect of heat treatment and adjunct culture Lactobacillus rhamnosus GG. Food Res. Int. 2019, 123, 393–402. [Google Scholar] [CrossRef]
- Galli, B.D.; Martin, J.G.P.; Da Silva, P.P.M.; Porto, E.; Spoto, M.H.F. Sensory quality of Camembert-type cheese: Relationship between starter cultures and ripening molds. Int. J. Food Microbiol. 2016, 234, 71–75. [Google Scholar] [CrossRef]
- Aziza, M.; Couriol, C.; Amrane, A.; Boutrou, R. Evidences for synergistic effects of Geotrichum candidum on Penicillium camembertii growing on cheese juice. Enzyme Microb. Technol. 2005, 37, 218–224. [Google Scholar] [CrossRef]
- Boutrou, R.; Guéguen, M. Interests in Geotrichum candidum for cheese technology. Int. J. Food Microbiol. 2005, 102, 1–20. [Google Scholar] [CrossRef]
- Boutrou, R.; Aziza, M.; Amrane, A. Enhanced proteolytic activities of Geotrichum candidum and Penicillium camembertii in mixed culture. Enzyme Microb. Technol. 2006, 39, 325–331. [Google Scholar] [CrossRef]
- Boutrou, R.; Kerriou, L.; Gassi, J.Y. Contribution of Geotrichum candidum to the proteolysis of soft cheese. Int. Dairy J. 2006, 16, 775–783. [Google Scholar] [CrossRef]
- Adour, L.; Couriol, C.; Amrane, A.; Prigent, Y. Growth of Geotrichum candidum and Penicillium camembertii in liquid media in relation with the consumption of carbon and nitrogen sources and the release of ammonia and carbon dioxide. Enzyme Microb. Technol. 2002, 31, 533–542. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, Characterization, and Bioactivity of Non-Dairy Kefir-Like Fermented Beverage Based on Flaxseed Oil Cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teh, S.S.; Niven, B.E.; Bekhit, A.E.D.A.; Carne, A.; Birch, J. Optimization of polyphenol extraction and antioxidant activities of extracts from defatted flax seed cake (Linum usitatissimum L.) using microwave-assisted and pulsed electric field (PEF) technologies with response surface methodology. Food Sci. Biotechnol. 2015, 24, 1649–1659. [Google Scholar] [CrossRef]
- Dzuvor, C.; Taylor, J.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of Functional Ingredients from Flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef] [Green Version]
- Tekin, Z.H.; Karasu, S. Cold-pressed flaxseed oil by-product as a new source of fat replacers in low-fat salad dressing formulation: Steady, dynamic and 3-ITT rheological properties. J. Food Process. Preserv 2020, e14650. [Google Scholar] [CrossRef]
- Singh, K.K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A potential source of food, feed and fiber. Crit. Rev. Food Sci. Nutr. 2011, 51, 210–222. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. Valorization of flaxseed oil cake residual from cold-press oil production as a material for preparation of spray-dried functional powders for food applications as emulsion stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A. Preparation and characterization of novel flaxseed oil cake yogurt-like plant milk fortified with inulin. J. Food Nutr. Res. 2020, 59, 61–70. [Google Scholar]
- Tong, T.; Liu, Y.-J.; Kang, J.; Zhang, C.-M.; Kang, S.-G. Antioxidant Activity and Main Chemical Components of a Novel Fermented Tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Wang, Y.; Guo, G.Y.; He, Y.L.; Lu, Y.; Ye, Y.W.; Yang, Q.H.; Yang, P.Z. Physicochemical characteristics and antioxidant activity of arginine-modified melanin from Lachnum YM-346. Food Chem. 2012, 135, 2490–2497. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Barac, M.; Vucic, T.; Zilic, S.; Pesic, M.; Sokovic, M.; Petrovic, J.; Kostic, A.; Ignjatovic, I.S.; Milincic, D. The effect of in vitro digestion on antioxidant, ACE-inhibitory and antimicrobial potentials of traditional Serbian white-brined cheeses. Foods 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- BN-74 8020-07 Norm. Fat Rancidity Tests—Determination of Peroxide Value; Polish Committee for Standardization: Warsaw, Poland, 1982. [Google Scholar]
- PN-93/A-86926 Norm. Edible Vegetable Fats—Determinations of Anisidine Value and Calculation of Total Oxidation Value Totox; Polish Committee for Standardization: Warsaw, Poland, 1994. [Google Scholar]
- PN-ISO 660 Norm. Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- PN-ISO 3961 Norm. Animal and Vegetable Fats and Oils—Determination of Iodine Value; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- Corsetti, A.; Rossi, J.; Gobbetti, M. Interactions between yeasts and bacteria in the smear surface-ripened cheeses. Int. J. Food Microbiol. 2001, 69, 1–10. [Google Scholar] [CrossRef]
- HadiNezhad, M.; Duc, C.; Han, N.F.; Hosseinian, F. Flaxseed Soluble Dietary Fibre Enhances Lactic Acid Bacterial Survival and Growth in Kefir and Possesses High Antioxidant Capacity. J. Food Res. 2013, 2, 152. [Google Scholar] [CrossRef] [Green Version]
- Leclercq-Perlat, M.-N. Cheese|Camembert, Brie, and Related Varieties. In Encyclopedia of Dairy Sciences, 2nd ed.; Academic Press: San Diego, CA, USA, 2011; pp. 773–782. ISBN 978-0-12-374407-4. [Google Scholar]
- Aziza, M.; Amrane, A. Commensalism during submerged mixed culture of Geotrichum candidum and Penicillium camembertii on glutamate and lactate. Process. Biochem. 2006, 41, 2452–2457. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Mahgoub, S.A.; El-Zahar, K.M. Soft cheese supplemented with black cumin oil: Impact on food borne pathogens and quality during storage. Saudi J. Biol. Sci. 2014, 21, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlesser, J.E.; Schmidt, S.J.; Speckman, R. Characterization of Chemical and Physical Changes in Camembert Cheese During Ripening. J. Dairy Sci. 1992, 75, 1753–1760. [Google Scholar] [CrossRef]
- Ong, L.; Dagastine, R.; Kentish, S.; Gras, S. Microstructure and Composition of Full Fat Cheddar Cheese Made with Ultrafiltered Milk Retentate. Foods 2013, 2, 310–331. [Google Scholar] [CrossRef] [Green Version]
- Ropars, J. Yeasts and Molds: Penicillium Camemberti; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780081005965. [Google Scholar]
- Petrova, P.; Petrov, K. Lactic acid fermentation of cereals and pseudocereals: Ancient nutritional biotechnologies with modern applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł. Waste from the harvesting of button mushroom (Agaricus bisporus) as a source of natural melanin. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2018, 343, 23–42. [Google Scholar] [CrossRef]
- Reale, A.; Ianniello, R.G.; Ciocia, F.; Di Renzo, T.; Boscaino, F.; Ricciardi, A.; Coppola, R.; Parente, E.; Zotta, T.; McSweeney, P.L.H. Effect of respirative and catalase-positive Lactobacillus casei adjuncts on the production and quality of Cheddar-type cheese. Int. Dairy J. 2016, 63, 78–87. [Google Scholar] [CrossRef]
- Michotte, D.; Rogez, H.; Chirinos, R.; Mignolet, E.; Campos, D.; Larondelle, Y. Linseed oil stabilisation with pure natural phenolic compounds. Food Chem. 2011, 129, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Batty, D.; Waite-Cusic, J.G.; Meunier-Goddik, L. Influence of cheese-making recipes on the composition and characteristics of Camembert-type cheese. J. Dairy Sci. 2019, 102, 164–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|
| Sample | 0 | 1 | 7 | 14 | 21 | 28 |
| pH | ||||||
| PC | 6.50 ± 0.01 Aa | 6.18 ± 0.02 Ba | 6.31 ± 0.01 Ca | 6.73 ± 0.02 Da | 6.78 ± 0.00 Ea | 7.35 ± 0.01 Fa |
| PC + GC | 6.45 ± 0.01 Ab | 6.01 ± 0.01 Bb | 6.25 ± 0.05 Cb | 6.82 ± 0.01 Db | 7.04 ± 0.01 Eb | 7.92 ± 0.01 Fb |
| TA (g lactic acid/100 g) | ||||||
| PC | 0.12 ± 0.02 Aa | 0.72 ± 0.01 Ba | 0.68 ± 0.00 Ca | 0.67 ± 0.04 Ba | 0.52 ± 0.07 Da | 0.38 ± 0.00 Ea |
| PC + GC | 0.10 ± 0.01 Aa | 0.80 ± 0.01 Bb | 0.72 ± 0.01 Ca | 0.60 ± 0.01 Db | 0.42 ± 0.05 Eb | 0.22 ± 0.08 Fb |
| TSC (%) | ||||||
| PC | 25.64 ± 0.40 Ab | 25.89 ± 2.06 Aa | 27.83 ± 0.33 Ba | 27.86 ± 1.29 Ba | 26.72 ± 0.37 ABa | 23.72 ± 0.39 Ca |
| PC + GC | 24.40 ±1.03 ABCc | 26.86 ± 1.54 Ba | 27.86 ± 1.15 Ba | 25.15 ± 0.35 ABb | 25.25 ± 0.56 ABb | 23.36 ± 0.48 Ca |
| AC (%) | ||||||
| PC | 1.63 ± 0.01 Ab | 1.88 ± 0.02 Ba | 2.05 ± 0.03 Ba | 2.34 ± 0.03 Ca | 2.00 ± 0.06 Ba | 2.10 ± 0.06 BCa |
| PC + GC | 1.54 ± 0.04 Ab | 2.30 ± 0.03 Bb | 1.99 ± 0.04 Ca | 2.23 ± 0.35 BCb | 2.19 ± 0.01 BCa | 2.05 ± 0.07 Ca |
| PC (g/100 g) | ||||||
| PC | 4.50 ± 0.01 Ab | 5.11 ± 0.01 Ba | 4.65 ± 0.03 Ca | 5.84 ± 0.01 Da | 6.10 ± 0.04 Ea | 4.60 ± 0.00 Fa |
| PC + GC | 4.66 ± 0.00 Ac | 4.35 ± 0.00 Bb | 5.17 ± 0.03 Cb | 5.12 ± 0.00 Db | 4.73 ± 0.01 Eb | 4.48 ± 0.00 Fb |
| TFAAL (mg Gly/g) | ||||||
| PC | 6.84 ± 0.00 Aa | 7.44 ± 0.00 Ba | 8.25 ± 0.00 Ca | 8.28 ± 0.00 Da | 8.40 ± 0.05 Ea | 9.36 ± 0.01 Fa |
| PC + GC | 7.02 ± 0.00 Ab | 7.49 ± 0.00 Bb | 8.39 ± 0.01 Cb | 8.42 ± 0.01 Db | 8.57 ± 0.02 Eb | 9.40 ± 0.01 Fb |
| Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|
| Sample | 0 | 1 | 7 | 14 | 21 | 28 |
| TPC (mg GAE/g) | ||||||
| PC | 14.68 ± 0.08 Aa | 16.84 ± 0.18 Ba | 17.60 ± 0.20 Ca | 24.46 ± 0.00 Da | 27.00 ± 0.66 Ea | 31.02± 0.03 Fa |
| PC + GC | 14.44 ± 0.11 Aa | 16.40 ± 0.11 Bb | 18.49 ± 0.43 Cb | 30.34 ± 0.11 Db | 32.87 ± 0.11 Eb | 35.78 ± 0.02 Fb |
| TFC (mg QE/g) | ||||||
| PC | 7.67 ± 0.22 Aa | 7.90 ± 0.05 Aa | 9.83 ± 0.05 Ba | 12.52 ± 0.05 Ca | 11.00 ± 0.27 Da | 9.24 ± 0.00 Ea |
| PC + GC | 7.55 ± 0.05 Ab | 7.93 ± 0.03 Ba | 9.79 ± 0.10 Ca | 9.24 ± 0.00 Cb | 10.03 ± 0.05 Db | 9.72 ± 0.10 Cb |
| RSC (mg/g) | ||||||
| PC | 22.12 ± 0.03 Aa | 31.92 ± 0.04 Ba | 26.40 ± 0.05 Ca | 20.27 ± 0.02 Da | 19.92 ± 0.03 Ea | 18.99 ± 0.03 Ea |
| PC + GC | 23.19 ± 0.00 Aa | 33.22 ± 0.02 Bb | 21.10 ± 0.01 Cb | 19.58 ± 0.01 Db | 19.54 ± 0.01 Eb | 16.44 ± 0.01 Eb |
| Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|
| Sample | 0 | 1 | 7 | 14 | 21 | 28 |
| DPPH inhibition (%) | ||||||
| PC | 77.46 ± 0.00 Aa | 94.00 ± 0.20 Ba | 94.11 ± 0.16 Ba | 93.07 ± 0.33 Ca | 89.64 ± 0.16 Da | 81.55 ± 0.21 Ea |
| PC + GC | 76.23 ± 0.00 Ab | 93.76 ± 0.11 Ba | 94.23 ± 0.04 Ca | 93.30 ± 0.47 Da | 87.38 ± 0.19 Eb | 80.36 ± 0.07 Fb |
| ABTS inhibition (%) | ||||||
| PC | 42.84 ± 0.00 Aa | 52.97 ± 0.00 Ba | 55.74 ± 0.00 Ca | 57.43 ± 0.00 Da | 66.93 ± 0.00 Ea | 57.63 ± 0.09 Fa |
| PC + GC | 45.18 ± 0.09 Ab | 56.10 ± 0.00 Bb | 56.43 ± 0.09 Cb | 56.47 ± 0.09 Cb | 61.65 ± 0.09 Db | 59.04 ± 0.00 Eb |
| O2− inhibition (%) | ||||||
| PC | 40.59 ± 0.90 Aa | 54.93 ± 0.74 Ba | 59.13± 0.86 Ca | 67.90 ± 0.99 Da | 65.31 ± 0.12 Ea | 54.17 ± 0.16 Ba |
| PC + GC | 41.65 ± 0.41 Aa | 59.13 ± 0.99 Bb | 52.66 ± 0.35 Cb | 65.52 ± 0.06 Db | 64.65 ± 1.20 Eb | 54.87 ± 0.40 Fa |
| RP 700 nm | ||||||
| PC | 0.139 ± 0.02 Aa | 0.176 ± 0.01 Ba | 0.233 ± 0.01 Ca | 0.208 ± 0.03 Da | 0.276 ± 0.01 Ea | 0.168 ± 0.01 Fa |
| PC + GC | 0.140 ± 0.01 Aa | 0.161 ± 0.02 Bb | 0.183 ± 0.02 Cb | 0.182 ± 0.01 Cb | 0.202 ± 0.01 Db | 0.171 ± 0.01 Eb |
| ·OH inhibition (%) | ||||||
| PC | 35.48 ± 0.02 Aa | 40.17 ± 0.03 Ba | 53.60 ± 0.00 Ca | 63.17 ± 0.00 Da | 77.77 ± 0.02 Ea | 67.49 ± 0.05 Fa |
| PC + GC | 34.57 ± 0.01 Ab | 48.57 ± 0.01 Bb | 53.45 ± 0.01 Cb | 61.45 ± 0.05 Db | 71.38 ± 0.01 Eb | 69.48 ± 0.01 Fb |
| Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|
| Sample | 0 | 1 | 7 | 14 | 21 | 28 |
| Oil Content (%) | ||||||
| PC | 3.00 ± 0.03 Aa | 3.30 ± 0.04 Ba | 3.61 ± 0.05 Ca | 3.11 ± 0.02 Da | 2.91 ± 0.01 Ea | 2.41 ± 0.04 Fa |
| PC + GC | 3.00 ± 0.03 Aa | 2.90 ± 0.05 Bb | 3.03 ± 0.02 Ab | 2.91 ± 0.05 Bb | 2.81 ± 0.05 Cb | 2.70 ± 0.05 Db |
| Peroxide Value (mg O/100 g) | ||||||
| PC | 4.59 ± 0.74 Aa | 11.32 ± 3.30 Ba | 17.70 ± 0.33 Ca | 8.19 ± 1.43 Aa | 12.38 ± 0.31 Ba | 55.18 ± 2.32 Da |
| PC + GC | 6.89 ± 0.99 Aa | 16.42 ± 1.01 Bb | 22.06 ± 2.12 BCb | 21.56 ± 0.48 BCb | 23.34 ± 2.35 Cb | 40.08 ± 0.00 Db |
| Anisidine Value (-) | ||||||
| PC | 2.17 ± 0.33 Aa | 2.24 ± 0.23 Aa | 3.71 ± 0.59 Ba | 3.62 ± 0.58 Ba | 9.00 ± 0.05 | 3.95 ± 0.35 Ba |
| PC + GC | 1.02 ± 0.21 Ab | 8.79 ± 0.82 Bb | 7.73 ± 0.15 Cb | 10.18 ± 0.05 Db | 6.57 ± 0.62 Eb | 2.95 ± 0.29 Fb |
| TOTOX (-) | ||||||
| PC | 2.28 ± 0.31 Aa | 2.53 ± 0.26 Aa | 4.17 ± 0.59 Ba | 3.84 ± 0.54 Ba | 9.32 ± 0.06 Ca | 3.33 ± 0.30 Da |
| PC + GC | 1.20 ± 0.23 Ab | 9.22 ± 0.74 Bb | 8.30 ± 0.21 Cb | 10.74 ± 0.07 Db | 7.18 ± 0.68 Eb | 10.13 ± 0.44 Fb |
| Acid Value (mg NaOH/g) | ||||||
| PC | 65.96 ± 0.07 Aa | 88.12 ± 0.44 Ba | 84.68 ± 0.14 Ca | 79.08 ± 0.64 Da | 62.43 ± 0.52 Ea | 59.31 ± 0.43 Fa |
| PC + GC | 75.66 ± 0.83 Ab | 74.99 ± 0.44 Bb | 82.14 ± 0.67 Cb | 80.37 ± 0.18 Db | 78.59 ± 0.36 Eb | 58.72 ± 0.38 Fa |
| Iodine Value (g/100 g) | ||||||
| PC | 156.95 ± 1.03 Aa | 173.37 ± 2.67 Ba | 171.84 ± 3.36 Ba | 189.99 ± 2.81 Ca | 204.08 ± 3.28 Da | 183.59 ± 0.00 Ea |
| PC + GC | 160.60 ± 7.43 Aa | 160.21 ± 1.12 Ab | 182.80 ± 0.27 Bb | 194.48 ± 3.33 Cb | 200.49 ± 1.15 Cb | 173.47 ± 2.11 Db |
| Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|
| Sample | 0 | 1 | 7 | 14 | 21 | 28 |
| Springness (N) | ||||||
| PC | 1.33 ± 0.15 Aa | 1.45 ± 0.08 Aa | 1.64 ± 0.21 Ba | 1.55 ± 0.22 Ca | 2.01 ± 0.11 Da | 2.08 ± 0.18 Da |
| PC + GC | 1.34 ± 0.10 Aa | 1.42 ± 0.10 Aa | 1.58 ± 0.92 Bb | 1.83 ± 0.18 Cb | 1.87 ± 0.16 Cb | 1.66 ± 0.16 Da |
| Gumminess (N) | ||||||
| PC | 0.75 ± 0.05 Aa | 0.87 ± 0.12 Aa | 1.38 ± 0.09 Ba | 1.53 ± 0.17 Ca | 1.72 ± 0.12 Da | 1.92 ± 0.32 Ea |
| PC + GC | 0.72 ± 0.03 Aa | 0.78 ± 0.21 Aa | 0.82 ± 0.09 Ab | 1.02 ± 0.10 Bb | 1.45 ± 0.32 Cb | 1.23 ± 0.10 Bb |
| Chewiness (N) | ||||||
| PC | 1.01 ± 0.20 Aa | 1.50 ± 0.14 Aa | 1.65 ± 0.27 Aa | 2.34 ± 0.29 Ba | 3.45 ± 0.68 Ca | 3.17 ± 0.64 Ca |
| PC + GC | 0.99 ± 0.23 Aa | 1.18 ± 0.55 Aa | 1.20 ± 0.09 Aa | 1.86 ± 0.23 Ba | 2.70 ± 0.13 Cb | 2.05 ± 0.22 Bb |
| Cohesiveness (N) | ||||||
| PC | 0.54 ± 0.07 Aa | 0.48 ± 0.05 Ba | 0.35 ± 0.02 Ca | 0.27± 0.02 Da | 0.30 ± 0.04 CDa | 0.25 ± 0.04 Da |
| PC + GC | 0.56 ± 0.02 Aa | 0.46 ± 0.01 Ba | 0.34 ± 0.07 Ca | 0.27± 0.05 Da | 0.27 ± 0.01 Da | 0.25 ± 0.04 Da |
| Hardness (N) | ||||||
| PC | 2.11 ± 0.44 Aa | 2.20 ± 0.16 Aa | 2.47 ± 0.55 Aa | 3.86 ± 0.20 Ba | 3.92 ± 0.74 Ba | 5.37 ± 0.26 Ca |
| PC + GC | 2.10 ± 0.52 Aa | 2.15 ± 0.23 Aa | 2.28 ± 0.49 Aa | 2.85 ± 0.15 Bb | 3.66 ± 0.79 Ca | 3.62 ± 0.57 Cb |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewicz, Ł.; Drozłowska, E.; Tarnowiecka-Kuca, A.; Bartkowiak, A.; Mazurkiewicz-Zapałowicz, K.; Salachna, P. Biotransformation of Flaxseed Oil Cake into Bioactive Camembert-Analogue Using Lactic Acid Bacteria, Penicillium camemberti and Geotrichum candidum. Microorganisms 2020, 8, 1266. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8091266
Łopusiewicz Ł, Drozłowska E, Tarnowiecka-Kuca A, Bartkowiak A, Mazurkiewicz-Zapałowicz K, Salachna P. Biotransformation of Flaxseed Oil Cake into Bioactive Camembert-Analogue Using Lactic Acid Bacteria, Penicillium camemberti and Geotrichum candidum. Microorganisms. 2020; 8(9):1266. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8091266
Chicago/Turabian StyleŁopusiewicz, Łukasz, Emilia Drozłowska, Alicja Tarnowiecka-Kuca, Artur Bartkowiak, Kinga Mazurkiewicz-Zapałowicz, and Piotr Salachna. 2020. "Biotransformation of Flaxseed Oil Cake into Bioactive Camembert-Analogue Using Lactic Acid Bacteria, Penicillium camemberti and Geotrichum candidum" Microorganisms 8, no. 9: 1266. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8091266