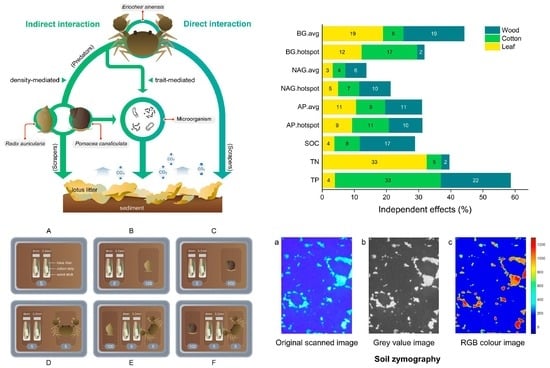
Top-Down Effect of Arthropod Predator Chinese Mitten Crab on Freshwater Nutrient Cycling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Feeding Conditions
2.2. Crab Feeding Preference Experiment
2.3. Litter Decomposition Experiment
2.3.1. Experimental Design
2.3.2. Sample Recovery and Testing
2.4. Data Analysis
3. Results
3.1. Predation of Chinese Mitten Crab on Both Snail Species
3.2. Dynamic Changes in the Water Quality Parameters of the Decomposition System
3.3. Decomposition Rate of Lotus Leaf and Standard Litter
3.4. Correlation between Sediment Properties and Litter Decomposition
3.5. Changes in the Microbial Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velthuis, M.; Kosten, S.; Aben, R.; Kazanjian, G.; Hilt, S.; Peeters, E.T.H.M.; van Donk, E.; Bakker, E.S. Warming enhances sedimentation and decomposition of organic carbon in shallow macrophyte-dominated systems with zero net effect on carbon burial. Glob. Chang. Biol. 2018, 24, 5231–5242. [Google Scholar] [CrossRef] [Green Version]
- Battin, T.J.; Kaplan, L.A.; Findlay, S.; Hopkinson, C.S.; Marti, E.; Packman, A.I.; Newbold, J.D.; Sabater, F. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 2008, 1, 95–100. [Google Scholar] [CrossRef]
- Blagodatsky, S.; Smith, P. Soil physics meets soil biology: Towards better mechanistic prediction of greenhouse gas emissions from soil. Soil Biol. Biochem. 2012, 47, 78–92. [Google Scholar] [CrossRef]
- Bastviken, S.K.; Eriksson, P.G.; Premrov, A.; Tonderski, K. Potential denitrification in wetland sediments with different plant species detritus. Ecol. Eng. 2005, 25, 183–190. [Google Scholar] [CrossRef]
- Bishop, M.J.; Kelaher, B.P. Replacement of native seagrass with invasive algal detritus: Impacts to estuarine sediment communities. Biol. Invasions 2013, 15, 45–59. [Google Scholar] [CrossRef]
- Alam, M.K.; Negishi, J.N.; Pongsivapai, P.; Yamashita, S.; Nakagawa, T. Additive Effects of Sediment and Nutrient on Leaf Litter Decomposition and Macroinvertebrates in Hyporheic Zone. Water 2021, 13, 1340. [Google Scholar] [CrossRef]
- Wang, F.; Lin, D.M.; Li, W.; Dou, P.P.; Han, L.; Huang, M.F.; Qian, S.H.; Yao, J.M. Meiofauna promotes litter decomposition in stream ecosystems depending on leaf species. Ecol. Evol. 2020, 10, 9257–9270. [Google Scholar] [CrossRef]
- Chauvet, E.; Ferreira, V.; Giller, P.S.; McKie, B.G.; Tiegs, S.D.; Woodward, G.; Elosegi, A.; Dobson, M.; Fleituch, T.; Graça, M.A.S.; et al. Chapter Three—Litter Decomposition as an Indicator of Stream Ecosystem Functioning at Local-to-Continental Scales: Insights from the European RivFunction Project. In Advances in Ecological Research; Dumbrell, A.J., Kordas, R.L., Woodward, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 55, pp. 99–182. [Google Scholar]
- Tank, J.L.; Rosi-Marshall, E.J.; Griffiths, N.A.; Entrekin, S.A.; Stephen, M.L. A review of allochthonous organic matter dynamics and metabolism in streams. J. N. Am. Benthol. Soc. 2010, 29, 118–146. [Google Scholar] [CrossRef] [Green Version]
- Dangles, O.; Gessner, M.O.; Guerold, F.; Chauvet, E. Impacts of stream acidification on litter breakdown: Implications for assessing ecosystem functioning. J. Appl. Ecol. 2004, 41, 365–378. [Google Scholar] [CrossRef] [Green Version]
- Migliorini, G.H.; Romero, G.Q. Warming and leaf litter functional diversity, not litter quality, drive decomposition in a freshwater ecosystem. Sci. Rep. 2020, 10, 20333. [Google Scholar] [CrossRef] [PubMed]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; Mckie, B.G.; Bardgett, R.D.; Wall, D.H.; Hattenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, M.; Malmqvist, B. Ecosystem process rate increases with animal species richness: Evidence from leaf-eating, aquatic insects. Oikos 2000, 89, 519–523. [Google Scholar] [CrossRef]
- Santonja, M.; Pellan, L.; Piscart, C. Macroinvertebrate identity mediates the effects of litter quality and microbial conditioning on leaf litter recycling in temperate streams. Ecol. Evol. 2018, 8, 2542–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornut, J.; Ferreira, V.; Gonçalves, A.L.; Chauvet, E.; Canhoto, C. Fungal alteration of the elemental composition of leaf litter affects shredder feeding activity. Freshw. Biol. 2015, 60, 1755–1771. [Google Scholar] [CrossRef]
- Usio, N. Effects of crayfish on leaf processing and invertebrate colonisation of leaves in a headwater stream: Decoupling of a trophic cascade. Oecologia 2000, 124, 608–614. [Google Scholar] [CrossRef]
- Hicks, L.C.; Frey, B.; Kjoller, R.; Lukac, M.; Moora, M.; Weedon, J.T.; Rousk, J. Toward a function-first framework to make soil microbial ecology predictive. Ecology 2021, 103, e03594. [Google Scholar] [CrossRef]
- Carvalho, F.; Pascoal, C.; Cássio, F.; Teixeira, A.; Sousa, R. Combined per-capita and abundance effects of an invasive species on native invertebrate diversity and a key ecosystem process. Freshw. Biol. 2022, 67, 828–841. [Google Scholar] [CrossRef]
- Villanueva, V.D.; Albariño, R.; Canhoto, C. Positive effect of shredders on microbial biomass and decomposition in stream microcosms. Freshw. Biol. 2012, 57, 2504–2513. [Google Scholar] [CrossRef]
- Juette, T.; Cucherousset, J.; Cote, J. Animal personality and the ecological impacts of freshwater non-native species. Curr. Zool. 2014, 60, 417–427. [Google Scholar] [CrossRef]
- Carvalho, F.; Pascoal, C.; Cássio, F.; Sousa, R. Effects of intrapopulation phenotypic traits of invasive crayfish on leaf litter processing. Hydrobiologia 2018, 819, 67–75. [Google Scholar] [CrossRef]
- Luo, Z.; Tan, X.; Chen, Y.; Wang, W.; Zhou, G. Apparent digestibility coefficients of selected feed ingredients for Chinese mitten crab Eriocheir sinensis. Aquaculture 2008, 285, 141–145. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Xu, N.; Lin, H.; Yu, F.; Huang, C.; Li, Z. Signs of claw asymmetry appear in a homochelate crab. Appl. Anim. Behav. Sci. 2021, 246, 105537. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Liu, S.W.; He, C.; Yu, X.P. Distribution and the origin of invasive apple snails, Pomacea canaliculata and P. maculata (Gastropoda: Ampullariidae) in China. Sci. Rep. 2018, 8, 1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; Molinos, J.G.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S.; et al. Responses of Marine Organisms to Climate Change across Oceans. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef] [Green Version]
- Persson, T.; Bååth, E.; Clarholm, M.; Lundkvist, H.; Söderström, B.; Sohlenius, B. Trophic structure, biomass dynamics and carbon metabolism of soil organisms in a Scots pine forest. In Structure and Function of Nothern Coniferous Forests—An Ecosystem Study; Persson, T., Ed.; Swedish Natural Science Research Council (NFR): Stockholm, Sweden; Ecological Bulletins: Stockholm, Sweden, 1980; Volume 32, pp. 419–462. [Google Scholar]
- Yamada, K.; Toyohara, H. Function of meiobenthos and microorganisms in cellulose breakdown in sediments of wetlands with different origins in Hokkaido. Fish. Sci. 2012, 78, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, L.; Cao, T.; He, W.; Lu, S.; Li, F.; Zhang, Z.; Chang, T.; Tian, X. Legacy effect of plant chemical defence substances on litter decomposition. Plant Soil 2023, 487, 93–108. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, L.; Cao, T.; Chen, J.; Lv, M.; Wei, S.; Lu, S.; Tian, X. Microplastics are transferred by soil fauna and regulate soil function as material carriers. Sci. Total Environ. 2023, 857, 159690. [Google Scholar] [CrossRef]
- Tiegs, S.D.; Costello, D.M.; Isken, M.W.; Woodward, G.; McIntyre, P.B.; Gessner, M.O.; Chauvet, E.; Griffiths, N.A.; Flecker, A.S.; Acuna, V.; et al. Global patterns and drivers of ecosystem functioning in rivers and riparian zones. Sci. Adv. 2019, 5, eaav0486. [Google Scholar] [CrossRef] [Green Version]
- Jamei, M.; Ahmadianfar, I.; Chu, X.; Yaseen, Z.M. Prediction of surface water total dissolved solids using hybridized wavelet-multigene genetic programming: New approach. J. Hydrol. 2020, 589, 125335. [Google Scholar] [CrossRef]
- Spohn, M.; Kuzyakov, Y. Distribution of microbial- and root-derived phosphatase activities in the rhizosphere depending on P availability and C allocation—Coupling soil zymography with C-14 imaging. Soil Biol. Biochem. 2013, 67, 106–113. [Google Scholar] [CrossRef]
- Kraychenko, A.N.; Guber, A.K.; Razavi, B.S.; Koestel, J.; Quigley, M.Y.; Robertson, G.P.; Kuzyakov, Y. Microbial spatial footprint as a driver of soil carbon stabilization. Nat. Commun. 2019, 10, 3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Quan, Z.; Sun, Y.; Du, J.; Liu, B. Excess sulfur and Fe elements drive changes in soil and vegetation at abandoned coal gangues, Guizhou China. Sci. Rep. 2020, 10, 10456. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Shi, L.; Wang, H.; Fu, X.; Kou, L.; Meng, W.; Dai, X. Soil enzyme activities and their stoichiometry of typical plantations in mid-subtropical China. Chin. J. Appl. Ecol. 2020, 31, 1980–1988. [Google Scholar] [CrossRef]
- Amano, T.; Székely, T.; Wauchope, H.S.; Sandel, B.; Nagy, S.; Mundkur, T.; Langendoen, T.; Blanco, D.; Michel, N.L.; Sutherland, W.J. Responses of global waterbird populations to climate change vary with latitude. Nat. Clim. Chang. 2020, 10, 959–964. [Google Scholar] [CrossRef]
- Turke, M.; Schmidt, A.; Schadler, M.; Hotes, S.; Weisser, W.W. Are invasive apple snails important neglected decomposers of rice straw in paddy fields? Biol. Agric. Hortic. 2018, 34, 245–257. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bellingham, P.J.; Fukami, T.; Mulder, C.P.H. Promotion of ecosystem carbon sequestration by invasive predators. Biol. Lett. 2007, 3, 479–482. [Google Scholar] [CrossRef] [Green Version]
- Paterson, R.A.; Pritchard, D.W.; Dick, J.T.A.; Alexander, M.E.; Hatcher, M.J.; Dunn, A.M. Predator cue studies reveal strong trait-mediated effects in communities despite variation in experimental designs. Anim. Behav. 2013, 86, 1301–1313. [Google Scholar] [CrossRef] [Green Version]
- Bolnick, D.I.; Preisser, E.L. Resource competition modifies the strength of trait-mediated predator-prey interactions: A meta-analysis. Ecology 2005, 86, 2771–2779. [Google Scholar] [CrossRef]
- Ewers, C.; Beiersdorf, A.; Wieski, K.; Pennings, S.C.; Zimmer, M. Predator/Prey-Interactions Promote Decomposition of Low-Quality Detritus. Wetlands 2012, 32, 931–938. [Google Scholar] [CrossRef]
- Chen, X.X.; Wiesmeier, M.; Sardans, J.; Van Zwieten, L.; Fang, Y.Y.; Gargallo-Garriga, A.; Chen, Y.Y.; Chen, S.Y.; Zeng, C.S.; Penuelas, J.; et al. Effects of crabs on greenhouse gas emissions, soil nutrients, and stoichiometry in a subtropical estuarine wetland. Biol. Fertil. Soils 2021, 57, 131–144. [Google Scholar] [CrossRef]
- Hawlena, D.; Strickland, M.S.; Bradford, M.A.; Schmitz, O.J. Fear of predation slows plant-litter decomposition. Science 2012, 336, 1434–1438. [Google Scholar] [CrossRef]
- Holway, D.A.; Cameron, E.K. The importance of scavenging in ant invasions. Curr. Opin Insect Sci. 2021, 46, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Ferlian, O.; Eisenhauer, N.; Aguirrebengoa, M.; Camara, M.; Ramirez-Rojas, I.; Santos, F.; Tanalgo, K.; Thakur, M.P. Invasive earthworms erode soil biodiversity: A meta-analysis. J. Anim. Ecol. 2018, 87, 162–172. [Google Scholar] [CrossRef] [Green Version]
- Berger, E.; Fror, O.; Schafer, R.B. Salinity impacts on river ecosystem processes: A critical mini-review. Philos. Trans. R. Soc. B 2019, 374. [Google Scholar] [CrossRef] [Green Version]
- Wright, M.S.; Covich, A.P. The effect of macroinvertebrate exclusion on leaf breakdown rates in a tropical headwater stream. Biotropica 2005, 37, 403–408. [Google Scholar] [CrossRef]
- Duffy, J.E.; Cardinale, B.J.; France, K.E.; McIntyre, P.B.; Thebault, E.; Loreau, M. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 2007, 10, 522–538. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.X.; Richardson, J.S.; Negishi, J.N. Detritus processing, ecosystem engineering and benthic diversity: A test of predator-omnivore interference. J. Anim. Ecol. 2004, 73, 756–766. [Google Scholar] [CrossRef]
- Green, P.T.; Lake, P.S.; O’Dowd, D.J. Monopolization of litter processing by a dominant land crab on a tropical oceanic island. Oecologia 1999, 119, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Fang, Y.; Chen, Y.; Kong, X.; Yang, J.; Alharbi, H.; Kuzyakov, Y.; Tian, X. Synergy of saprotrophs with mycorrhiza for litter decomposition and hotspot formation depends on nutrient availability in the rhizosphere. Geoderma 2022, 410, 115662. [Google Scholar] [CrossRef]
- Cao, T.; Kong, X.; He, W.; Chen, Y.; Fang, Y.; Li, Q.; Chen, Q.; Luo, Y.; Tian, X. Spatiotemporal characteristics of enzymatic hotspots in subtropical forests: In situ evidence from 2D zymography images. Catena 2022, 216, 106365. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Guber, A.; Kravchenko, A.; Razavi, B.S.; Uteau, D.; Peth, S.; Blagodatskaya, E.; Kuzyakov, Y. Quantitative soil zymography: Mechanisms, processes of substrate and enzyme diffusion in porous media. Soil Biol. Biochem. 2018, 127, 156–167. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, S.; Wang, Z.; Chen, D.; Dai, G.; Feng, B.; Su, X.; Hu, H.; Li, K.; Han, W.; et al. Divergent accumulation of microbial necromass and plant lignin components in grassland soils. Nat. Commun. 2018, 9, 3480. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.S.; Jiang, J.Q.; Zhao, Q.L.; Wang, K.; Yu, H. Analysis of functional genomes from metagenomes: Revealing the accelerated electron transfer in microbial fuel cell with rhamnolipid addition. Bioelectrochemistry 2018, 119, 59–67. [Google Scholar] [CrossRef]
- Zheng, M.Q.; Shi, J.X.; Xu, C.Y.; Han, Y.X.; Zhang, Z.W.; Han, H.J. Insights into electroactive biofilms for enhanced phenolic degradation of coal pyrolysis wastewater (CPW) by magnetic activated coke (MAC): Metagenomic analysis in attached biofilm and suspended sludge. J. Hazard. Mater. 2020, 395, 122688. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, H.; Carvalho, F.; Chen, Y.; Lai, L.; Ge, J.; Tian, X.; Luo, Y. Top-Down Effect of Arthropod Predator Chinese Mitten Crab on Freshwater Nutrient Cycling. Animals 2023, 13, 2342. https://0-doi-org.brum.beds.ac.uk/10.3390/ani13142342
Wang L, Liu H, Carvalho F, Chen Y, Lai L, Ge J, Tian X, Luo Y. Top-Down Effect of Arthropod Predator Chinese Mitten Crab on Freshwater Nutrient Cycling. Animals. 2023; 13(14):2342. https://0-doi-org.brum.beds.ac.uk/10.3390/ani13142342
Chicago/Turabian StyleWang, Lin, Hongjun Liu, Francisco Carvalho, Yunru Chen, Linshiyu Lai, Jiachun Ge, Xingjun Tian, and Yunchao Luo. 2023. "Top-Down Effect of Arthropod Predator Chinese Mitten Crab on Freshwater Nutrient Cycling" Animals 13, no. 14: 2342. https://0-doi-org.brum.beds.ac.uk/10.3390/ani13142342