3.1. Acoustic Analysis of Digesters
Acoustic analyses of the digesters were performed during the summer of 2019 rather than the summer of 2018. This was because of a persistent 60-Hz hum that occurred in the recordings which we were unable to locate the source of until we disconnected the float switches controlling the PVC float valves. After identifying the source of the 60-cycle hum, power to the float switches and valves was shut off at the electrical breaker box except during digester feeding.
Initially, it was planned to compare the performance of speakers placed facing towards the bottom of the tank to those placed facing transversely at the bottom of the tank to determine if this would affect relative biogas production. We realized that this was not possible, since the system would exhibit hysteresis, and the amount of biogas produced would partially depend on the degree to which the previous sound treatment had affected sludge breakdown. Therefore, the pair of speakers that were operated were simply alternated week to week, and no significant difference in biogas production was noted due to speaker placement.
Previously [
5], wastewater was exposed to 1000-Hz sine waves as a means of enhancing biogas production, as well as sludge breakdown. One of the interesting aspects of this study was that the bubble harmonics of the excitation frequency were evident in acoustic spectra of the wastewater, often followed by subharmonics symptomatic of incipient inertial cavitation. In the present study, bubble harmonics at 2
f0, 3
f0, 4
f0,…, and, presumably, beyond the recording capability of our equipment, were also evident. We never observed the appearance of subharmonics, nor did we ever note the appearance of broadband noise indicative of cavitation cloud collapse.
In the previous study [
5], biogas production was enhanced by 12 percent as compared to control digesters. We speculated that the effectiveness of sound treatment may have been limited by the speakers being placed in the sludge layer of the digester. The observation of widespread or chaotic cavitation may have been a direct consequence of the speaker placement, leading to dramatic acoustic effects but also limiting the effectiveness of the treatment.
Figure 2 illustrates the fundamental excitation frequency and first four harmonics for 100-, 500-, 1000-, 2000-, and 3000-Hz sine waves played at one-half volume, as observed in the primary anaerobic digester. These frequencies were chosen as being well within the optimum frequency range of the speakers (Supplemental Material). The 1000-Hz sine wave was the most powerful used, the fundamental frequency having a peak intensity of −1.5 dB relative to the full scale (dBFS) in our recordings, while the fundamental frequencies of 100, 500, 2000, and 3000 Hz had much lower intensities despite the speaker having a fundamentally flat frequency response from 500 to 4000 Hz in our tests. It is also interesting to note that the odd-numbered harmonics were more intense than even-numbered harmonics for all five sine waves.
While the peak intensity of the fundamental frequency and harmonics were quite high in the digester, overall acoustic intensity at sonic frequencies was quite low and comparable to that of ambient sound in the digester due to the narrow frequency range of the fundamentals and harmonics. As stated in Loughrin et al. [
5], we envision anerobic digestate as a gas-saturated liquid which, in addition to solvated gases, contains a continuum of bubble sizes, each of which will have a resonant frequency proportional to its radius. Therefore, in the expectation of affecting the vibrational state of the greatest possible numbers of bubbles, we decided to use audio files with greater frequency coverage than was possible with single-frequency or even multiple-frequency sine waves. By varying the frequencies and intensities of the audio excitation, we also hoped to avoid the occurrence of standing waves, wherein the point of peak amplitude of a given frequency does not vary in space. This was particularly important considering the wavelength of the frequencies used. Assuming a sound velocity in salt water of 1400 m s
−1, a 1000-Hz sound wave would have a wavelength of 1.4 m. Since the primary anaerobic digesters only had a radius of approximately 1.2 m, rapid variations in wavelength would be more likely to transfer energy efficiently to the digester, since the presence of standing waves would be avoided and fixed points within the digester where sound amplitude were at maximum and minimum would be eliminated.
Music with broad frequency coverage was chosen for routine excitation of the digestate, rather than recordings of white or other broadband noise with similar or greater frequency coverage. When playing music to the digester, it was easier to spot the occurrence of cavitation events, since broadband noise obscured the frequencies induced by cavitation. We chose to use “classical” music, or what might be more properly termed orchestral compositions, since these works are often characterized by wide and often abrupt variations in amplitude and are harmonically complex. We felt that these characteristics would have a high likelihood of exciting a wide range of intrinsic bubble resonant frequencies and that variations in amplitude would allow us to more easily observe cavitation events. Musical compositions afforded higher amplitude in the range of 0–4000 Hz than did individual sine waves when played to the digestate, even when accounting for bubble harmonics of the excitation frequency (
Figure 3). As a further benefit of using music rather than sine waves or broadband noise, we found that music was less likely to be transmitted through the concrete base of the system and be detected by the hydrophones in the primary control digester than were high-intensity, narrow-band sine waves. While some “cross-contamination” of sound did occur via transmission of sound from the sound-treated tank to the control tank, transmitted sounds recorded in the control digester were 30–40 dB less intense than in the sound-treated digester.
We did find that music, rather than sine waves or broadband white noise, was more likely to induce noticeable cavitational events. This seemed to confirm that the harmonic and amplitude variations of the music were more likely to perturb the bubbles than were broadband noise or sine waves. Some of the cavitation events were quite violent, with amplitudes of as high as 104 greater than the level of the music being played to the digester when the amplifier was at one-quarter volume. This represents a 104 increase in energy input into the system, although without calibrated hydrophones, we were unable to quantify the intensity of these events.
Hydrophones were also installed in the primary control digester so that we might compare acoustics between the two tanks. After several weeks of exposure to sound on a two-h sound, one-h silence schedule, background recordings of the sound-treated primary digester were louder than those of the control primary digester (
Figure 4). When sound was played to the primary sound-treated digester at half-volume, peak amplitude reached as high as −8 dBFS. When no sound was played to the primary digester, amplitude averaged roughly −58 dBFS but only about −87 dBFS in the control primary digester. There did not seem so much that there were more cavitation events in the sound-treated digester but that these events were louder than in the control digester. However, this may still be an indication of a greater number of cavitation events, since we do not know the distance at which the hydrophones were able to detect cavitation. Therefore, the apparent louder cavitation in the sound-treated digester may have been an indication of more cavitation inception or collapse occurring in the immediate vicinity of the hydrophone.
In addition to the louder cavitation events in the sound-treated primary digester, the base amplitude was greater than that of the control digester, especially in the region from approximately 1500 to 3500 Hz. This may have been due to more bubble resonance in the sound-treated digester or simply due to a greater number of cavitation events that increase the background sound level of the sound-treated digester. It is interesting to speculate that a greater number, or greater average intensity, of cavitation event flows could be induced in the digester due to both cavitation inception and collapse and to drag-induced flows resulting from rising bubbles [
20]. This would be similar to biogas recirculation, which has been used to provide some degree of mixing in anaerobic digesters [
21].
The most likely explanation for the higher base amplitude of the sound-treated digester is greater metabolic activity than in the control digester. This could be due to a combination of physical and biological effects. Among the physical factors that could enhance biological activity in the digester are vibrational energy imparted to sludge, which would act to accelerate its breakdown; acoustic streaming, which would accelerate nutrient mixing; and accelerated sludge breakup due to acoustically-induced cavitation. All these factors could enhance microbial colonization and utilization of wastewater and sludge. Sound has been shown to enhance the growth of microorganisms such as
Brevibacillus parabrevis,
Escherichia coli, and
Saccharomyces cerevisiae [
22,
23,
24]. It is unclear whether accelerated microbial growth in the presence of sound in these or our experiments is proximally due to physiological responses to the sound itself or greater nutrient availability due to mixing and matrix breakdown. Microbial community analyses are planned to determine if microbial numbers and/or populations were affected by the sound treatment.
Background sound levels in the secondary anaerobic sound-treated digester were also much lower than that of the primary sound-treated digester. Since gas production in the secondary sound-treated digester was only about 4% that of the primary sound-treated digester, this was not unexpected. Examples of recordings made from the digesters are given in the supplemental materials.
3.2. Digester Performance
Initially, the primary digesters were each seeded with approximately 750 L of swine waste obtained from the lagoon of a farrow to finish operation, along with 22.7-kg poultry litter and 4.5-kg cracked corn. From that point onwards, the digesters were fed 22.7 kg cracked corn or defatted soybean meal weekly until July 17, after which they were fed twice weekly. From April 10 through July 10, which we term the initial evaluation period, gas production from the digesters was low, averaging 3500 and 1910 L per week from the primary and secondary sound-treated digesters, respectively, and 2890 and 1250 L per week from the primary and secondary control digesters, respectively (
Table 1). Gas quality was also poor, with CH
4 averaging only 39% and 59% of the combined total for CH
4 and CO
2 in the primary sound-treated and control digesters, respectively.
This may have been due to inadequate microbial seeding of the digesters but was more likely the result of poor bicarbonate buffering and the resultant low pH. Bicarbonate buffering averaged only 2.53 and 3.26 mM in the primary sound-treated and control digesters, respectively, during this period, and pH averaged only 6.30 and 6.33. Gas quality was much higher in the secondary sound-treated and control digesters, with CH4 averaging 77% in the secondary sound-treated digester and 75% in the secondary control digester. Bicarbonate buffering and pH were greater in the secondary digesters, which largely accounted for the better gas quality; on the other hand, the digesters were designed to retain as much sludge in the primary tanks as possible so that we could evaluate the effects of sound on sludge degradation. Due to this, gas production in the secondary sound-treated and control digesters averaged only 54% and 43% that of their respective primary digesters during this period.
On June 29, 750 L of digestate from a commercial thermophilic digester located on a poultry farm was added to each of the primary anaerobic digesters. This was done both for additional microbial seeding and to raise the pH of the digesters. This digestate had a high buffering capacity with HCO3−, averaging 341 ± 100 mM, and with an average pH of 7.32 ± 0.14.
After feeding of digestate from the thermophilic digester, gas production in the primary sound-treated digester increased by 895% and decreased by 58% in the secondary sound-treated digester, whereas gas production increased by 368% in the primary control digester and deceased by 25% in the secondary control digester (
Table 2). The increase in gas production in the primary digesters was likely due both to microbial seeding and enhanced HCO
3− buffering. As stated, in the initial evaluation period, HCO
3−-buffering averaged 2.5 and 3.1 mM in the primary sound-treated and control digesters, respectively, and 17.3 and 14.4 mM after addition of wastewater from the commercial digester. We ascribe the decrease in gas production by the secondary digesters after the initial evaluation period to more complete digestion of wastewater in the primary digesters.
Whether due to increased buffering or microbial seeding, from July 3 to October 16, gas production from the primary sound-treated digester was over twice that of the primary control digester (
Figure 5). This difference was seen even though the primary control digester, due to a more southerly exposure, had significantly higher water temperatures (29.4 ± 0.3 °C) than did the primary sound-treated digester (28.2 ± 0.3 °C); t (15) and
p = 0.0002.
Water quality parameters in the primary sound-treated and control digesters were similar during both the preliminary and main evaluation periods. During the initial evaluation period of 12 weeks, total suspended solids (TSS) averaged 401 and 656 mg L
−1 in the primary sound-treated and control digesters, respectively, and 1000 and 1020 mg L
−1, respectively, during the main evaluation period (
Table 3). During the preliminary evaluation period, the chemical oxygen demand (COD) averaged 4770 and 4370 mg L
−1 in the primary sound-treated and control digesters, respectively. COD concentrations decreased during the main evaluation period as biogas production increased. The higher COD concentrations in the sound-treated digesters as compared to the control digesters was likely due to enhanced sludge breakdown by sound, as noted in our previous research [
5]. Obtaining representative sludge samples would have entailed opening the digesters and the mixing of the tanks for homogenization of the tank contents, so this analysis was not performed.
The oxidation-reduction potential (ORP) was less negative in the sound-treated digesters than in the control digesters (
Table 3). This was somewhat unexpected given that average COD concentrations were somewhat higher in the sound-treated digesters. The chemical oxygen demand test usually overestimates the bioavailability of solutes in wastewater, since it relies on strong oxidizing agents. Due to this, we believe that the ORP is a more reliable indication that the wastewater was more degraded in the sound-treated digesters than in the control digesters.
Gas quality during the principal evaluation period was good, with CO
2 averaging 11,000 µM L
−1 (489,000 µg L
−1) in the primary sound-treated digester and 10,700 µM L
−1 (471,000 µg L
−1) in the control primary digester. Methane during this same period averaged 38,200 µM L
−1 (613,000 µg L
−1) in the primary sound-treated digester and 36,800 µM L
−1 (591,000 µg L
−1) in the primary control digester. Again, this is similar to our previous research, in which biogas CH
4 concentrations were somewhat higher in sound-treated digesters [
5]. Given greater pressure in the sound-treated digesters as a consequence of higher gas production, this was not unexpected.
Dissolved gas concentrations were similar during the preliminary and principal digester evaluation periods. During the principal evaluation period, aqueous CH4 concentrations were 18% lower in the primary sound-treated digester than in the primary control digester. Although this difference was not statistically significant, it may have indicated some degassing of the digestate due to sound.
Dissolved gas concentrations in the secondary anaerobic digesters were likewise comparable to those of the primary control and sound-treated digesters. Most of the subsurface gas in the digesters is likely in the form of bubbles rather than in a dissolved state, however, so our method of estimating subsurface gases is likely to contain considerable measurement uncertainty by failing to obtain a representative sample of bubbles.
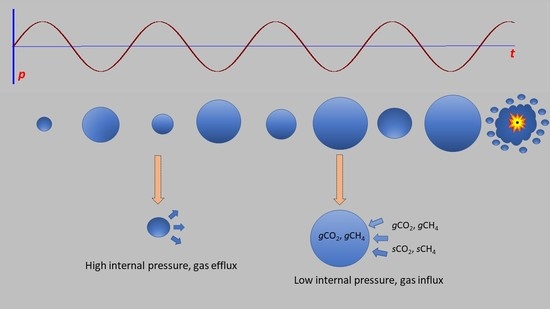
Gas production in the primary sound-treated digester averaged about 2700 L day−1. This translates to a flux of about 40 µL cm−2 min−1 of biogas. With an active gas flux, it is hard to imagine that most of the subsurface gas does not exist in the form of bubbles with the flux largely released during bubble collapse at the digestate surface. These bubbles are subject to sonic manipulation, as we saw in our experiments.
3.4. Advantages of Acoustic Enhancement of Anaerobic Digestion
Cracked corn was used to feed the digesters due to the time and expense of hauling animal wastes from distant producers. We felt this would serve as an adequate substitute for animal wastewater in our experiments and contribute to rapid-sludge formation in the digesters. The hydrolysis of sludge is usually the rate-limiting step in the anaerobic degradation of wastewater to produce biogas [
25,
26]. This has led to various pretreatments to disrupt sludge by mechanical means, such as ultrasonic disruption; treatment with surfactants to disrupt bacterial extracellular matrices [
27]; alkaline chemical hydrolysis, which may or may not be coupled with ultrasonification [
28,
29]; and biological treatments such as incubation with bacteria that produce hydrolytic enzymes [
30].
In the present study, sludge reduction without pretreatment in situ was attempted. This simplifies and reduces the expense of sludge handling. The results compare favorably to those of the ultrasonic pretreatment. For instance, Neis et al. [
31] were able to improve biogas production by 30% over that of controls by using a 31-KHz ultrasonification pretreatment at a full-scale wastewater treatment plant, and Geng et al. [
29] were able to improve biogas production by 69% by using pretreatment consisting of 20-kHz ultrasonification in conjunction with calcium oxide hydrolysis.
Cavitational collapse of bubbles with radii that are resonant at ultrasonic frequencies differs from that of bubbles resonant at sonic frequencies. Cavitational collapse induced at ultrasonic frequencies releases more energy than that induced at sonic frequencies, and temperatures as high as 6500
0K have been measured [
9]. These temperatures are not generated during cavitational collapse with large bubble radii. This should result in less deleterious effects on the microflora of the digester and, given the longer wavelength of the sound excitation, less sound attenuation [
32]. Thus, while ultrasonification would be the preferred method for treatment of sewage influent, sonification would be preferred in the larger volumes of a digester.
Another common practice in anaerobic digestion is to operate the digesters at either mesophilic (30–40 °C) or thermophilic (50–60 °C) temperatures to accelerate biogas production and reduce sludge volumes [
33]. It has been estimated that the heating requirements for thermophilic digestion are approximately twice that of mesophilic digestion, though considerable savings may have been realized due to shorter wastewater retention times and smaller digester volumes [
3]. In this study, biogas production was more than doubled over that of the control at an average wastewater temperature of 28.2 °C and a maximum recorded temperature of 32.1 °C. The energy required to raise the temperature of one of the primary digesters from 28.1 °C to the midrange of mesophilic operating conditions (35 °C), assuming 100 percent efficiency, would be approximately 62,700 kcal, with considerable energy required to maintain the elevated temperature.
In contrast, the power requirements for acoustic treatments are low. In our experiments, only about 72 ± 46, 62 ± 30, 68 ± 0.3, and 140 ± 0.8 W were consumed by the amplifiers at half-volume when playing The Rite of Spring; Beethoven’s Symphony No. 6, Movement 5; white noise; and a 1,000-Hz sine wave, respectively. Average energy consumption of the stereo system operated at one-half volume was 68.4 W. The sound system was typically operated 16 h day−1, which translates to approximately 1150 kcal day−1.
The water temperature during the principal evaluation period did not go below 15.5 °C and that was just as the experiment was terminated, so it is unknown to what extent sound treatment of anaerobic digestate might act as a substitute for wastewater heating. Nevertheless, the results of this experiment clearly show that audio treatment of digestate has the potential to decrease heating requirements for anaerobic digesters.