Multi-Year Monitoring of Ecosystem Metabolism in Two Branches of a Cold-Water Stream
Abstract
:1. Introduction
2. Materials and Methods
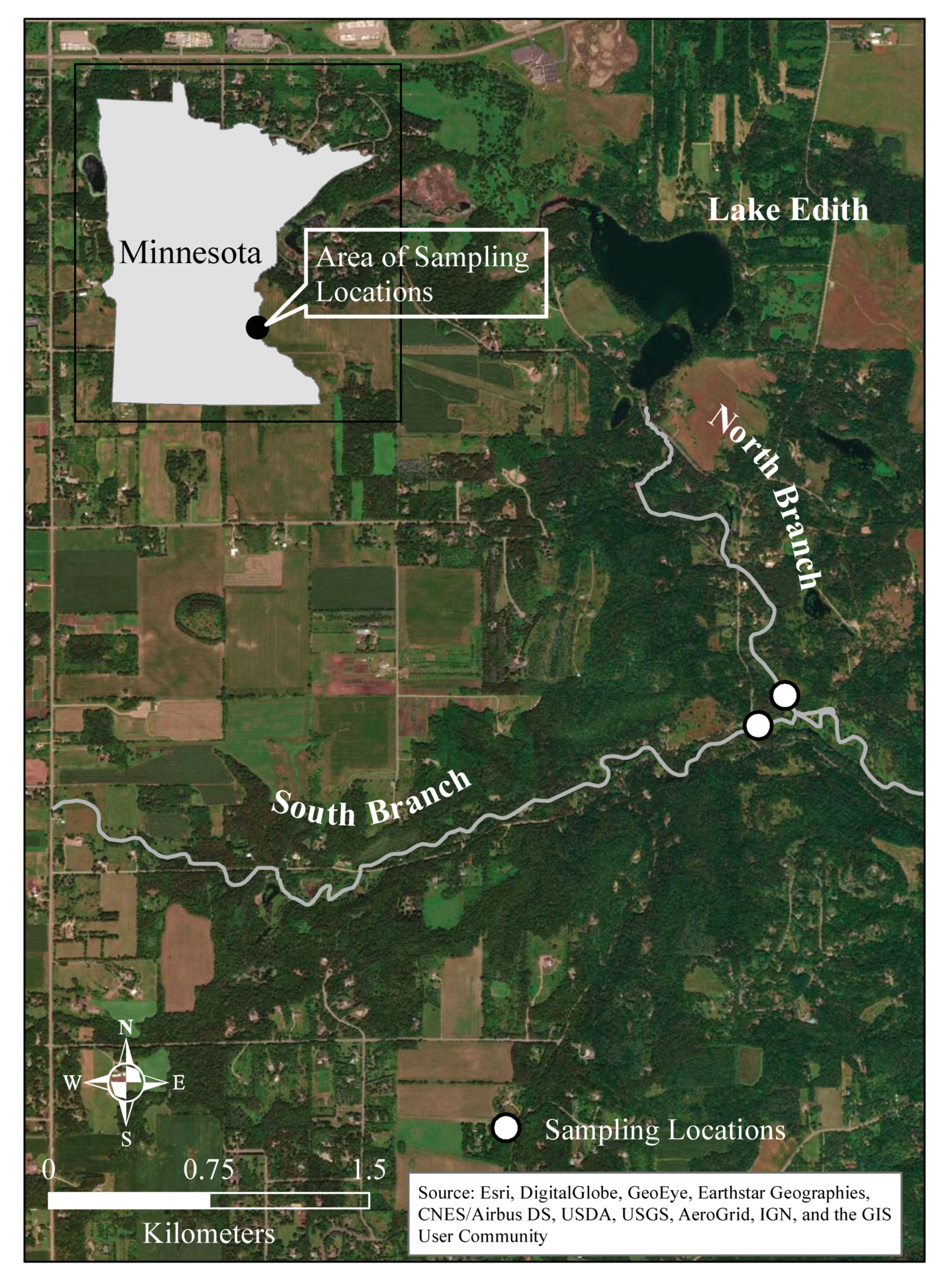
2.1. Sampling Location
2.2. Physical and Chemical Data
2.3. Ecosystem Metabolism
3. Results
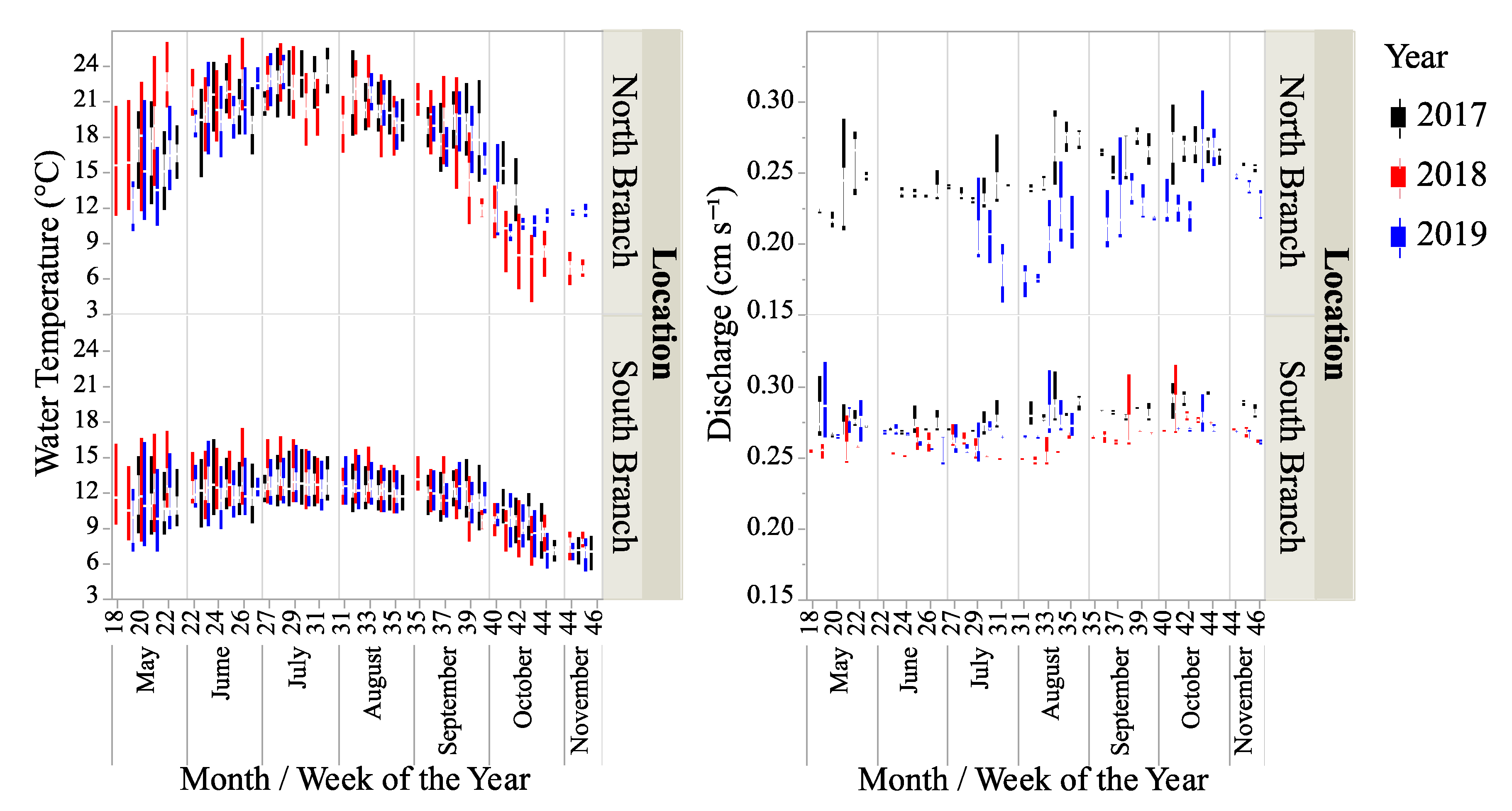
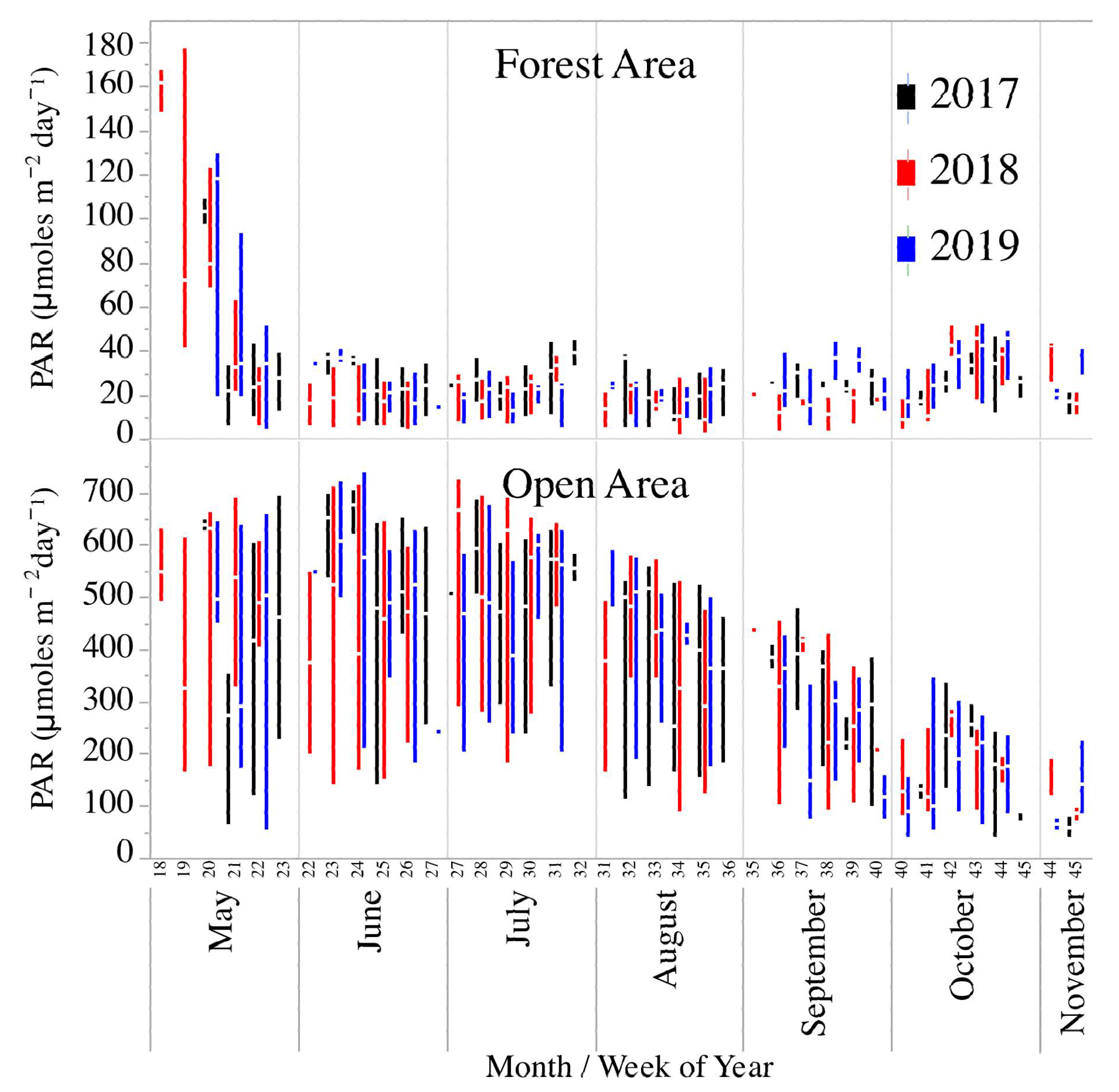
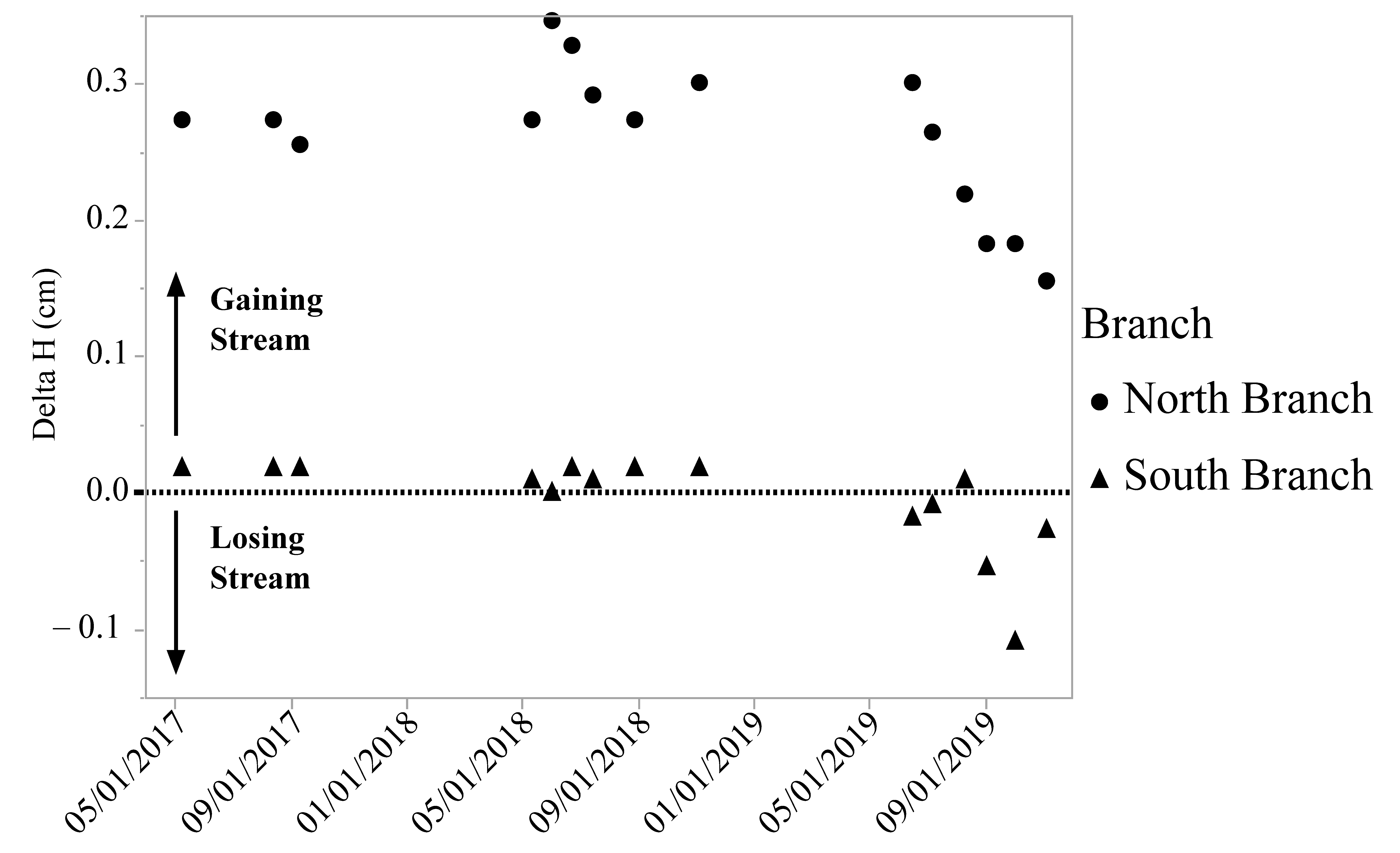
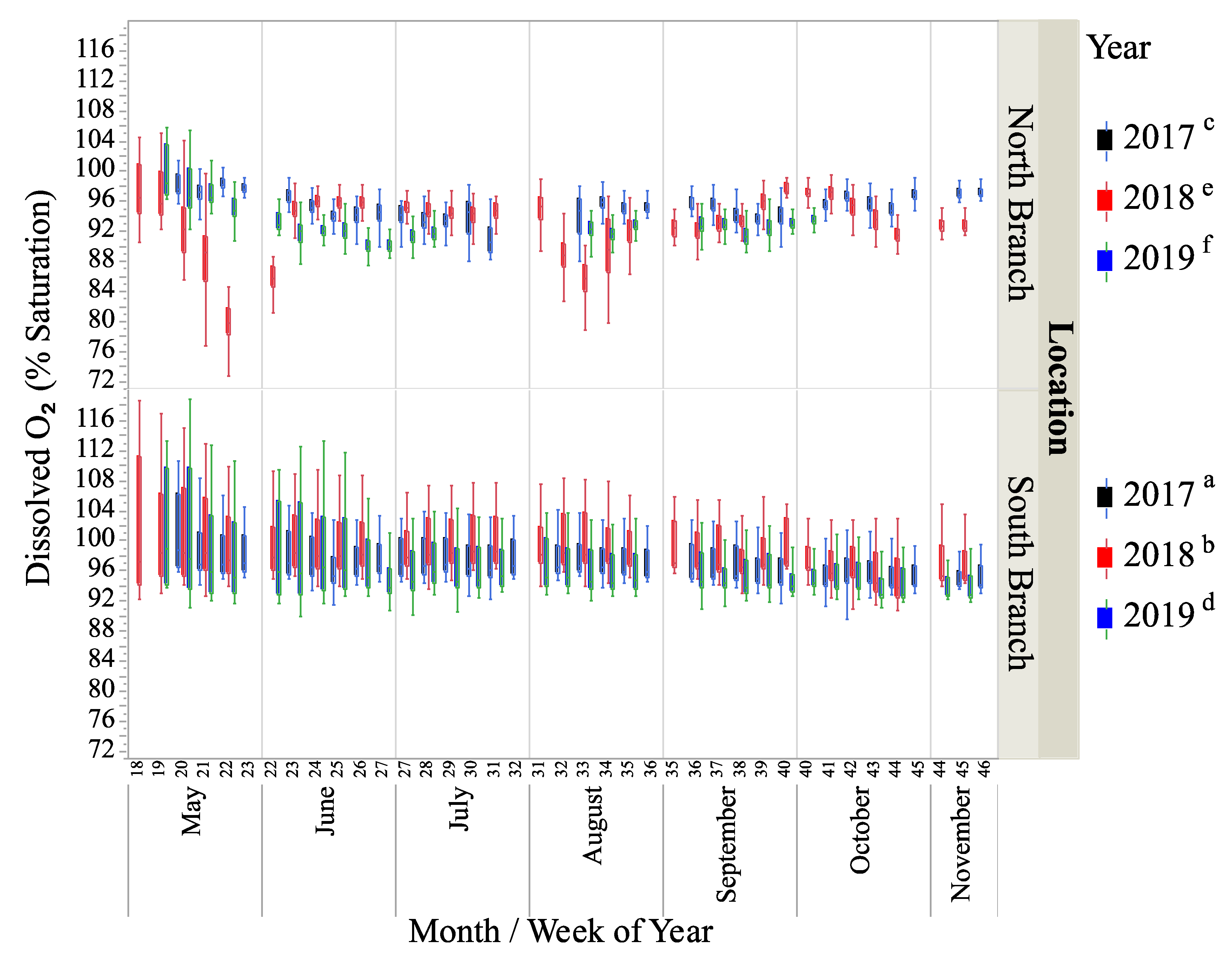
3.1. Physical–Chemical Parameters
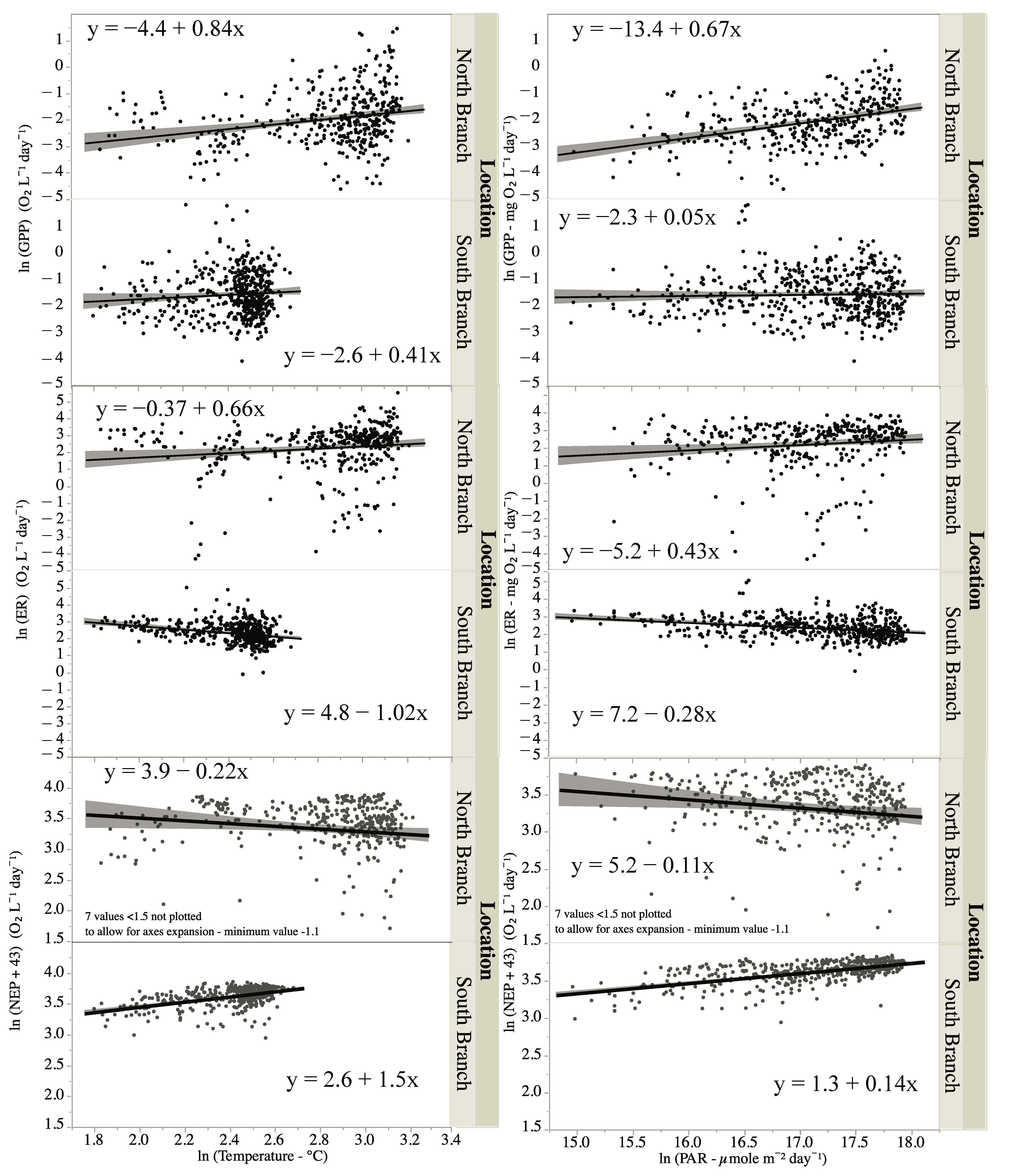
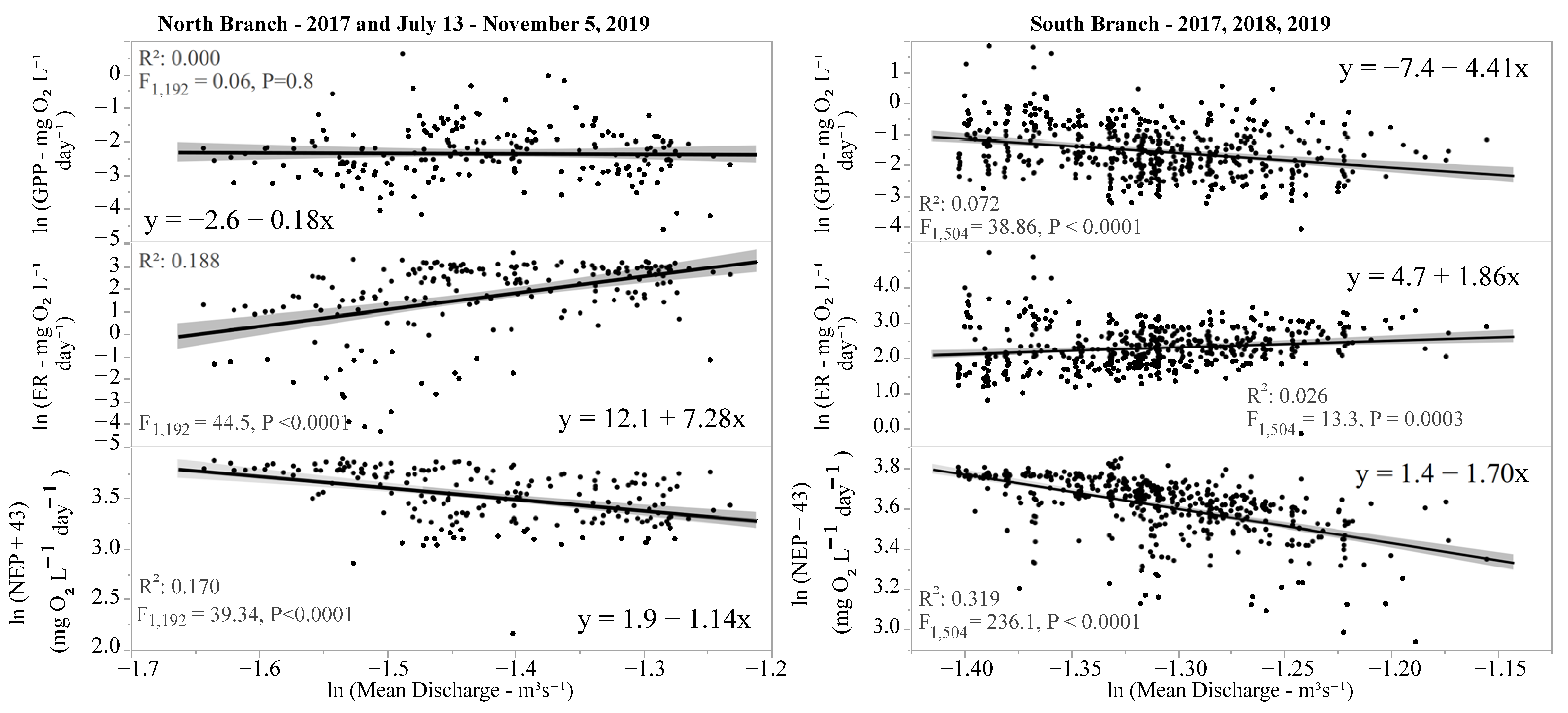
3.2. Ecosystem Metabolism
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caissie, D. The thermal regime of rivers: A review. Freshw. Biol. 2006, 51, 1389–1406. [Google Scholar] [CrossRef]
- Heino, J.; Virkkala, R.; Toivonen, H. Climate change and freshwater biodiversity: Detected patterns, future trends, and adaptations in northern regions. Biol. Rev. 2009, 84, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Markovic, D.; Carrizo, S.F.; Kärcher, O.; Walz, A.; David, J.N.W. Vulnerability of European freshwater catchments to climate change. Glob. Change Biol. 2017, 23, 3567–3580. [Google Scholar] [CrossRef]
- Pyne, M.I.; Poff, L. Vulnerability of stream community composition and function to projected thermal warming and hydrologic change across ecoregions in the western United States. Glob. Change Biol. 2017, 23, 77–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhardt, E.S.; Heffernan, J.B.; Grimm, N.B.; Stanley, E.H.; Harvey, J.W.; Arroita, M.; Appling, A.P.; Cohen, M.J.; McDowell, W.H.; Hall, R.O., Jr.; et al. The metabolic regimes of flowing waters. Limnol. Oceanogr. 2018, 63, S99–S118. [Google Scholar] [CrossRef] [Green Version]
- Coffey, R.; Paul, M.J.; Stamp, J.; Hamilton, A.; Johnson, T. A review of water quality responses to air temperature and precipitation changes 2: Nutrients, algal blooms, sediment, pathogens. J. Am. Water Resour. Assoc. 2019, 55, 844–868. [Google Scholar] [CrossRef]
- Paul, M.J.; Coffey, R.; Stamp, J.; Johnson, T. A review of water quality responses to air temperature and precipitation changes 1: Flow, water temperature, saltwater intrusion. J. Am. Water Resour. Assoc. 2019, 55, 824–843. [Google Scholar] [CrossRef]
- O’Briain, R. Climate change and European rivers: An eco-hydromorphological perspective. Ecohydrology 2019, 12. [Google Scholar] [CrossRef]
- Knouft, J.H.; Ficklin, D.L. The potential impacts of climate change on biodiversity in flowing water systems. Ann. Rev. Ecol. Syst. 2017, 48, 111–133. [Google Scholar] [CrossRef]
- Niedrist, G.H.; Füreder, L. Real-time warming of alpine streams: (re)defining invertebrates’ temperature preferences. River Res. Applic. 2020, 1–11. [Google Scholar] [CrossRef]
- Bogardi, J.J.; Leentvaar, J.; Sebesvárl, Z. Biologia Futura: Integrating freshwater ecosystem health in water resources management. Biol. Futur. 2020, 71, 337–358. [Google Scholar] [CrossRef]
- O’Gorman, E.J.; Pichler, D.E.; Adams, G.; Benstead, J.P.; Cohen, H.; Craig, N.; Cross, W.F.; Demars, B.O.L.; Friberg, N.; Gislason, G.M.; et al. Impacts of warming on the structure and functioning of aquatic communities: Individual-to-ecosystem-level responses. Adv. Ecol. Res. 2012, 47, 81–176. [Google Scholar] [CrossRef] [Green Version]
- Fullerton, A.H.; Torgersen, C.E.; Lawler, J.J.; Steel, E.A.; Ebersole, J.L.; Lee, S.Y. Longitudinal thermal heterogeneity in rivers and refugia for coldwater species: Effect of scale and climate change. Aquat. Sci. 2018, 80, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, S.; Bo, T.; Cucco, M.; Mercalli, L.; Malacarne, G. Effects of global climate change on freshwater biota: A review with special emphasis on the Italian situation. Ital. J. Zool. 2010, 77, 374–383. [Google Scholar] [CrossRef]
- Haase, P.; Pilotto, F.; Li, F.; Sunderman, A.; Lorenz, A.W.; Tonkin, J.D.; Stoll, S. Moderate warming over the past 25 years has already reorganized stream invertebrate communities. Sci. Total Environ. 2019, 658, 1531–1538. [Google Scholar] [CrossRef]
- Isaak, D.J.; Wollrab, S.; Horan, D.; Chandler, G. Climate change effects on stream and river temperatures across the northwest U.S. from 1980–2009 and implications for salmonid fishes. Climactic Chang. 2012, 113, 499–524. [Google Scholar] [CrossRef] [Green Version]
- Selbig, W.R. Simulating the effect of climate change on stream temperature in the Trout Lake Watershed, Wisconsin. Sci. Total Environ. 2015, 521, 11–18. [Google Scholar] [CrossRef]
- Lynch, A.J.; Myers, B.J.E.; Chu, C.X.; Eby, L.A.; Falke, J.A.; Kovach, R.P.; Krabbenhoft, T.J.; Kwak, T.J.; Lyons, J.; Paukert, B.P.; et al. Climate change effects on North American inland fish populations and assemblages. Fisheries 2016, 41, 346–361. [Google Scholar] [CrossRef]
- Biswas, S.R.; Vogt, R.J.; Sharma, S. Projected compositional shifts and loss of ecosystem services in freshwater fish communities under climate change scenarios. Hydrobiologia 2017, 799, 135–149. [Google Scholar] [CrossRef]
- Mitro, M.G.; Lyons, J.D.; Stewart, J.S.; Cunningham, P.K.; Griffin, J.D.T. Projected changes in brook trout and brown trout distribution in Wisconsin streams in the mid-twenty-first century in response to climate change. Hydrobiologia 2019, 840, 215–226. [Google Scholar] [CrossRef]
- Johnson, Z.C.; Snyder, C.D.; Hitt, N.P. Landform and season precipitation predict shallow groundwater influence on temperature in headwater streams. Water Resour. Res. 2017, 53, 5788–5812. [Google Scholar] [CrossRef]
- Leach, J.A.; Moore, R.D. Empirical stream thermal sensitivities may underestimate stream temperature response to climate warming. Water Resour. Res. 2019, 55, 5453–5467. [Google Scholar] [CrossRef]
- Carlson, A.K.; Taylor, W.W.; Infante, D.M. Modelling effects of climate change on Michigan brown trout and rainbow trout: Precipitation and groundwater as key predictors. Ecol. Freshw. Fish 2020, 29, 433–449. [Google Scholar] [CrossRef]
- Tank, J.L.; Rosi-Marshall, E.J.; Griffiths, N.A.; Entrekin, S.A.; Stephen, M.L. A review of allochthonous organic matter dynamics and metabolism in streams. J. N. Am. Benthol. Soc. 2010, 29, 118–146. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.O. Metabolism of streams and rivers: Estimation, controls and application. In Stream Ecosystems in a Changing Environment; Jones, J.B., Stanley, E.H., Eds.; Academic Press: New York, NY, USA, 2016; pp. 151–180. [Google Scholar] [CrossRef]
- Demars, B.O.L.; Thompson, J.; Manson, J.R. Stream metabolism and the open diel oxygen method: Principles, practice, and perspectives. Limnol. Oceanogr. Methods 2015, 13, 356–374. [Google Scholar] [CrossRef] [Green Version]
- Siders, A.C.; Larson, D.M.; Rüegg, J.; Dodds, W.K. Probing whole-stream metabolism: Influence of spatial and heterogeneity on rate estimates. Freshw. Biol. 2017, 62, 711–723. [Google Scholar] [CrossRef]
- Rodríguez-Castillo, T.; Estévez, E.; González-Ferreras, A.M.; Barquín, J. Estimating ecosystem metabolism to entire river networks. Ecosystems 2019, 22, 892–911. [Google Scholar] [CrossRef]
- Young, R.G.; Matthaei, C.D.; Townsend, C.R. Organic matter breakdown and ecosystem metabolism: Functional indicators for assessing river ecosystem health. J. North Am. Benthol. Soc. 2008, 27, 605–625. [Google Scholar] [CrossRef]
- Irwin, C.E.; Culp, J.M.; Yates, A.G. Spatio-temporal variation of benthic metabolism in a large, regulated river. Can. Water Resour. J. 2020, 45, 144–157. [Google Scholar] [CrossRef]
- Staehr, P.A.; Testa, J.M.; Kemp, W.M.; Cole, J.J.; Sand-Jensen, K.; Smith, S.V. The metabolism of aquatic ecosystems: History, applications and future challenges. Aquat. Sci. 2012, 74, 15–29. [Google Scholar] [CrossRef]
- Young, R.G.; Collier, K.J. Contrasting responses to catchment modification among a range of functional and structural indicators of river ecosystem health. Freshw. Biol. 2009, 54, 2155–2170. [Google Scholar] [CrossRef] [Green Version]
- Silva-Junior, E.F. Land use effects and stream metabolic rates: A review of ecosystem response. Acta Limnol. Bras. 2016, 28, e2016103. [Google Scholar] [CrossRef] [Green Version]
- von Schiller, D.; Acuña, V.; Aristi, I.; Arroita, M.; Basaguren, A.; Bellin, A.; Boyero, L.; Butturini, A.; Ginebreda, A.; Kalogianni, E.; et al. River ecosystem processes; a synthesis of approaches, criteria of use and sensitivity to environmental stressors. Sci. Total Environ. 2017, 569–597, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, X.B.; Peng, W.Q.; Wu, L.X.; Yano, S.; Zhang, J.M.; Zhao, F. Periphyton and ecosystem metabolism as indicators of river ecosystem response to environmental flow restoration in a flow-reduced river. Ecol. Indic. 2018, 92, 394–401. [Google Scholar] [CrossRef]
- Clapcott, J.E.; Young, R.G.; Neale, M.W.; Doehring, K.; Barmuta, L.A. Land use affects temporal variation in stream metabolism. Freshw. Sci. 2016, 35, 1164–1175. [Google Scholar] [CrossRef] [Green Version]
- Pearce, N.J.T.; Yates, A.G. Agricultural best management practice abundance and location does not influence stream ecosystem function or water quality in the summer season. Water 2015, 7, 6861–6876. [Google Scholar] [CrossRef] [Green Version]
- Qasem, K.; Vitousek, S.; O’Connor, B.; Hoellein, T. The effect of floods on ecosystem metabolism in suburban streams. Freshwater Sci. 2019, 38, 412–424. [Google Scholar] [CrossRef]
- Battin, T.J.; Luyssaert, S.; Kaplan, L.A.; Aufdenkampe, A.K.; Richter, A.; Tranvik, L.J. The boundless carbon cycle. Nat. Geosci. 2009, 2, 598–600. [Google Scholar] [CrossRef]
- Hefferman, J.B. Stream metabolism heats up. Nat. Geosci. 2018, 11, 384–385. [Google Scholar] [CrossRef]
- Song, C.; Dodds, W.K.; Rüegg, J.; Argerich, A.; Baker, C.L.; Bowden, W.B.; Douglas, M.M.; Farrell, K.J.; Flinn, M.B.; Garcia, E.A.; et al. Continential-scale decrease in net primary productivity in streams due to climate warming. Nat. Geosci. 2018, 11, 415–420. [Google Scholar] [CrossRef]
- Koenig, L.E.; Helton, A.M.; Savoy, P.; Bertuzzo, E.; Heffernan, J.B.; Hall, R.O., Jr.; Bernhardt, E.S. Emergent productivity regimes of river networks. Limnol. Oceanogr. Lett. 2019, 4, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Uehlinger, U.R.S. Annual cycle and inter-annual variability in gross primary production and ecosystem respiration in a flood prone river during a 15-year period. Freshw. Biol. 2006, 51, 938–950. [Google Scholar] [CrossRef]
- Roberts, B.J.; Mulholland, P.J.; Hill, W.R. Multiple scales of temporal variability in ecosystem metabolism rates: Results from 2 years of continuous monitoring in a forested headwater stream. Ecosystems 2007, 10, 588–606. [Google Scholar] [CrossRef]
- Summers, B.M.; Van Horn, D.J.; González-Pinzón, R.; Bixby, R.J.; Grace, M.R.; Sherson, L.R.; Crossey, L.J.; Stone, M.C.; Parmenter, R.R.; Compton, T.S.; et al. Long-term data reveal highly-variable metabolism and transitions in trophic status in a montane stream. Freshw. Sci. 2020, 39, 241–255. [Google Scholar] [CrossRef]
- Hornbach, D.M.; Hove, M.; Agata, M.; Arnold, E.; Cavazos, E.; Friedman, C.; Jay, K.; Johnson, E.; Johnson, K.; Staudenmaier, A. Ecosystem Structure and Function in two branches of an eastern Minnesota trout stream. J. Freshw. Ecol. 2016, 31, 487–507. [Google Scholar] [CrossRef] [Green Version]
- Fellows, C.D.; Valett, H.M.; Dahm, C.N.; Mulholland, P.J.; Thomas, S. Coupling nutrient uptake and energy flow in headwater streams. Ecosystems 2006, 9, 788–804. [Google Scholar] [CrossRef] [Green Version]
- Mulholland, P.J.; Thomas, S.A.; Valett, H.M.; Webster, J.R.; Beaulieu, J. Effects of light on NO3- uptake in small forested streams: Diurnal and day-to-day variations. J. N. Am. Benthol. Soc. 2006, 25, 583–595. [Google Scholar] [CrossRef]
- Rasmussen, J.J.; Baattrup-Pedersen, A.; Riis, T.; Friberg, N. Stream ecosystem properties and processes along a temperature gradient. Aquat. Ecol. 2011, 45, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Metropolitan Council. Comprehensive Water Quality Assessment of Select Metropolitan Area Streams. St. Paul: Metropolitan Council. 2014. Available online: https://metrocouncil.org/Wastewater-Water/Services/Water-Quality-Management/Stream-Monitoring-Assessment/St-Croix-River-Tributary-Streams-Assessment/St-Croix-Trib-Assessment-Reports/Valley-Creek-Section.aspx (accessed on 22 May 2020).
- Almendinger, J.E. Watershed Hydrology of Valley Creek and Browns Creek: Trout Streams Influenced By Agriculture and Urbanization in Eastern Washington County, Minnesota, 1998_99. 2003. Marine on St. Croix (MN): St. Croix. Watershed Research Station, Science Museum of Minnesota. Available online: https://www.smm.org/sites/default/files/public/attachments/2003_almendinger._watershed.pdf (accessed on 22 May 2020).
- Baxter, C.; Hauer, F.C.; Woessner, W. Measuring groundwater-stream water exchange: New techniques for installing minipiezometers and estimating hydraulic conductivity. Trans Am. Fish Soc. 2003, 132, 493–502. [Google Scholar] [CrossRef]
- Odum, H.T. Primary production in flowing waters. Limnol. Oceanogr. 1956, 1, 102–117. [Google Scholar] [CrossRef]
- Grace, M.; Giling, D.P.; Hladyz, S.; Caron, V.; Thompson, R.M.; Mac Nally, R. Fast processing of diel oxygen curves: Estimating stream metabolism with BASE (BAyesian Single-station Estimation). Limnol. Oceanogr. Methods 2015, 13, 103–114. [Google Scholar] [CrossRef]
- Uehlinger, U. Resistance and resilience of ecosystem metabolism in a flood prone river system. Freshw. Biol. 2000, 4, 319–332. [Google Scholar] [CrossRef]
- Roberts, B.J.; Mulholland, P.J. In-stream biotic control on nutrient biochemistry in a forested stream, West Fork of Walker Branch. J. Geophys. Res. 2007, 112, G04002. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, J.R.; Bellmore, J.R.; Watson, G.A. Response of ecosystem metabolism to low densities of spawning Chinook Salmon. Freshw. Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Jabiol, J.; Gossiaux, A.; Lecerf, A.; Rota, T.; Guérold, F.; Danger, M.; Poupin, P.; Gilbert, F.; Chauvet, E. Variable temperature effects between heterotrophic stream processes and organisms. Freshw. Biol. 2020, 65, 1543–1554. [Google Scholar] [CrossRef]
- Pearce, N.J.T.; Thomas, K.E.; Chambers, P.A.; Venkiteswaran, J.J.; Yates, A.G. Metabolic regimes of three mid-order streams in southern Ontario, Canada exposed to contrasting sources of nutrients. Hydrobiologia 2020, 847, 1925–1942. [Google Scholar] [CrossRef]
- Rojano, F.; Huber, D.H.; Ugwuanyi, I.R.; Noundou, V.L.; Kemajou-Tchamba, A.L.; Chavarria-Palma, J.E. Net ecosystem production of a river relying on hydrology, hydrodynamics and water quality monitoring stations. Water 2020, 12, 783. [Google Scholar] [CrossRef] [Green Version]
- Hoellein, T.J.; Bruesewitz, D.A.; Richardson, D.C. Revisiting Odum (1956): A synthesis of aquatic ecosystem metabolism. Limnol. Oceanogr. 2013, 58, 2089–2100. [Google Scholar] [CrossRef]
- Hauer, F.R.; Hill, W.R. Temperature, light and oxygen. In Methods in Stream Ecology, 2nd ed.; Hauer, F.R., Lamberti, G.A., Eds.; Academic Press: San Diego, CA, USA, 2007; pp. 103–117. [Google Scholar]
- He, J.; Chu, A.; Ryan, M.C.; Valeo, C.; Zaitlin, B. Abiotic influences on dissolved oxygen in a riverine environment. Ecol. Eng. 2011, 37, 1804–1814. [Google Scholar] [CrossRef]
- Hall, R.O.; Tank, J.L. Correcting whole-stream estimates of metabolism for groundwater input. Limnol. Oceanogr. Methods 2005, 3, 222–229. [Google Scholar] [CrossRef]
- Nebgen, E.L.; Herrman, K.S. Effects of shading on stream ecosystem metabolism and water termperature in and agriculturally influence stream in central Wisconsin, USA. Ecol. Eng. 2019, 126, 16–24. [Google Scholar] [CrossRef]
- Schilling, K.E.; Jacobson, P.J. Temporal variations in dissolved oxygen concentrations observed in a shallow floodplain aquifer. Riv. Res. Appl. 2015, 31, 576–589. [Google Scholar] [CrossRef]
- Behar, S. Testing the Waters: Chemical and Physical Vital Signs of a River; River Watch Network: Montpelier, VT, USA, 1996. [Google Scholar]
- Kaylor, M.J.; White, S.M.; Saunders, W.C.; Warren, D.R. Relating spatial patterns of stream metabolism to distributions of juvenile salmonids at the river network scale. Ecosphere 2019, 10, e02781. [Google Scholar] [CrossRef] [Green Version]
- Zapp, M.J.; Almendinger, J.E. Nutrient Dynamics and Water Quality of Valley Creek, a High Quality Trout Stream in Southeastern Washington County. St. Paul (MN): Water Resources Science, University of Minnesota. Final Project Report to the Valley Branch Watershed District. 2001. Available online: https://www.smm.org/sites/default/files/public/attachments/2001_zapp._nutrient.pdf (accessed on 15 January 2021).
- Padfield, D.; Lowe, C.; Buckling, A.; French-Constant, R.; Student Research Team; Jennings, S.; Shelley, F.; Ólafsson, J.S.; Ynov-Durocher, G. Metabolic compensation constrains the temperature dependence of gross primary production. Ecol. Lett. 2017, 20, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Savoy, P.; Appling, A.P.; Heffernan, J.B.; Stets, E.G.; Read, J.S.; Harvey, J.W.; Bernhardt, E.S. Metabolic rhythms in flowing waters: An approach for classifying river productivity regimes. Limnol. Oceanogr. 2019, 64, 1835–1851. [Google Scholar] [CrossRef]
- Alberts, J.M.; Beaulieu, J.J.; Buffam, I. Watershed land use and seasonal variation constrain the influence of riparian canopy cover on stream ecosystem metabolism. Ecosystems 2017, 20, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.J.; Arango, C.P.; Balz, D.A.; Shuster, W.D. Continuous monitoring reveals multiple controls on ecosystem metabolism in a suburban stream. Freshw. Biol. 2013, 58, 918–937. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Fellows, C.J.; Tank, J.L.; Grimm, N.B.; Webster, J.R.; Hamilton, S.K.; Marti, E.; Ashkenas, L.; Bowden, W.B.; Dodds, W.K.; et al. Inter-biome comparison of factors controlling stream metabolism. Freshw. Biol. 2001, 46, 1503–1517. [Google Scholar] [CrossRef] [Green Version]
- Dodds, W.K.; Veach, A.; Ruffing, C.M.; Larson, D.M.; Fischer, J.L.; Costigan, K.H. Abiotic controls and temporal variability of river metabolism: Multiyear analyses of Mississippi and Chattahoochee River data. Freshw. Sci. 2013, 32, 1073–1087. [Google Scholar] [CrossRef] [Green Version]
- Huryn, A.D.; Benstead, J.P.; Parker, S.M. Seasonal changes in light availability modify the temperature dependence of ecosystem metabolism in an arctic stream. Ecology 2014, 95, 2826–2839. [Google Scholar] [CrossRef] [Green Version]
- Bernot, M.J.; Sobota, D.J.; Hall, R.O.; Mulholland, P.J.; Dodds, W.K.; Webster, J.R.; Tank, J.L.; Ashkenas, L.R.; Cooper, L.W.; Dahm, C.N.; et al. Inter-regional comparison of land-use effects on stream metabolism. Freshw. Biol. 2010, 55, 1874–1890. [Google Scholar] [CrossRef]
- Demars, B.O.L.; Manson, J.R.; Ólafsson, J.S.; Gíslason, G.M.; Gudmundsdóttir, R.; Woodward, G.; Reiss, J.; Pichler, D.E.; Rasmussen, J.J.; Friberg, N. Temperature and the metabolic balance of streams. Freshw. Biol. 2011, 56, 1106–1121. [Google Scholar] [CrossRef]
- Jane, S.F.; Rose, K.C. Carbon quality regulates the temperature dependence of aquatic ecosystem respiration. Freshw. Biol. 2018, 63, 1407–1419. [Google Scholar] [CrossRef]
- Demars, B.O.L. Hydrological pulses and burning of dissolved organic carbon by stream respiration. Limnol. Oceanogr. 2019, 64, 406–421. [Google Scholar] [CrossRef] [Green Version]
- Escoffier, N.; Bensoussan, N.; Vilmin, L.; Flipo, N.; Rocher, V.; David, A.; Métivier, F.; Groleau, A. Estimating ecosystem metabolism from continuous multi-sensor measurements in the Seine River. Environ. Sci. Pollut. Res. 2018, 25, 23451–23467. [Google Scholar] [CrossRef]

| Parameter | North Branch | South Branch | ||||
|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2017 | 2018 | 2019 | |
| Depth (m) | 0.33 a (0.001) | 0.19 b (0.001) | 0.35 c (0.001) | 0.29 d (0.001) | 0.27 e (0.001) | 0.37 f (0.001) |
| Discharge (m3 s−1) 1 | 0.23 a (0.001) | — | 0.21 b (0.002) | 0.27 c (0.001) | — | 0.26 d (0.001) |
| Temperature (°C) 2 | 17.6 a (0.21) | 17.7 b (0.21) | 17.2 c (0.21) | 11.3 d (0.21) | 11.6 e (0.21) | 11.1 f (0.21) |
| Dissolved oxygen (% saturation) 2 | 95.5 a (0.05) | 93.5 b (0.06) | 93.0 c (0.06) | 96.3 d (0.05) | 96.8 e (0.04) | 95.1 f (0.05) |
| Conductivity (µS cm−1) 2 | 451.0 a (2.0) | — | — | 436.6 b (2.0) | 427.9 (0.1) | — |
| Dissolved P (μg L−1) 3 | 11.3 a (2.7) | 18.2 a (9.3) | 11.6 a (3.1) | 10.0 a (0.7) | 12.6 a (0.1) | 14.1 a (5.7) |
| Dissolved N (mg L−1) 3 | 2.75 a (0.99) | 2.9 a (0.92) | 2.79 a (0.79) | 8.34 b (0.25) | 9.28 b (0.21) | 9.78 b (0.46) |
| Variable | Factor | F Ratio | Degrees of Freedom | Probability > F |
|---|---|---|---|---|
| Water depth | Year | 128,744.2 | 2, 154,114 | <0.0001 |
| Location | 10,356.5 | 1, 154,097 | <0.0001 | |
| Year * Location | 25,347.5 | 2, 154,083 | <0.0001 | |
| Discharge | Year | 188.4 | 1, 690 | <0.0001 |
| Location | 1445.9 | 1, 1446 | <0.0001 | |
| Year * Location | 9.3 | 1, 573 | 0.002 | |
| Temperature | Year | 599.8 | 2, 148,717 | <0.0001 |
| Location | 311,070.6 | 1, 311,070 | <0.0001 | |
| Year * Location | 56.5 | 2, 148,707 | <0.0001 | |
| % DO | Year | 5143.7 | 2, 125,124 | <0.0001 |
| Location | 21,036.5 | 1, 125,129 | <0.0001 | |
| Year * Location | 2807.5 | 2, 125,066 | <0.0001 | |
| GPP | Year | 12.8 | 2, 809 | <0.0001 |
| Location | 578.2 | 1, 808 | <0.0001 | |
| Year * Location | 23.5 | 2, 795 | <0.0001 | |
| ER | Year | 38.0 | 2, 844 | <0.0001 |
| Location | 1.3 | 1, 845 | 0.3 | |
| Year * Location | 67.0 | 2, 832 | <0.0001 | |
| NEP | Year | 5.4 | 2, 855 | 0.0046 |
| Location | 128.6 | 1, 859 | <0.0001 | |
| Year * Location | 33.9 | 2, 846 | <0.0001 |
| Variable | Year | |||||
|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | ||||
| North Branch | South Branch | North Branch | South Branch | North Branch | South Branch | |
| GPP | 1.3 (1.4) a | 3.6 (1.9) c | 1.6 (2.2) a | 10.5 (17.0) d | 3.0 (1.5) b | 5.5 (3.6) c |
| ER | 13.8 (6.1) a,b | 10.8 (4.7) b,c | 17.9 (9.5) a | 12.9 (16.6) c | 5.6 (8.1) d | 12.6 (5.8) b,c |
| NEP | −12.5 (5.9) a | −7.2 (4.2) c | −16.3 (9.3) b | −2.4 (3.3) d | −2.7 (8.8) a,c | −7.1 (5.7) c |
| Variable | Factor | F Ratio | Degrees of Freedom | Probability > F |
|---|---|---|---|---|
| GPP | ln (temperature) | 15.5 | 1, 868 | <0.0001 |
| Location | 97.8 | 1, 868 | <0.0001 | |
| ln (temperature) * location | 0.5 | 1, 868 | 0.47 | |
| ER | ln (temperature) | 3.5 | 1, 868 | 0.06 |
| Location | 0.7 | 1, 868 | 0.4 | |
| ln (temperature) * location | 22.9 | 1, 868 | <0.0001 | |
| NEP | ln (temperature) | 3.5 | 1, 868 | 0.06 |
| Location | 75.3 | 1, 868 | <0.0001 | |
| ln (temperature) * location | 22.9 | 1, 868 | <0.0001 |
| Variable | Factor | F Ratio | Degrees of Freedom | Probability > F |
|---|---|---|---|---|
| GPP | ln (PAR) | 45.0 | 1, 868 | <0.0001 |
| Location | 83.1 | 1, 868 | <0.0001 | |
| ln (PAR) * location | 32.9 | 1, 868 | <0.0001 | |
| ER | ln (PAR) | 0.1 | 1, 868 | 0.8 |
| Location | 3.9 | 1, 868 | 0.05 | |
| ln (PAR) * location | 29.3 | 1, 868 | <0.0001 | |
| NEP | ln (PAR) | 0.6 | 1, 868 | 0.4 |
| Location | 107.2 | 1, 868 | <0.0001 | |
| ln (PAR) * location | 28.4 | 1, 868 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hornbach, D.J. Multi-Year Monitoring of Ecosystem Metabolism in Two Branches of a Cold-Water Stream. Environments 2021, 8, 19. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8030019
Hornbach DJ. Multi-Year Monitoring of Ecosystem Metabolism in Two Branches of a Cold-Water Stream. Environments. 2021; 8(3):19. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8030019
Chicago/Turabian StyleHornbach, Daniel J. 2021. "Multi-Year Monitoring of Ecosystem Metabolism in Two Branches of a Cold-Water Stream" Environments 8, no. 3: 19. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8030019






