The Brown Seaweeds of Scotland, Their Importance and Applications
Abstract
:1. Introduction
1.1. The Distribution of Brown Seaweeds in Scotland
1.2. The Biodiversity of the Brown Seaweed Habitat and Their Ecological Importance
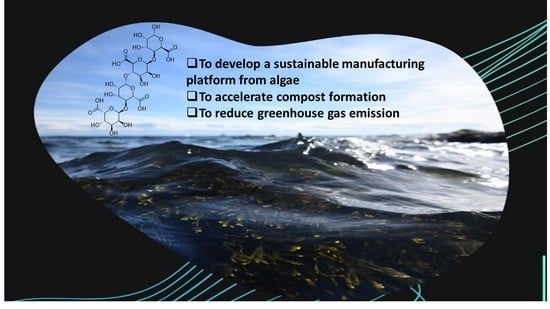
2. Some of the Important Uses of the Seaweeds
2.1. Seaweed as Food Supplements
2.2. Seaweeds Used in Green Waste Management
2.3. Role of Seaweeds in Agriculture
3. Current and Future Seaweed Harvesting Activity in Scotland
4. Phytochemistry of Seaweeds
4.1. Overview of Some Seaweed Derived Phytochemicals and Their Biological Activity
4.2. Symbiotic Microorganisms
5. Extraction Techniques for Algal Derived Compounds
5.1. Extraction of Proteins
5.2. Microwave-Assisted Extraction
5.3. Green Method to Extract Sodium Alginate by Ultra-Sonication
5.4. Extraction of Alginates Using Formaldehyde to Remove Phenolic Compounds
6. Characterization of Alginate
7. Conclusions and Future Vision
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rebours, C.; Marinho-Soriano, E.; Zertuche-González, J.A.; Hayashi, L.; Vásquez, J.A.; Kradolfer, P.; Soriano, G.; Ugarte, R.; Abreu, M.H.; Bay-Larsen, I. Seaweeds: An opportunity for wealth and sustainable livelihood for coastal communities. J. Appl. Phycol. 2014, 26, 1939–1951. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, V.; Barsanti, L.; Frassanito, A.M.; Passarelli, V.; Gualtieri, P. Algal Toxins: Nature, Occurrence, Effect and Detection; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- van Ginneken, V. Some mechanism seaweeds employ to cope with salinity stress in the harsh euhaline oceanic environment. Am. J. Plant Sci. 2018, 9, 1191–1211. [Google Scholar] [CrossRef] [Green Version]
- Fernández, P.A.; Roleda, M.Y.; Rautenberger, R.; Hurd, C.L. Carbonic anhydrase activity in seaweeds: Overview and recommendations for measuring activity with an electrometric method, using Macrocystis pyrifera as a model species. Mar. Biol. 2018, 165, 1–12. [Google Scholar] [CrossRef]
- Baweja, P.; Sahoo, D. Classification of algae. In The Algae World; Springer: Berlin/Heidelberg, Germany, 2015; pp. 31–55. [Google Scholar]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Davenport, J.; Davenport, J.L. Effects of shore height, wave exposure and geographical distance on thermal niche width of intertidal fauna. Mar. Ecol. Prog. Ser. 2005, 292, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Ounsley, J.P.; Gallego, A.; Morris, D.J.; Armstrong, J.D. Regional variation in directed swimming by Atlantic salmon smolts leaving Scottish waters for their oceanic feeding grounds—A modelling study. ICES J. Mar. Sci. 2020, 77, 315–325. [Google Scholar] [CrossRef]
- Scotland, M. Marine Scotland Information. Available online: http://marine.gov.scot/data/facts-and-figures-about-scotlands-sea-area-coastline-length-sea-area-sq-kms (accessed on 20 May 2021).
- Rae, G.H. Sea louse control in Scotland, past and present. Pest Manag. Sci. Former. Pestic. Sci. 2002, 58, 515–520. [Google Scholar] [CrossRef]
- Howard, P.M. Environment, Labour and Capitalism at Sea:‘Working the Ground’ in Scotland; Manchester University Press: Manchester, UK, 2017. [Google Scholar]
- Directorate, M.S. Scotland’s Marine Economic Statistics 2016. Available online: https://www.gov.scot/publications/scotlands-marine-economic-statistics/pages/7/ (accessed on 20 May 2021).
- Roberts, T.; Upham, P. Prospects for the use of macro-algae for fuel in Ireland and the UK: An overview of marine management issues. Mar. Policy 2012, 36, 1047–1053. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Morrissey, M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar]
- Lucarini, M.; Zuorro, A.; Di Lena, G.; Lavecchia, R.; Durazzo, A.; Benedetti, B.; Lombardi-Boccia, G. Sustainable Management of Secondary Raw Materials from the Marine Food-Chain: A Case-Study Perspective. Sustainability 2020, 12, 8997. [Google Scholar] [CrossRef]
- Coull, J.R. Fish farming in the Highlands and Islands: Boom industry of the 1980s. Scott. Geogr. Mag. 1988, 104, 4–13. [Google Scholar] [CrossRef]
- Wippelhauser, G.S. Ecology and Management of Maine’s Eelgrass, Rockweeds, and Kelps; US Department of Commerce NOAA Coastal Services Center Library: Charleston, SC, USA, 1996. [Google Scholar]
- Hill, J.; White, N. Marine Life Information Network: Biology and Sensitivity Key Information Sub-programme. Available online: https://www.marlin.ac.uk/ (accessed on 20 April 2021).
- Carlson, L. Seasonal variation in growth, reproduction and nitrogen content of Fucus vesiculosus L. in the Öresund, Southern Sweden. Bot. Mar. 1991, 34, 447–454. [Google Scholar] [CrossRef]
- Morrissey, J.; Kraan, S.; Guiry, M.D. A Guide to Commercially Important Seaweeds on the Irish Coast; Irish Bord Iascaigh Mhara/Iirish Sea Fisheries Board: Dublin, Ireland, 2001. [Google Scholar]
- McLachlan, J. Seaweed Resources in Europe: Uses and Potential; Taylor & Francis: Boca Raton, FL, USA, 1992. [Google Scholar]
- Guiry, M.D.; Blunden, G. Seaweed Resources in Europe: Uses and Potential; John Wiley & Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- Williams, G.A. Seasonal variation in a low shore Fucus serratus (Fucales, Phaeophyta) population and its epiphytic fauna. Hydrobiology 1996, 326, 191–197. [Google Scholar] [CrossRef]
- Anderson, C.; Scott, G. The occurrence of distinct morphotypes within a population of Fucus spiralis. J. Mar. Biol. Assoc. UK 1998, 78, 1003–1006. [Google Scholar] [CrossRef]
- Bond, P.; Brown, M.; Moate, R.; Gledhill, M.; Hill, S.; Nimmo, M. Arrested development in Fucus spiralis (Phaeophyceae) germlings exposed to copper. Eur. J. Phycol. 1999, 34, 513–521. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Teng, D.; Zhang, Y.; Mao, R.; Xi, D.; Wang, J. Candidacidal mechanism of the arenicin-3-derived peptide NZ17074 from Arenicola marina. Appl. Microbiol. Biotechnol. 2014, 98, 7387–7398. [Google Scholar] [CrossRef] [PubMed]
- Chock, J.S.; Mathieson, A.C. Physiological ecology of Ascophyllum nodosum (L.) Le Jolis and its detached ecad scorpioides (Hornemann) Hauck (Fucales, Phaeophyta). Bot. Mar. 1979, 22, 21–26. [Google Scholar] [CrossRef]
- Gibb, D.C. The free-living forms of Ascophyllum nodosum (L.) Le Jol. J. Ecol. 1957, 45, 49–83. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 1–24. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundene, O. Growth and Reproduction in Ascophyllum Nodosum (Phaeophyceae). Nor. J. Bot. 1973, 20, 249–255. [Google Scholar]
- Halat, L.; Galway, M.E.; Garbary, D.J. Cell wall structural changes lead to separation and shedding of biofouled epidermal cell wall layers by the brown alga Ascophyllum nodosum. Protoplasma 2020, 257, 1–13. [Google Scholar] [CrossRef]
- Filion-Myklebust, C. Epidermis shedding in the brown seaweed Ascophyllum nodosum (L.) Le Jolis and its ecological significance. Mar. Biol. Lett. 1981, 2, 45–51. [Google Scholar]
- Boaden, P.J.; Dring, M. A quantitative evaluation of the effects of Ascophyllum harvesting on the littoral ecosystem. Helgoländer Meeresunters. 1980, 33, 700–710. [Google Scholar] [CrossRef] [Green Version]
- Hill, J. Ascophyllum Nodosum. Knotted Wrack. In Marine Life Information Network: Biology and Sensitivity Key Information Reviews [on-line]; Tyler-Walters, H., Hiscock, K., Eds.; Marine Biological Association of the United Kingdom: Plymouth, UK, 2008. [Google Scholar] [CrossRef]
- Bush, L.; Davies, A.; Maggs, C.A.; Yesson, C.; Brodie, J.A. A Review for the Crown Estate July 2013. Available online: https://macroalgalresearchgroupcom.files.wordpress.com/2017/03/bush_2013_reviewofloss.pdf (accessed on 20 April 2021).
- White, N. Pelvetia Canaliculata. Channelled Wrack. In Marine Life Information Network: Biology and Sensitivity Key Information Reviews [on-line]; Tyler-Walters, H., Hiscock, K., Eds.; Marine Biological Association of the United Kingdom: Plymouth, UK, 2008. [Google Scholar]
- Stengel, D.; Wilkes, R.; Guiry, M. Seasonal growth and recruitment of Himanthalia elongata Fucales, Phaeophycota) in different habitats on the Irish west coast. Eur. J. Phycol. 1999, 34, 213–221. [Google Scholar] [CrossRef]
- De Schryver, A.M.; Brakkee, K.W.; Goedkoop, M.J.; Huijbregts, M.A. Characterization Factors for Global Warming in Life Cycle Assessment Based on Damages to Humans and Ecosystems; ACS Publications: Washington, DC, USA, 2009. [Google Scholar]
- Gårdmark, A.; Huss, M. Individual variation and interactions explain food web responses to global warming. Philos. Trans. R. Soc. B 2020, 375, 20190449. [Google Scholar] [CrossRef]
- Botkin, D.B.; Saxe, H.; Araujo, M.B.; Betts, R.; Bradshaw, R.H.; Cedhagen, T.; Chesson, P.; Dawson, T.P.; Etterson, J.R.; Faith, D.P. Forecasting the effects of global warming on biodiversity. Bioscience 2007, 57, 227–236. [Google Scholar] [CrossRef]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The seaweed holobiont: Understanding seaweed–bacteria interactions. Fems Microbiol. Rev. 2013, 37, 462–476. [Google Scholar] [CrossRef] [Green Version]
- Suryanarayanan, T. Fungal endosymbionts of seaweeds. In Biology of Marine Fungi; Springer: Berlin/Heidelberg, Germany, 2012; pp. 53–69. [Google Scholar]
- Nybakken, J.W. Marine Biology: An Ecological Approach; Benjamin Cummings: San Francisco, CA, USA, 2001. [Google Scholar]
- Burrows, M.T.; Smale, D.; Connor, N.O.; Van Rein, H.; Moore, P. Marine Strategy Framework Directive Indicators for UK Kelp Habitats Part 1: Developing Proposals for Potential Indicators; Joint Nature Conservation Committee: Peterborough, UK, 2014. [Google Scholar]
- Jones, L.A.; Hiscock, K.; Connor, D.W. Marine Habitat Reviews: A summary of Ecological Requirements and Sensitivity Characteristics for the Conservation and Management of Marine SACs; Joint Nature Conservation Committee: Peterborough, UK, 2000. [Google Scholar]
- Christie, H.; Jørgensen, N.M.; Norderhaug, K.M.; Waage-Nielsen, E. Species distribution and habitat exploitation of fauna associated with kelp (Laminaria hyperborea) along the Norwegian coast. Mar. Biol. Assoc. UK J. Mar. Biol. Assoc. 2003, 83, 687. [Google Scholar] [CrossRef]
- Adams, J.; Toop, T.; Donnison, I.S.; Gallagher, J.A. Seasonal variation in Laminaria digitata and its impact on biochemical conversion routes to biofuels. Bioresour. Technol. 2011, 102, 9976–9984. [Google Scholar] [CrossRef] [PubMed]
- Kain, J.M. A view of the genus Laminaria. Oceanogr. Mar. Biol. 1979, 17, 101–161. [Google Scholar]
- Pavia, H.; Carr, H.; Åberg, P. Habitat and feeding preferences of crustacean mesoherbivores inhabiting the brown seaweed Ascophyllum nodosum (L.) Le Jol. and its epiphytic macroalgae. J. Exp. Mar. Biol. Ecol. 1999, 236, 15–32. [Google Scholar] [CrossRef]
- Wilkinson, M. Information Review on the Impact of Kelp Harvesting; Scottish Natural Heritage: Edinburgh, UK, 1995. [Google Scholar]
- Marzinelli, E.M.; Leong, M.R.; Campbell, A.H.; Steinberg, P.D.; Vergés, A. Does restoration of a habitat-forming seaweed restore associated faunal diversity? Restor. Ecol. 2016, 24, 81–90. [Google Scholar] [CrossRef]
- Pereira, R.C.; Da Gama, B.A.P.; Teixeira, V.L.; Yoneshigue-Valentin, Y. Ecological roles of natural products of the Brazilian red seaweed Laurencia obtusa. Braz. J. Biol. 2003, 63, 665–672. [Google Scholar] [CrossRef]
- McClintock, J.B.; Baker, B.J. Marine Chemical Ecology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Kumari, P.; Reddy, C. Antimicrobial compounds from seaweeds-associated bacteria and fungi. Appl. Microbiol. Biotechnol. 2015, 99, 1571–1586. [Google Scholar] [CrossRef]
- Pringgenies, D.; Retnowati, E.I.; Ariyanto, D.; Dewi, K.; Viharyo, M.A.; Susilowati, R. Symbiotic microbes from various seaweeds with antimicrobial and fermentative properties. Aquac. Aquar. Conserv. Legis. 2020, 13, 2211–2217. [Google Scholar]
- Hoey, G.v.; Drent, J.; Ysebaert, T.; Herman, P. The Benthic Ecosystem Quality Index (BEQI), Intercalibraton and Assessment of Dutch Coastal and Transitional Waters for the Water Frame Directive. Final Report. 2008. Available online: https://www.semanticscholar.org/paper/The-Benthic-Ecosystem-Quality-Index-(BEQI)%2C-and-of-Hoey-Drent/fda18bba6b2c10924aa62d96d9a2f4768b5422a6 (accessed on 20 April 2021).
- Alonso, P.D. Water European Law And The Watershed Management. Int. Bus. Econ. Res. J. 2012, 11, 1545–1548. [Google Scholar] [CrossRef] [Green Version]
- The Scottish, G. Wild Seaweed Harvesting: Strategic Environmental Assessment Environmental Report; APS Group Scotland: Edinburgh, UK, 2016. [Google Scholar]
- Juanes, J.; Guinda, X.; Puente, A.; Revilla, J. Macroalgae, a suitable indicator of the ecological status of coastal rocky communities in the NE Atlantic. Ecol. Indic. 2008, 8, 351–359. [Google Scholar] [CrossRef]
- Alami, A.H.; Alasad, S.; Ali, M.; Alshamsi, M. Investigating algae for CO2 capture and accumulation and simultaneous production of biomass for biodiesel production. Sci. Total Environ. 2021, 759, 143529. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.D.-W.; Chen, P.H.; Ramaraj, R. The potential of carbon dioxide capture and sequestration with algae. Ecol. Eng. 2017, 98, 17–23. [Google Scholar] [CrossRef]
- Muraoka, D. Seaweed resources as a source of carbon fixation. Bull. Fish. Res. Agency Jpn. 2004, (Supplement No. 1), 59–64. [Google Scholar]
- Zou, D.; Gao, K. Physiological responses of seaweeds to elevated atmospheric CO2 concentrations. In Seaweeds and their Role In Globally Changing Environments; Springer: Berlin/Heidelberg, Germany, 2010; pp. 115–126. [Google Scholar]
- Fernández, P.A.; Hurd, C.L.; Roleda, M.Y. Bicarbonate uptake via an anion exchange protein is the main mechanism of inorganic carbon acquisition by the giant kelp M acrocystis pyrifera (L aminariales, P haeophyceae) under variable pH. J. Phycol. 2014, 50, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Change.org. Do Not Allow Mechanical Kelp Dredging in Scottish Waters. Available online: https://www.change.org/p/scottish-parliament-ensure-that-mechanical-kelp-dredging-does-not-happen-in-scotland (accessed on 20 May 2021).
- Oaten, J.; Hull, S.; Roberts, C.; Brooks, T.; San Martin, E.; Smedley, M. Wild Seaweed Harvesting; ABP mer: Southampton, UK, 2018. [Google Scholar]
- Scotland, M. Seaweed Cultivation Policy Statement. 2017. Available online: https://www.gov.scot/binaries/content/documents/govscot/publications/speech-statement/2017/03/seaweed-cultivation-policy-statement-2017/documents/00515518-pdf/00515518-pdf/govscot%3Adocument/00515518.pdf (accessed on 20 April 2021).
- Chakdar, H.; Jadhav, S.D.; Dhar, D.W.; Pabbi, S. Potential applications of blue green algae. J. Sci. Indust. Res. 2012, 71, 13–20. [Google Scholar]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Fleurence, J.; Morançais, M.; Dumay, J. Seaweed proteins. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 245–262. [Google Scholar]
- Sho, H. History and characteristics of Okinawan longevity food. Asia Pac. J. Clin. Nutr. 2001, 10, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Allen, V.G.; Pond, K.R.; Saker, K.E.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Brown, C.P.; Miller, M.F.; Montgomery, J.L. Tasco-Forage: III. Influence of a seaweed extract on performance, monocyte immune cell response, and carcass characteristics in feedlot-finished steers. J. Anim. Sci. 2001, 79, 1032–1040. [Google Scholar] [CrossRef]
- Montgomery, J.L.; Allen, V.G.; Pond, K.R.; Miller, M.F.; Wester, D.B.; Brown, C.P.; Evans, R.; Bagley, C.P.; Ivy, R.L.; Fontenot, J.P. Tasco-Forage: IV. Influence of a seaweed extract applied to tall fescue pastures on sensory characteristics, shelf-life, and vitamin E status in feedlot-finished steers. J. Anim. Sci. 2001, 79, 884–894. [Google Scholar] [CrossRef]
- Saker, K.E.; Allen, V.G.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Wester, D.B. Tasco-Forage: II. Monocyte immune cell response and performance of beef steers grazing tall fescue treated with a seaweed extract. J. Anim. Sci. 2001, 79, 1022–1031. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, I.A.; Seal, C.J.; Wilcox, M.; Dettmar, P.W.; Pearson, J.P. Applications of alginates in food. In Alginates: Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 211–228. [Google Scholar]
- Brownlee, I.; Allen, A.; Pearson, J.; Dettmar, P.; Havler, M.; Atherton, M.; Onsøyen, E. Alginate as a source of dietary fiber. Crit. Rev. Food Sci. Nutr. 2005, 45, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.M.; Jorge, K.; Nogueira, L.C.; Silva, F.; Trugo, L.C. Effects of the combination of hydrophobic polypeptides, iso-α acids, and malto-oligosaccharides on beer foam stability. J. Agric. Food Chem. 2005, 53, 4976–4981. [Google Scholar] [CrossRef]
- Huang, X.; Kakuda, Y.; Cui, W. Hydrocolloids in emulsions: Particle size distribution and interfacial activity. Food Hydrocoll. 2001, 15, 533–542. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hwang, S.; Park, J.; Park, H.J. Preparation and release characteristics of polymer-coated and blended alginate microspheres. J. Microencapsul. 2003, 20, 179–192. [Google Scholar] [CrossRef] [PubMed]
- de Vos, P.; Faas, M.M.; Strand, B.; Calafiore, R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27, 5603–5617. [Google Scholar] [CrossRef]
- Uludag, H.; De Vos, P.; Tresco, P.A. Technology of mammalian cell encapsulation. Adv. Drug Deliv. Rev. 2000, 42, 29–64. [Google Scholar] [CrossRef]
- Wang, L.Z.; Liu, L.; Holmes, J.; Kerry, J.F.; Kerry, J.P. Assessment of film-forming potential and properties of protein and polysaccharide-based biopolymer films. Int. J. Food Sci. Technol. 2007, 42, 1128–1138. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Salmieri, S.; Saucier, L.; Lacroix, M. Antimicrobial effects of alginate-based films containing essential oils on Listeria monocytogenes and Salmonella typhimurium present in bologna and ham. J. Food Prot. 2007, 70, 901–908. [Google Scholar] [CrossRef]
- Datta, S.; Janes, M.; Xue, Q.G.; Losso, J.; La Peyre, J. Control of Listeria monocytogenes and Salmonella anatum on the surface of smoked salmon coated with calcium alginate coating containing oyster lysozyme and nisin. J. Food Sci. 2008, 73, M67–M71. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.-H. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Nouri, S.; Yorghanlou, R.A.; Roufegarinejad, L. Development of hydroxypropyl methylcellulose/sodium alginate blend active film incorporated with Dracocephalum moldavica L. essential oil for food preservation. J. Thermoplast. Compos. Mater. 2020, 0892705720962153. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Cherry, P. Seaweeds as a Source of Non-Digestible Complex Polysaccharide Components for the Development of Novel Prebiotic Ingredients for the Functional Food Industry; Ulster University: Coleraine, Ireland, 2020. [Google Scholar]
- Fao, F. Food and Agriculture Organisation of the United Nations. Available online: http://www.fao.org/home/en/ (accessed on 18 June 2021).
- Nisizawa, K.; Noda, H.; Kikuchi, R.; Watanabe, T. The main seaweed foods in Japan. Hydrobiologia 1987, 151, 5–29. [Google Scholar] [CrossRef]
- Fujiwara-Arasaki, T.; Mino, N.; Kuroda, M. The Protein Value in Human Nutrition of Edible Marine Algae in Japan; Springer: Berlin/Heidelberg, Germany, 1984; pp. 513–516. [Google Scholar]
- Hall, A.C.; Fairclough, A.C.; Mahadevan, K.; Paxman, J.R. Ascophyllum nodosum enriched bread reduces subsequent energy intake with no effect on post-prandial glucose and cholesterol in healthy, overweight males. A pilot study. Appetite 2012, 58, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Hoad, C.L.; Rayment, P.; Spiller, R.C.; Marciani, L.; Alonso, B.d.C.; Traynor, C.; Mela, D.J.; Peters, H.P.; Gowland, P.A. In vivo imaging of intragastric gelation and its effect on satiety in humans. J. Nutr. 2004, 134, 2293–2300. [Google Scholar] [CrossRef] [Green Version]
- Pelkman, C.L.; Navia, J.L.; Miller, A.E.; Pohle, R.J. Novel calcium-gelled, alginate-pectin beverage reduced energy intake in nondieting overweight and obese women: Interactions with dietary restraint status. Am. J. Clin. Nutr. 2007, 86, 1595–1602. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Angell, A.R.; Angell, S.F.; de Nys, R.; Paul, N.A. Seaweed as a protein source for mono-gastric livestock. Trends Food Sci. Technol. 2016, 54, 74–84. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Nutritional quality of some wild and cultivated seaweeds: Nutrient composition, total phenolic content and in vitro digestibility. J. Appl. Phycol. 2016, 28, 3575–3585. [Google Scholar] [CrossRef]
- de Beukelaar, M.F.A.; Zeinstra, G.G.; Mes, J.J.; Fischer, A.R.H. Duckweed as human food. The influence of meal context and information on duckweed acceptability of Dutch consumers. Food Qual. Prefer. 2019, 71, 76–86. [Google Scholar] [CrossRef]
- Van der Spiegel, M.; Noordam, M.Y.; Van der Fels-Klerx, H.J. Safety of novel protein sources (insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [Green Version]
- Sousa, I.; Gouveia, L.; Batista, A.P.; Raymundo, A.; Bandarra, N.M. Microalgae in novel food products. Food Chem. Res. Dev. 2008, 75–112. [Google Scholar]
- Van Krimpen, M.M.; Bikker, P.; Van der Meer, I.M.; Van der Peet-Schwering, C.M.C.; Vereijken, J.M. Cultivation, Processing and Nutritional Aspects for Pigs and Poultry of European Protein Sources as Alternatives for Imported Soybean Products; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2013. [Google Scholar]
- Trentacoste, E.M.; Martinez, A.M.; Zenk, T. The place of algae in agriculture: Policies for algal biomass production. Photosynth. Res. 2015, 123, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Handler, R.M.; Shi, R.; Shonnard, D.R. Land use change implications for large-scale cultivation of algae feedstocks in the United States Gulf Coast. J. Clean. Prod. 2017, 153, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D.; Houser, J.; Preiss, E.; White, O.; Fang, H.; Mesnick, L.; Barsky, T.; Tariche, S.; Schreck, J.; Alpert, S. Water resources: Agriculture, the environment, and society. BioScience 1997, 47, 97–106. [Google Scholar] [CrossRef]
- Iwamoto, H. Industrial production of microalgal cell-mass and secondary products-major industrial species. Handb. Microalgal Cult. Biotechnol. Appl. Phycol. 2004, 255, 263. [Google Scholar]
- Champenois, J.; Marfaing, H.; Pierre, R. Review of the taxonomic revision of Chlorella and consequences for its food uses in Europe. J. Appl. Phycol. 2015, 27, 1845–1851. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Hernandez-Gonzalez, M.d.C.; Varela, D. Seaweed future cultivation in Chile: Perspectives and challenges. Int. J. Environ. Pollut. 2008, 33, 432–456. [Google Scholar] [CrossRef]
- Aaron-Amper, J.; Largo, D.B.; Handugan, E.R.B.; Nini, J.L.; Alingasa, K.M.A.; Gulayan, S.J. Culture of the tropical brown seaweed Sargassum aquifolium: From hatchery to field out-planting. Aquac. Rep. 2020, 16, 100265. [Google Scholar] [CrossRef]
- Joubert, Y.; Fleurence, J. Simultaneous extraction of proteins and DNA by an enzymatic treatment of the cell wall of Palmaria palmata (Rhodophyta). J. Appl. Phycol. 2008, 20, 55–61. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.F.; Sary, H.H. Selective isolation of glycoprotein materials from the green seaweed Ulva lactuca. Pak. J. Biochem. 1987, 20, 61. [Google Scholar]
- Polprasert, C.; Koottatep, T. Organic Waste Recycling: Technology, Management and Sustainability; IWA Publishing: London, UK, 2017. [Google Scholar]
- Wosnitza, T.M.A.; Barrantes, J.G. Utilization of seaweed Ulva sp. in Paracas Bay (Peru): Experimenting with compost. J. Appl. Phycol. 2006, 18, 27. [Google Scholar] [CrossRef]
- Eyras, M.C.; Defosse, G.; Dellatorre, F. Seaweed compost as an amendment for horticultural soils in Patagonia, Argentina. Compos. Sci. Util. 2008, 16, 119–124. [Google Scholar] [CrossRef]
- Haug, R. The Practical Handbook of Compost Engineering; Routledge: London, UK, 2018. [Google Scholar]
- Chang, J.I.; Hsu, T.-E. Effects of compositions on food waste composting. Bioresour. Technol. 2008, 99, 8068–8074. [Google Scholar] [CrossRef]
- Eiland, F.; Klamer, M.; Lind, A.-M.; Leth, M.; Bååth, E. Influence of initial C/N ratio on chemical and microbial composition during long term composting of straw. Microb. Ecol. 2001, 41, 272–280. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Paull, J.; Rathjen, A. Shoot mineral composition and yield of wheat genotypes grown on a sodic and a non-sodic soil. Aust. J. Exp. Agric. 2000, 40, 69–78. [Google Scholar] [CrossRef]
- EKOGEA. BCx for Compost. Available online: https://www.ekogea-int.com/compost (accessed on 20 May 2021).
- Hasznos, G. Compost/Biofertiliser Certification Schemes’ Annual Report 2018; Renewable Energy Assurance Ltd.: London, UK, 2019. [Google Scholar]
- Jayaraman, J.; Norrie, J.; Punja, Z.K. Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J. Appl. Phycol. 2011, 23, 353–361. [Google Scholar] [CrossRef]
- Lola-Luz, T.; Hennequart, F.; Gaffney, M. Effect on yield, total phenolic, total flavonoid and total isothiocyanate content of two broccoli cultivars (Brassica oleraceae var italica) following the application of a commercial brown seaweed extract (Ascophyllum nodosum). Agric. Food Sci. 2014, 23, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Rayorath, P.; Jithesh, M.N.; Farid, A.; Khan, W.; Palanisamy, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J. Appl. Phycol. 2008, 20, 423–429. [Google Scholar] [CrossRef]
- Somai-Jemmali, L.; Siah, A.; Randoux, B.; Magnin-Robert, M.; Halama, P.; Hamada, W.; Reignault, P. Brown alga Ascophyllum nodosum extract-based product, Dalgin Active®, triggers defense mechanisms and confers protection in both bread and durum wheat against Zymoseptoria tritici. J. Appl. Phycol. 2020, 32, 3387–3399. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2019, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Ervin, E.H. Cytokinins and seaweed extracts for summer putting green health. Golfdom 2013, 69, 36–39. [Google Scholar]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Zodape, S.; Gupta, A.; Bhandari, S.; Rawat, U.; Chaudhary, D.; Eswaran, K.; Chikara, J. Foliar application of seaweed sap as biostimulant for enhancement of yieldand quality of tomato (Lycopersicon esculentum Mill.). J. Sci. Ind. Res. 2011, 70, 215–219. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Zhang, J.; Kirby, C.W.; Ji, X.; Locke, S.J.; Critchley, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Angus, S. Modern Seaweed Harvesting and Gathering in Scotland: The Legal and Ecological Context. Scott. Geogr. J. 2017, 133, 1–14. [Google Scholar] [CrossRef]
- VisitScotland. Scotland Map. Available online: https://www.visitscotland.com/destinations-maps/ (accessed on 18 May 2021).
- Angus, S. Dé tha cearr air a’mhachaire? Biodiversity issues for Scottish machair: An initial appraisal. Glasg. Nat. 2009, 25, 53–62. [Google Scholar]
- Marine, S. Draft Seaweed Policy Statement Consultation Paper; APS Group Scotland: Scotland, UK, 2013. [Google Scholar]
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Radulovich, R.; Neori, A.; Valderrama, D.; Reddy, C.; Cronin, H.; Forster, J. Farming of seaweeds. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 27–59. [Google Scholar]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Valderrama, D.; Cai, J.; Hishamunda, N.; Ridler, N. Social and Economic Dimensions of Carrageenan Seaweed Farming; FAO: Rome, Italy, 2013. [Google Scholar]
- Burrows, M.T.; Macleod, M.; Orr, K. Mapping the Intertidal Seaweed Resources of the Outer Hebrides; Scottish Association for Marine Science and Hebridean Seaweed Company: Scotland, UK, 2010. [Google Scholar]
- MacLeod, A.K.; Orr, K.K.; Greenhill, L.; Burrows, M.T. Understanding the Potential Effects of Wave Energy Devices on Kelp Biotopes; Scottish Natural Heritage: Inverness, UK, 2014.
- Byrnes, J.E.; Reed, D.C.; Cardinale, B.J.; Cavanaugh, K.C.; Holbrook, S.J.; Schmitt, R.J. Climate-driven increases in storm frequency simplify kelp forest food webs. Glob. Chang. Biol. 2011, 17, 2513–2524. [Google Scholar] [CrossRef] [Green Version]
- Burrows, M.T.; Fox, C.J.; Moore, P.; Smale, D.; Sotheran, I.; Benson, A.; Greenhill, L.; Martino, S.; Parker, A.; Thompson, E.; et al. Wild Seaweed Harvesting as a Diversification Opportunity for Fishermen; A Report by SRSL for Highlands and Islands Enterprise: Scotland, UK, 2018. [Google Scholar]
- Kain, J.M.; Dawes, C. Useful European seaweeds: Past hopes and present cultivation. In Proceedings of the Twelfth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 1987; pp. 173–181. [Google Scholar]
- Andreakis, N.; Schaffelke, B. Invasive marine seaweeds: Pest or prize? In Seaweed Biology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 235–262. [Google Scholar]
- Angulo-Valdés, J.A.; Hatcher, B.G. A new typology of benefits derived from marine protected areas. Mar. Policy 2010, 34, 635–644. [Google Scholar] [CrossRef]
- Tyler-Walters, H.; James, B.; Carruthers, M.; Wilding, C.; Durkin, O.; Lacey, C.; Philpott, E.; Adams, L.; Chaniotis, P.D.; Wilkes, P.T.V.; et al. Descriptions of Scottish Priority Marine Features (PMFs); Scottish Natural Heritage: Scotland, UK, 2016. [Google Scholar]
- Marine, S. Scotland’s National Marine Plan A Single Framework for Managing Our Seas; The Scottish Government: Edinburgh, UK, 2015. [Google Scholar]
- Wood, D.; Capuzzo, E.; Kirby, D.; Mooney-McAuley, K.; Kerrison, P. UK macroalgae aquaculture: What are the key environmental and licensing considerations? Mar. Policy 2017, 83, 29–39. [Google Scholar] [CrossRef]
- Kelly, C.; Gray, L.; Shucksmith, R.; Tweddle, J.F. Review and evaluation of marine spatial planning in the Shetland Islands. Mar. Policy 2014, 46, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, M.; Pico, Y. Chapter 2—Gas Chromatography and Mass Spectroscopy Techniques for the Detection of Chemical Contaminants and Residues in Foods. In Chemical Contaminants and Residues in Food, 2nd ed.; Schrenk, D., Cartus, A., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 15–50. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D.; Praveen, N.K. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J. Food Sci. Technol. 2015, 52, 1924–1935. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef]
- Abel, U.; Koch, C.; Speitling, M.; Hansske, F.G. Modern methods to produce natural-product libraries. Curr. Opin. Chem. Biol. 2002, 6, 453–458. [Google Scholar] [CrossRef]
- Ranger, S.; Rose, C. Seaweed in the Daily Diet and Nutrition. Available online: http://www.seagreens.co.uk/Documents/Daily_Diet_and_Nutrtion_article_plus_products_10.13.pdf (accessed on 18 May 2021).
- Restani, P.; Persico, A.; Ballabio, C.; Moro, E.; Fuggetta, D.; Colombo, M.L. Analysis of food supplements containing iodine: A survey of Italian market. Clin. Toxicol. 2008, 46, 282–286. [Google Scholar] [CrossRef]
- Roy, M.-C.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a commercially-available algal phlorotannins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res. Int. 2011, 44, 3026–3029. [Google Scholar] [CrossRef]
- Paradis, M.-E.; Couture, P.; Lamarche, B. A randomised crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on postchallenge plasma glucose and insulin levels in men and women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef]
- Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Dietary fiber and antioxidant capacity in Fucus vesiculosus products. Int. J. Food Sci. Nutr. 2009, 60, 23–34. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liang, H.; Ji, X.; Zhou, Z.; Liu, Y.; Sun, T.; Zhang, L. Effects of fucoidan on gut flora and tumor prevention in 1, 2-dimethylhydrazine-induced colorectal carcinogenesis. J. Nutr. Biochem. 2020, 82, 108396. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-C.; Huang, R.-Y.; Chou, T.-C. Oligo-Fucoidan Improves Diabetes-Induced Renal Fibrosis via Activation of Sirt-1, GLP-1R, and Nrf2/HO-1: An In Vitro and In Vivo Study. Nutrients 2020, 12, 3068. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Hina, S.; Masood, S.; Kalim, I.; Saeed, M.K.; Ahmad, I.; Arshad, M. Exploration of Locally Grown Yellow and Green Pumpkin as a Potential Source of b-Carotene and Vitamin A. Biol. Sci. Pjsir 2020, 63, 238–241. [Google Scholar] [CrossRef]
- Coronel, J.; Pinos, I.; Amengual, J. β-carotene in obesity research: Technical considerations and current status of the field. Nutrients 2019, 11, 842. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chen, X.; Nakamura, Y.; Yu, C.; Qi, H. Fucoxanthin activities motivate its nanoencapsulation for food or nutraceutical application: A review. Food Funct. 2020, 11, 9338–9358. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Takahashi, S.; Nishimura, R.; Kubota, A.; Kojima, H.; Ohta, T.; Hamada, J.; Kuramitsu, Y.; Maeda, H.; Miyashita, K. A Marine Carotenoid of Fucoxanthinol Accelerates the Growth of Human Pancreatic Cancer PANC-1 Cells. Nutr. Cancer 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, H. Neoxanthin and fucoxanthinol in Fucus vesiculosus. Biochim. Biophys. Acta Gen. Subj. 1974, 338, 572–576. [Google Scholar] [CrossRef]
- Jin, W.; Yang, L.; Yi, Z.; Fang, H.; Chen, W.; Hong, Z.; Zhang, Y.; Zhang, G.; Li, L. Anti-Inflammatory Effects of Fucoxanthinol in LPS-Induced RAW264. 7 Cells through the NAAA-PEA Pathway. Mar. Drugs 2020, 18, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnett, R.; Mallams, A.; Spark, A.; Tee, J.; Weedon, B.; McCormick, A. Carotenoids and related compounds. Part XX. Structure and reactions of fucoxanthin. J. Chem. Soc. C Org. 1969, 3, 429–454. [Google Scholar] [CrossRef]
- Budzikiewicz, H.; Taraz, K. Chlorophyll c. Tetrahedron 1971, 27, 1447–1460. [Google Scholar] [CrossRef]
- Glombitza, K.-W.; Lentz, G. Antibiotics from algae—XXVIII: Cleavage of high molecular phlorotannin derivatives from the brown alga fucus vesiculosus L. Tetrahedron 1981, 37, 3861–3866. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Fisch, K.M.; Wright, A.D.; König, G.M. A new antioxidant isobenzofuranone derivative from the algicolous marine fungus Epicoccum sp. Planta Med. 2003, 69, 831–834. [Google Scholar]
- McInnes, A.; Walter, J.; Wright, J. 13C NMR Spectra of Δ 24 (28) phytosterols. Org. Magn. Reson. 1980, 13, 302–303. [Google Scholar] [CrossRef]
- Ioannou, E.; Zervou, M.; Ismail, A.; Ktari, L.; Vagias, C.; Roussis, V. 2, 6-Cyclo-xenicanes from the brown algae Dilophus fasciola and Dilophus spiralis. Tetrahedron 2009, 65, 10565–10572. [Google Scholar] [CrossRef]
- Song, L.; Qu, D.; Zhang, Q.; Zhou, H.; Jiang, R.; Li, Y.; Zhang, Y.; Yan, H. Phytosterol esters attenuate hepatic steatosis in rats with non-alcoholic fatty liver disease rats fed a high-fat diet. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Heilbron, I.; Phipers, R.; Wright, H. 343. The chemistry of the algæ. Part I. The algal sterol fucosterol. J. Chem. Soc. 1934, 1572–1576. [Google Scholar] [CrossRef]
- Knapp, F.; Greig, J.; Goad, L.; Goodwin, T. The conversion of 24-ethylidene-sterols into poriferasterol by Ochromonas malhamensis. J. Chem. Soc. D Chem. Commun. 1971, 707–709. [Google Scholar] [CrossRef]
- Buedenbender, L.; Astone, F.A.; Tasdemir, D. Bioactive Molecular Networking for Mapping the Antimicrobial Constituents of the Baltic Brown Alga Fucus vesiculosus. Mar. Drugs 2020, 18, 311. [Google Scholar] [CrossRef] [PubMed]
- de Souza, N.J.; Nes, W.R. The presence of phytol in brown and blue-green algae and its relationship to evolution. Phytochemistry 1969, 8, 819–822. [Google Scholar] [CrossRef]
- Parys, S.; Kehraus, S.; Krick, A.; Glombitza, K.-W.; Carmeli, S.; Klimo, K.; Gerhäuser, C.; König, G.M. In vitro chemopreventive potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry 2010, 71, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Gite, S.; Ross, R.P.; Kirke, D.; Guihéneuf, F.; Aussant, J.; Stengel, D.B.; Dinan, T.G.; Cryan, J.F.; Stanton, C. Nutraceuticals to promote neuronal plasticity in response to corticosterone-induced stress in human neuroblastoma cells. Nutr. Neurosci. 2019, 22, 551–568. [Google Scholar] [CrossRef]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Budzikiewicz, H.; Brzezinka, H.; Johannes, B. Zur photosynthese grüner pflanzen, 2. Mitt.: Massenspektroskopische untersuchungen an carotinoiden. Mon. Für Chem. Chem. Mon. 1970, 101, 579–609. [Google Scholar] [CrossRef]
- Haugan, J.A.; Liaaen-Jensen, S. Isolation and characterisation of four allenic (6′ S)-isomers of fucoxanthin. Tetrahedron Lett. 1994, 35, 2245–2248. [Google Scholar] [CrossRef]
- Kim, M.-M.; Kim, S.-K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-H.; Qian, Z.-J.; Ryu, B.; Karadeniz, F.; Kim, D.; Kim, S.-K. Antioxidant peptides from protein hydrolysate of microalgae Navicula incerta and their protective effects in HepG2/CYP2E1 cells induced by ethanol. Phytother. Res. 2012, 26, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-S. Antithrombotic and profibrinolytic activities of phloroglucinol. Food Chem. Toxicol. 2011, 49, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Michel, T.; Nde, C.B.M.; Njuh, A.N.; Cisilotto, J.; Ndinteh, D.T.; Clyne, C.; Fernandez, X.; Creczynski-Pasa, T.B.; Njamen, D. Estrogen-like and tissue-selective effects of 7-methoxycoumarin from Ficus umbellata (Moraceae): An in vitro and in vivo study. BMC Complementary Altern. Med. 2017, 17, 383. [Google Scholar] [CrossRef] [Green Version]
- Sancheti, S.; Sancheti, S.; Seo, S.-Y. Ameliorative effects of 7-methylcoumarin and 7-methoxycoumarin against CCl4-induced hepatotoxicity in rats. Drug Chem. Toxicol. 2013, 36, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Daletos, G.; Proksch, P.; Andrade, P.B.; Valentão, P. Anti-inflammatory potential of monogalactosyl diacylglycerols and a monoacylglycerol from the edible brown seaweed Fucus spiralis Linnaeus. Mar. Drugs 2014, 12, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Fathalipour, M.; Fathalipour, H.; Safa, O.; Nowrouzi-Sohrabi, P.; Mirkhani, H.; Hassanipour, S. The therapeutic role of carotenoids in diabetic retinopathy: A systematic review. DiabetesMetab. Syndr. Obes. Targets Ther. 2020, 13, 2347. [Google Scholar] [CrossRef]
- Rai, M.; Rathod, D.; Ingle, A.; Proksch, P.; Kon, K. 5 Biocidal Metabolites from Endophytes that Occur in Medicinal Plants. In Natural Antioxidants and Biocides from Wild Medicinal Plants; CAB International: Wallingford, UK, 2013. [Google Scholar]
- Santiago, C.; Fitchett, C.; Munro, M.H.; Jalil, J.; Santhanam, J. Cytotoxic and antifungal activities of 5-hydroxyramulosin, a compound produced by an endophytic fungus isolated from Cinnamomum mollisimum. Evid. Based Complementary Altern. Med. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sarfati, L. Stopping the Clock: Antiaging treatments and care for clients of every age. Ski. Deep 2017, 16, 48–53. [Google Scholar]
- He, L.; Shang, Z.; Liu, H.; Yuan, Z.-X. Alginate-Based Platforms for Cancer-Targeted Drug Delivery. Biomed Res. Int. 2020, 1–17. [Google Scholar] [CrossRef]
- Jiang, Z.; Okimura, T.; Yamaguchi, K.; Oda, T. The potent activity of sulfated polysaccharide, ascophyllan, isolated from Ascophyllum nodosum to induce nitric oxide and cytokine production from mouse macrophage RAW264.7 cells: Comparison between ascophyllan and fucoidan. Nitric Oxide 2011, 25, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Jensen, A.; Ragan, M.A. 1, 2, 3, 5-tetrahydroxybenzene 2, 5-disulfate ester: The “phenolic precursor” in gelbstoff-forming exudates from the marine brown alga Ascophyllum nodosum (L.) Lejol. Tetrahedron Lett. 1978, 19, 847–850. [Google Scholar] [CrossRef]
- Grosse-Damhues, J.; Glombitza, K.-W.; Schulten, H.-R. An eight-ring phlorotannin from the brown alga Himanthalia elongata. Phytochemistry 1983, 22, 2043–2046. [Google Scholar] [CrossRef]
- Liu, J.; Luthuli, S.; Wu, Q.; Wu, M.; Choi, J.-I.; Tong, H. Pharmaceutical and Nutraceutical Potential Applications of Sargassum fulvellum. Biomed Res. Int. 2020, 1–12. [Google Scholar] [CrossRef]
- Borst, E.M.; Ständker, L.; Wagner, K.; Schulz, T.F.; Forssmann, W.-G.; Messerle, M. A peptide inhibitor of cytomegalovirus infection from human hemofiltrate. Antimicrob. Agents Chemother. 2013, 57, 4751–4760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr. Res. 2006, 341, 238–245. [Google Scholar] [CrossRef]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Carotenoids. In Natural Food Colorants; Springer: Berlin/Heidelberg, Germany, 1996; pp. 197–243. [Google Scholar]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Heilbron, I.M.; Phipers, R.F. The algae: The lipochromes of Fucus vesiculosus. Biochem. J. 1935, 29, 1369–1375. [Google Scholar] [CrossRef] [Green Version]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Mounien, L.; Tourniaire, F.; Landrier, J.-F. Anti-obesity effect of carotenoids: Direct impact on adipose tissue and adipose tissue-driven indirect effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef] [Green Version]
- Hussain, H.; Krohn, K.; Schulz, B.; Draeger, S.; Nazir, M.; Saleem, M. Two new antimicrobial metabolites from the endophytic fungus, Seimatosporium sp. Nat. Prod. Commun. 2012, 7, 1934578X1200700305. [Google Scholar] [CrossRef] [Green Version]
- Lei, G.; Song, C.; Luo, Y. Chemical composition of hydrosol volatiles of flowers from ten Paeonia× suffruticosa Andr. cultivars from Luoyang, China. Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Naveen, J.; Baskaran, V. Antidiabetic plant-derived nutraceuticals: A critical review. Eur. J. Nutr. 2018, 57, 1275–1299. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Ulbricht, C. Focus: Diabetes. J. Diet. Suppl. 2011, 8, 239–256. [Google Scholar] [CrossRef]
- Haugan, J.A.; Aakermann, T.; Liaaen-Jensen, S. [20] Isolation of fucoxanthin and peridinin. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1992; Volume 213, pp. 231–245. [Google Scholar]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation. J. Cell. Biochem. 2006, 97, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.S.; Soler-Vila, A.; Rai, D.K.; Croft, A.K.; Brunton, N.P.; Smyth, T.J. UPLC-MS profiling of low molecular weight phlorotannin polymers in Ascophyllum nodosum, Pelvetia canaliculata and Fucus spiralis. Metabolomics 2014, 10, 524–535. [Google Scholar] [CrossRef]
- Osterhage, C.; König, G.M.; Jones, P.G.; Wright, A.D. 5-Hydroxyramulosin, a new natural product produced by Phoma tropica, a marine-derived fungus isolated from the alga Fucus spiralis. Planta Med. 2002, 68, 1052–1054. [Google Scholar] [CrossRef]
- Williams, R.; Goodwin, T. The occurrence of mutatochrome in green tissues. Phytochemistry 1967, 6, 1037–1039. [Google Scholar] [CrossRef]
- Apostolidis, E.; Lee, C. In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated α-glucosidase and α-amylase inhibition. J. Food Sci. 2010, 75, H97–H102. [Google Scholar] [CrossRef]
- Zhang, J.; Tiller, C.; Shen, J.; Wang, C.; Girouard, G.S.; Dennis, D.; Barrow, C.J.; Miao, M.; Ewart, H.S. Antidiabetic properties of polysaccharide- and polyphenolic-enriched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007, 85, 1116–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarfati, L. AGE Beautifully. Ski. Deep 2018, 17, 70–77. [Google Scholar]
- Dutot, M.; Fagon, R.; Hemon, M.; Rat, P. Antioxidant, anti-inflammatory, and anti-senescence activities of a phlorotannin-rich natural extract from brown seaweed Ascophyllum nodosum. Appl. Biochem. Biotechnol. 2012, 167, 2234–2240. [Google Scholar] [CrossRef]
- Nakayasu, S.; Soegima, R.; Yamaguchi, K.; Oda, T. Biological activities of fucose-containing polysaccharide ascophyllan isolated from the brown alga Ascophyllum nodosum. Biosci. Biotechnol. Biochem. 2009, 73, 961–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakubo, A.; Makino, H.; Ohnishi, J.-i.; Hirohara, H.; Hori, K. The marine red alga Eucheuma serra J. Agardh, a high yielding source of two isolectins. J. Appl. Phycol. 1997, 9, 331. [Google Scholar] [CrossRef]
- Weis, W.I.; Drickamer, K. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 1996, 65, 441–473. [Google Scholar] [CrossRef]
- Ziółkowska, N.E.; Wlodawer, A. Structural studies of algal lectins with anti-HIV activity. Acta Biochim. Pol. 2006, 53, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Naeem, A.; Saleemuddin, M.; Hasan Khan, R. Glycoprotein targeting and other applications of lectins in biotechnology. Curr. Protein Pept. Sci. 2007, 8, 261–271. [Google Scholar] [CrossRef]
- Alavi, M.; Asare-Addo, K.; Nokhodchi, A. Lectin Protein as a Promising Component to Functionalize Micelles, Liposomes and Lipid NPs against Coronavirus. Biomedicines 2020, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Stratil, S.B.; Neulinger, S.C.; Knecht, H.; Friedrichs, A.K.; Wahl, M. Salinity affects compositional traits of epibacterial communities on the brown macroalga Fucus vesiculosus. Fems Microbiol. Ecol. 2014, 88, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Ihua, M.W.; Guihéneuf, F.; Mohammed, H.; Margassery, L.M.; Jackson, S.A.; Stengel, D.B.; Clarke, D.J.; Dobson, A.D. Microbial population changes in decaying Ascophyllum nodosum result in macroalgal-polysaccharide-degrading bacteria with potential applicability in enzyme-assisted extraction technologies. Mar. Drugs 2019, 17, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of protein from the macroalga Palmaria palmata. Lwt Food Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Chemat, F.; Zille, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Kuhavichanan, A.; Kusolkumbot, P.; Sirisattha, S.; Areeprasert, C. Mechanical Extraction of Protein Solution from Microalgae by Ultrasonication. IOP Conf. Ser. Earth Environ. Sci. 2018, 159, 12009. [Google Scholar] [CrossRef]
- Mæhre, H.; Jensen, I.-J.; Eilertsen, K.-E. Enzymatic pre-treatment increases the protein bioaccessibility and extractability in Dulse (Palmaria palmata). Mar. Drugs 2016, 14, 196. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction and characterization of protein from Irish brown seaweed Ascophyllum nodosum. Food Res. Int. 2017, 99, 1021–1027. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro-and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Goettel, M.; Eing, C.; Gusbeth, C.; Straessner, R.; Frey, W. Pulsed electric field assisted extraction of intracellular valuables from microalgae. Algal Res. 2013, 2, 401–408. [Google Scholar] [CrossRef]
- Töpfl, S. Pulsed Electric Fields (PEF) for Permeabilization of Cell Membranes in Food-and Bioprocessing–Applications, Process and Equipment Design and Cost Analysis. Ph.D. Thesis, Technische Universität Berlin, Fakultät III, Berlin, Germnay, 2006. [Google Scholar]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Eng. Rev. 2015, 7, 45–62. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, N.; Ranjan, R.; Kumar, S.; Bhat, Z.F.; Jeong, D.K. Perspective of membrane technology in dairy industry: A review. Asian Australas. J. Anim. Sci. 2013, 26, 1347. [Google Scholar] [CrossRef] [Green Version]
- Macquarrie, D.J.; Clark, J.H.; Fitzpatrick, E. The microwave pyrolysis of biomass. BiofuelsBioprod. Biorefining 2012, 6, 549–560. [Google Scholar] [CrossRef]
- Michalak, I.; Tuhy, Ł.; Chojnacka, K. Seaweed extract by microwave assisted extraction as plant growth biostimulant. Open Chem. 2015, 13, 1183–1195. [Google Scholar] [CrossRef]
- van Hees, D.H.; Olsen, Y.S.; Wernberg, T.; Van Alstyne, K.L.; Kendrick, G.A. Phenolic concentrations of brown seaweeds and relationships to nearshore environmental gradients in Western Australia. Mar. Biol. 2017, 164, 74. [Google Scholar] [CrossRef] [Green Version]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurised liquid extraction and solid–liquid extraction methods on the phenolic content and antioxidant activities of I rish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on alpha-amylase, alpha-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef]
- Fertah, M. Chapter 2—Isolation and Characterization of Alginate from Seaweed. In Seaweed Polysaccharides; Venkatesan, J., Anil, S., Kim, S.-K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 11–26. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.m.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, N.; Jalsa, N.K.; Lee, K.-Y.; Ward, K. Multistage extraction and purification of waste Sargassum natans to produce sodium alginate: An optimization approach. Carbohydr. Polym. 2018, 198, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Hernández-carmona, G.; McHugh, D.J.; Arvizu-Higuera, D.L.; Rodríguez-montesinos, Y.E. Pilot plant scale extraction of alginate from Macrocystis pyrifera. 1. Effect of pre-extraction treatments on yield and quality of alginate. J. Appl. Phycol. 1998, 10, 507–513. [Google Scholar] [CrossRef]
- McHugh, D.J. Production and Utilization of Products from Commercial Seaweeds; FAO: Rome, Italy, 1987; pp. 1553–1561. [Google Scholar]
- Chee, S.Y.; Wong, P.K.; Wong, C.L. Extraction and characterisation of alginate from brown seaweeds (Fucales, Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. J. Appl. Phycol. 2011, 23, 191–196. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyraud, A.; Gey, C.; Leonard, C.; Rochas, C.; Girond, S.; Kloareg, B. NMR spectroscopy analysis of oligoguluronates and oligomannuronates prepared by acid or enzymatic hydrolysis of homopolymeric blocks of alginic acid. Application to the determination of the substrate specificity of Haliotis tuberculata alginate lyase. Carbohydr. Res. 1996, 289, 11–23. [Google Scholar] [CrossRef]
- Torres, M.R.; Sousa, A.P.A.; Silva Filho, E.A.T.; Melo, D.F.; Feitosa, J.P.A.; de Paula, R.C.M.; Lima, M.G.S. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074. [Google Scholar] [CrossRef] [Green Version]
- Draget, K.I.; Strand, B.; Hartmann, M.; Valla, S.; Smidsrød, O.; Skjåk-Bræk, G. Ionic and acid gel formation of epimerised alginates; the effect of AlgE4. Int. J. Biol. Macromol. 2000, 27, 117–122. [Google Scholar] [CrossRef]














| Code | Compound Name | Original Algal Source | Biological Activities | Ref. |
|---|---|---|---|---|
| 1 | Fucoidan | F. vesiculosus | Effects on Lewis lung carcinoma cells (LCCs), melanoma B16 cells. Activated the natural killer cell activity in vivo in mice model. | [167] |
| In vivo effect as a suppressor to induced colorectal carcinogenesis in rats. | [168] | |||
| Low-molecular-weight fucoidan showed protective effect against renal fibrosis in animal in vitro and in vivo studies. | [169] | |||
| 2 | β-carotene | F. vesiculosus | It is the pro-vitamin A with high antioxidant activities. | [170] |
| Important role in metabolism of fat and regulation of body weight. | [171] | |||
| 3 | Fucoxanthin | F. vesiculosus | Good intervention with some types of tumours. | [172] |
| Anticancer effects. | [173] | |||
| 4 | Fucoxanthinol | F. vesiculosus | Anti-inflammatory. | [174,175] |
| Anticancer effects. | [173] | |||
| 5 | Isofucoxanthin | F. vesiculosus and F. serratus | - | [176,177] |
| 6 | Isofucoxanthinol | F. vesiculosus | - | [176] |
| 7 | Neoxanthin | F. vesiculosus | - | [174] |
| 8 | 1,3,5-trimethoxybenzene | Controlled cleavage process of polyphenolic compounds extracted from F. vesiculosus | - | [178] |
| 9 | 3,5-dimethoxyphenol | |||
| 10 | 2,4,6-trimethoxyphenol | |||
| 11 | 2,2′,4,4′,6,6′-hexamethoxy-1,1′-biphenyl | |||
| 12 | 4-hydroxy-2,6,2′,4′,6′-pentamethoxybiphenyl | |||
| 13 | 2,6-dihydroxy-4,3′,5′-trimethoxydiphenyl ether | |||
| 14 | 4-hydroxy-2,6,3′,5′-tetramethoxydiphenyl ether | |||
| 15 | 4,3′-dihydroxy-2,6,4′,5′-tetramethoxydiphenyl ether | |||
| 16 | 2,4,6,2′,6′-pentamethoxybiphenyl | |||
| 17 | 2-hydroxy-4,6,3′,5′-tetramethoxydiphenyl ether | |||
| 18 | 4-hydroxy-2,6,3′,4′,5′-pentamethoxydiphenyl ether | |||
| 19 | 2,6,2′,4′,6′-pentamethoxy-4-(2,6-dihydroxy-4-methoxyphenoxy) biphenyl | |||
| 20 | 2,6,2′,4′,6′-pentamethoxy-4-(2-hydroxy-4,6-dimethoxyphenoxy) biphenyl | |||
| 21 | Trans-4-Hydroxymellein | From the endophyte Epicoccum sp. derived from the brown algae F. vesiculosus | Antioxidant activities | [179] |
| 22 | (3R)-5-hydroxymellein | |||
| 23 | 5-(acetoxymethyl)-furan-2-carboxylic acid | |||
| 24 | 4,5,6-trihydroxy-7-methylphthalide | |||
| 25 | (3β,24E)-stigmasta-5,24(28)-dien-3-ol (Isofucosterol) | F. vesiculosus and F. spiralis | Lowering total cholesterol and harmful low-density lipoproteins | [180,181,182] |
| 26 | Fucosterol | F. vesiculosus, F. spiralis, and P. canaliculata | [182,183,184] | |
| 27 | 1,2-Di-O-α-linolenoyl-3-O-β-galactopyranosyl-sn-glycerol | F. vesiculosus | - | [185] |
| 28 | 3,7,11-Trimethyl-2-dodecen-1-ol | F. vesiculosus | - | [186] |
| 29 | Heterosigma-glycolipid I | F. vesiculosus | - | [185] |
| 30 | Heterosigma-glycolipid II | |||
| 31 | Fucotriphlorethol A | F. vesiculosus L. | Free radical scavenging activities. | [187] |
| 32 | Trifucodiphlorethol A | F. vesiculosus L. | Free radical scavenging activities. | [187] |
| 33 | Trifucotriphlorethol A | F. vesiculosus L. | Free radical scavenging activities. | [187] |
| 34 | Quercetin | F. vesiculosus | Neuroprotective promoting the neuronal plasticity. | [188] |
| 35 | Curcumin | F. vesiculosus | Neuroprotective promoting the neuronal plasticity. | [188] |
| 36 | Fucose | F. vesiculosus | Sulfated polysaccharides have antioxidants properties | [189] |
| 37 | Glucose | |||
| 38 | Galactose | |||
| 39 | Xylose | |||
| 40 | zeaxanthin | F. serratus | - | [190] |
| 41 | Chlorophyll c1 | [177] | ||
| 42 | All-trans-fucoxanthin | [191] | ||
| 43 | Phloroglucinol | F. vesiculosus | Against the chronic inflammation consequences. | [178,192,193] |
| Antithrombotic and profibrinolytic. | [194] | |||
| 44 | 7-methoxycoumarin | F. spiralis | Estrogenic effects. | [195] |
| Hepatoprotective effects. | [196] | |||
| 45 | 1-monooleoyl glycerol | F. spiralis | [197] | |
| 46 | β-carotene 5,8-epoxide (Citroxanthin) | F. spiralis | Antidiabetic-retinopathy effect. | [198] |
| 47 | 5-hydroxyramulosin | Endophyte associated with F. spiralis | Strong antifungal effect IC50 1.56 µg/mL against Aspergillus niger. | [199,200] |
| 48 | Alginic acid | L. digitata and A. nodosum | Moisturise the skin | [201] |
| 49 | Alginate | A. nodosum | Anticancer drug vehicle | [202] |
| 50 | Glucuronic acid | A. nodosum | - | [203] |
| 51 | 1,2,3,5-tetrahydroxybenzol-2,5-disulfate ester | A. nodosum | - | [204] |
| 52 | Bis-fucopentaphlorethol A nonadecaactate | H. elongata | - | [205] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dulaimi, O.; Rateb, M.E.; Hursthouse, A.S.; Thomson, G.; Yaseen, M. The Brown Seaweeds of Scotland, Their Importance and Applications. Environments 2021, 8, 59. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8060059
Al-Dulaimi O, Rateb ME, Hursthouse AS, Thomson G, Yaseen M. The Brown Seaweeds of Scotland, Their Importance and Applications. Environments. 2021; 8(6):59. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8060059
Chicago/Turabian StyleAl-Dulaimi, Omar, Mostafa E. Rateb, Andrew S. Hursthouse, Gary Thomson, and Mohammed Yaseen. 2021. "The Brown Seaweeds of Scotland, Their Importance and Applications" Environments 8, no. 6: 59. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8060059