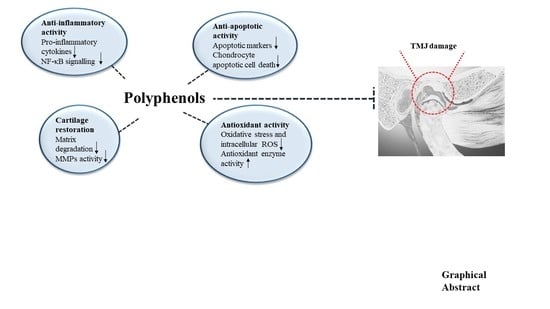
Polyphenols as Potential Agents in the Management of Temporomandibular Disorders
Abstract
:1. Introduction
2. The Role of Inflammation in TMJ
3. Polyphenols as Potential Agents in TMD
3.1. Molecular Mechanisms of Polyphenols Targeting Inflammation
3.1.1. Curcumin
3.1.2. Resveratrol
3.1.3. Epigallocatechin3-Gallate
3.1.4. Polyphenol-Based Extracts against TMJ Inflammation
3.2. Potential Polyphenols Application in TMJ Inflammation, In Vivo Studies
4. Alternative Therapies for TMD Management
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TMD | Temporomandibular disorders |
| TMJ | Temporomandibular joint |
| OA | Osteoarthritis |
| TMJOA | Temporomandibular joint Osteoarthritis |
| EGCG | Epigallocatechin3-gallate |
| CBCT | Cone-beam computed tomography |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| Nrf2 | Nuclear factor erythroid 2-like 2 |
| ROS | Reactive Oxygen Species |
| TNF-α | Tumor necrosis factor α |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin 6 |
| PG | Prostaglandins |
| MMPs | Matrix metalloproteinases |
| JNK | C-Jun N-terminal kinase |
| COX-2 | Cyclooxygenase-2 |
| NO | Nitric oxide |
| NF-κB | Nuclear factor-κB |
| MAPK | Mitogen-Activated Protein kinase |
| AP-1 | Activator protine-1 |
| LPS | Lipopolysaccharides |
| VEGF | Vascular endothelial growth factor |
| SCFAs | Short-chain fatty acids |
| SIRT-1 | Sirtuin 1 |
| FLS | Fibroblast-like synoviocytes |
| CIA | Murine model of Collagen-Induced Arthritis |
| PGA | Polyglycolic acid |
| PLA | Polylactic acid |
| ECM | Extracellular matrix |
| UBM | Decellularized urinary bladder matrix |
| Ahr | Aryl hydrocarbon receptor |
References
- Ibi, M. Inflammation and Temporomandibular Joint Derangement. Biol. Pharm. Bull. 2019, 42, 538–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalladka, M.; Quek, S.; Heir, G.; Eliav, E.; Mupparapu, M.; Viswanath, A. Temporomandibular joint osteoarthritis: Diagnosis and long-term conservative management: A topic review. J. Indian Prosthodont. Soc. 2014, 14, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.S.; Tang, Y.L.; Tang, Y.J.; Fei, W.; Liang, X.H. Multiple Treatment Meta-Analysis of Intra-Articular Injection for Temporomandibular Osteoarthritis. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2020, 78, 373 e371–373 e318. [Google Scholar] [CrossRef]
- Dental, S.; Minervini, G.; Nucci, L.; Lanza, A.; Femiano, F.; Contaldo, M.; Grassia, V. Temporomandibular disc displacement with reduction treated with anterior repositioning splint: A 2-year clinical and magnetic resonance imaging (MRI) follow-up. J. Biol. Regul. Homeost. Agents 2020, 34, 151–160. [Google Scholar]
- Minervini, G.; Lucchese, A.; Perillo, L.; Serpico, R.; Minervini, G. Unilateral superior condylar neck fracture with dislocation in a child treated with an acrylic splint in the upper arch for functional repositioning of the mandible. J. Craniomandib. Pract. 2017, 35, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Romano, A.; Petruzzi, M.; Maio, C.; Serpico, R.; Di Stasio, D.; Lucchese, A. Oral-facial-digital syndrome (OFD): 31-year follow-up management and monitoring. J. Biol. Regul. Homeost. 2018, 32, 127–130. [Google Scholar]
- Tchetina, E.V.; Markova, G.A. Regulation of energy metabolism in the growth plate and osteoarthritic chondrocytes. Rheumatol. Int. 2018, 38, 1963–1974. [Google Scholar] [CrossRef]
- Morel, M.; Ruscitto, A.; Pylawka, S.; Reeve, G.; Embree, M.C. Extracellular matrix turnover and inflammation in chemically-induced TMJ arthritis mouse models. PLoS ONE 2019, 14, e0223244. [Google Scholar] [CrossRef]
- Russo, A.; Lavorgna, L.; Silvestro, M.; Abbadessa, G.; Bisecco, A.; Trojsi, F.; Tessitore, A.; Tedeschi, G.; Bonavita, S. Readability Analysis of Online Headache and Migraine Information. Headache 2020. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, H.K.; Auh, Q.S.; Nah, H.; Lee, J.S.; Moon, H.J.; Heo, D.N.; Kim, I.S.; Kwon, I.K. Emerging Potential of Exosomes in Regenerative Medicine for Temporomandibular Joint Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 1541. [Google Scholar] [CrossRef] [Green Version]
- Di Stasio, D.; Lauritano, D.; Gritti, P.; Migliozzi, R.; Maio, C.; Minervini, G.; Petruzzi, M.; Serpico, R.; Candotto, V.; Lucchese, A. Psychiatric disorders in oral lichen planus: A preliminary case control study. J. Biol. Regul. Homeost. Agents 2018, 32, 97–100. [Google Scholar] [PubMed]
- Stoll, M.L.; Kau, C.H.; Waite, P.D.; Cron, R.Q. Temporomandibular joint arthritis in juvenile idiopathic arthritis, now what? Homeost. Agents. 2018, 16, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, G.; Casett, E.; Stuginski-Barbosa, J.; Guerra, E.N.S.; Fernandes, D.A.; Porporatti, A.L.; Flores-Mir, C.; De Luca Canto, G. Effects of glucosamine supplements on painful temporomandibular joint osteoarthritis: A systematic review. J. Oral Rehabil. 2018, 45, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Dolci, A.; Minervini, G.; Salerno, C.; D, D.I.S.; Minervini, G.; Laino, L.; Silvestre, F.; Serpico, R. Vulvovaginal gingival lichen planus: Report of two cases and review of literature. Oral Implantol. 2016, 9, 54–60. [Google Scholar] [CrossRef]
- Alves, S.M.; Freitas, R.S.; do Val, D.R.; Vieira, L.V.; de Assis, E.L.; Gomes, F.I.F.; Gadelha, C.A.A.; Gadelha, T.S.; de Lacerda, J.; Clemente-Napimoga, J.T.; et al. The efficacy of a lectin from Abelmoschus Esculentus depends on central opioid receptor activation to reduce temporomandibular joint hypernociception in rats. Biomed. Pharmacother. 2018, 101, 478–484. [Google Scholar] [CrossRef]
- Araujo, I.W.; Chaves, H.V.; Pacheco, J.M.; Val, D.R.; Vieira, L.V.; Santos, R.; Freitas, R.S.; Rivanor, R.L.; Monteiro, V.S.; Clemente-Napimoga, J.T.; et al. Role of central opioid on the antinociceptive effect of sulfated polysaccharide from the red seaweed Solieria filiformis in induced temporomandibular joint pain. Int. Immunopharmacol. 2017, 44, 160–167. [Google Scholar] [CrossRef]
- Coura, C.O.; Chaves, H.V.; do Val, D.R.; Vieira, L.V.; Silveira, F.D.; Dos Santos Lopes, F.M.; Gomes, F.I.; Frota, A.F.; Souza, R.B.; Clemente-Napimoga, J.T.; et al. Mechanisms involved in antinociception induced by a polysulfated fraction from seaweed Gracilaria cornea in the temporomandibular joint of rats. Int. J. Biol. Macromol. 2017, 97, 76–84. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.L.; Smith, B.J.; Lo, D.F.; Chyu, M.C.; Dunn, D.M.; Chen, C.H.; Kwun, I.S. Dietary polyphenols and mechanisms of osteoarthritis. J. Nutr. Biochem. 2012, 23, 1367–1377. [Google Scholar] [CrossRef]
- Bjorklund, G.; Aaseth, J.; Dosa, M.D.; Pivina, L.; Dadar, M.; Pen, J.J.; Chirumbolo, S. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition 2019, 66, 153–165. [Google Scholar] [CrossRef]
- Magni, G.; Marinelli, A.; Riccio, D.; Lecca, D.; Tonelli, C.; Abbracchio, M.P.; Petroni, K.; Ceruti, S. Purple Corn Extract as Anti-allodynic Treatment for Trigeminal Pain: Role of Microglia. Front. Cell. Neurosci. 2018, 12, 378. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [Green Version]
- da Conceicao Rivanor, R.L.; Chaves, H.V.; do Val, D.R.; de Freitas, A.R.; Lemos, J.C.; Rodrigues, J.A.; Pereira, K.M.; de Araujo, I.W.; Bezerra, M.M.; Benevides, N.M. A lectin from the green seaweed Caulerpa cupressoides reduces mechanical hyper-nociception and inflammation in the rat temporomandibular joint during zymosan-induced arthritis. Int. Immunopharmacol. 2014, 21, 34–43. [Google Scholar] [CrossRef] [Green Version]
- do Val, D.R.; Bezerra, M.M.; Silva, A.A.; Pereira, K.M.; Rios, L.C.; Lemos, J.C.; Arriaga, N.C.; Vasconcelos, J.N.; Benevides, N.M.; Pinto, V.P.; et al. Tephrosia toxicaria Pers. reduces temporomandibular joint inflammatory hypernociception: The involvement of the HO-1 pathway. Eur. J. Pain 2014, 18, 1280–1289. [Google Scholar] [CrossRef]
- Henrotin, Y.; Kurz, B. Antioxidant to treat osteoarthritis: Dream or reality? Curr. Drug Targets 2007, 8, 347–357. [Google Scholar] [CrossRef]
- Regan, E.; Flannelly, J.; Bowler, R.; Tran, K.; Nicks, M.; Carbone, B.D.; Glueck, D.; Heijnen, H.; Mason, R.; Crapo, J. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheum. 2005, 52, 3479–3491. [Google Scholar] [CrossRef] [Green Version]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef] [Green Version]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, H.; Cujec, T.P.; Yamanaka, H.; Kamatani, N. Molecular aspects of rheumatoid arthritis: Role of transcription factors. FEBS J. 2008, 275, 4463–4470. [Google Scholar] [CrossRef] [Green Version]
- Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.J.; Lu, H.P.; Gu, Z.Y.; Zhou, Y.Q. Involvement of the MAPK pathway in the pressure-induced synovial metaplasia procedure for the temporomandibular joint. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xie, J.; Sun, S.; Ren, X.; Kong, J.; Ji, P. Toll-Like Receptor 4 (TLR4) Stimulates Synovial Injury of Temporomandibular Joint in Rats Through the Activation of p38 Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 4405–4412. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Cantile, T.; Coda, M.; Alcidi, B.; Sangianantoni, G.; Ingenito, A.; Di Stasio, M.; Volpe, M.G. In Vivo Release Kinetics and Antibacterial Activity of Novel Polyphenols-Enriched Chewing Gums. Molecules 2016, 21, 1008. [Google Scholar] [CrossRef] [Green Version]
- Ferrazzano, G.F.; Scioscia, E.; Sateriale, D.; Pastore, G.; Colicchio, R.; Pagliuca, C.; Cantile, T.; Alcidi, B.; Coda, M.; Ingenito, A.; et al. In Vitro Antibacterial Activity of Pomegranate Juice and Peel Extracts on Cariogenic Bacteria. Biomed. Res. Int. 2017, 2017, 2152749. [Google Scholar] [CrossRef] [Green Version]
- Philip, N.; Walsh, L.J. Cranberry Polyphenols: Natural Weapons against Dental Caries. Dent. J. 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Lambert, C.; Couchourel, D.; Ripoll, C.; Chiotelli, E. Nutraceuticals: Do they represent a new era in the management of osteoarthritis?—A narrative review from the lessons taken with five products. Osteoarthr. Cartil. 2011, 19, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.J.; Rasool, M. Dietary component p-coumaric acid suppresses monosodium urate crystal-induced inflammation in rats. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2013, 62, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, A.; Shanbhag, R.; Chamallamudi, M.R.; Singh, V.P.; Mudgal, J. Ameliorative effect of caffeic acid against inflammatory pain in rodents. Eur. J. Pharmacol. 2011, 666, 80–86. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tome, S.M.; Silva, A.M.; Laufer, S.; Lima, J.L.; Fernandes, E. Flavonoids inhibit COX-1 and COX-2 enzymes and cytokine/chemokine production in human whole blood. Inflammation 2015, 38, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, J.; Mao, J.; Li, H.; Wang, M.; Zhang, H.; Li, H.; Chen, W. Genistein Ameliorates Non-alcoholic Fatty Liver Disease by Targeting the Thromboxane A2 Pathway. J. Agric. Food Chem. 2018, 66, 5853–5859. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.F.; Sun, W.X. Effect of Naringin on Monosodium Iodoacetate-Induced Osteoarthritis Pain in Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3746–3751. [Google Scholar] [CrossRef] [Green Version]
- Yowtak, J.; Lee, K.Y.; Kim, H.Y.; Wang, J.; Kim, H.K.; Chung, K.; Chung, J.M. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain 2011, 152, 844–852. [Google Scholar] [CrossRef] [Green Version]
- Filho, A.W.; Filho, V.C.; Olinger, L.; de Souza, M.M. Quercetin: Further investigation of its antinociceptive properties and mechanisms of action. Arch. Pharmacal Res. 2008, 31, 713–721. [Google Scholar] [CrossRef]
- Priebe, A.; Hunke, M.; Tonello, R.; Sonawane, Y.; Berta, T.; Natarajan, A.; Bhuvanesh, N.; Pattabiraman, M.; Chandra, S. Ferulic acid dimer as a non-opioid therapeutic for acute pain. J. Pain Res. 2018, 11, 1075–1085. [Google Scholar] [CrossRef] [Green Version]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complementary Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, G.L.; Spagnuolo, C.; Russo, M.; Tedesco, I.; Moccia, S.; Cervellera, C. Mechanisms of aging and potential role of selected polyphenols in extending healthspan. Biochem. Pharmacol. 2020, 173, 113719. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Priem, F.; Mobasheri, A. Curcumin: A new paradigm and therapeutic opportunity for the treatment of osteoarthritis: Curcumin for osteoarthritis management. SpringerPlus 2013, 2, 56. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Clutterbuck, A.L.; Allaway, D.; Lodwig, E.M.; Harris, P.; Mathy-Hartert, M.; Shakibaei, M.; Mobasheri, A. Biological actions of curcumin on articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Moon, D.O.; Kim, M.O.; Choi, Y.H.; Park, Y.M.; Kim, G.Y. Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int. Immunopharmacol. 2010, 10, 605–610. [Google Scholar] [CrossRef]
- Daily, J.W.; Yang, M.; Park, S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2016, 19, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Mathy-Hartert, M.; Jacquemond-Collet, I.; Priem, F.; Sanchez, C.; Lambert, C.; Henrotin, Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm. Res. 2009, 58, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, D.; Lauritano, D.; Minervini, G.; Paparella, R.S.; Petruzzi, M.; Romano, A.; Candotto, V.; Lucchese, A. Management of denture stomatitis: A narrative review. J. Biol. Regul. Homeost. Agents 2018, 32, 113–116. [Google Scholar] [PubMed]
- Lauritano, D.; Petruzzi, M.; Nardi, G.M.; Carinci, F.; Minervini, G.; Di Stasio, D.; Lucchese, A. Single Application of a Dessicating Agent in the Treatment of Recurrent Aphthous Stomatitis. J. Biol. Regul. Homeost. Agents 2015, 29, 59–66. [Google Scholar] [PubMed]
- Di Stasio, D.; Romano, A.; Gentile, C.; Maio, C.; Lucchese, A.; Serpico, R.; Paparella, R.; Minervini, G.; Candotto, V.; Laino, L. Systemic and topical photodynamic therapy (PDT) on oral mucosa lesions: An overview. J. Biol. Regul. Homeost. Agents 2018, 32, 123–126. [Google Scholar] [PubMed]
- Contaldo, M.; Luzzi, V.; Ierardo, G.; Raimondo, E.; Boccellino, M.; Ferati, K.; Bexheti-Ferati, A.; Inchingolo, F.; Di Domenico, M.; Serpico, R.; et al. Bisphosphonate-related osteonecrosis of the jaws and dental surgery procedures in children and young people with osteogenesis imperfecta: A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef]
- Feng, K.; Ge, Y.; Chen, Z.; Li, X.; Liu, Z.; Li, X.; Li, H.; Tang, T.; Yang, F.; Wang, X. Curcumin Inhibits the PERK-eIF2alpha-CHOP Pathway through Promoting SIRT1 Expression in Oxidative Stress-induced Rat Chondrocytes and Ameliorates Osteoarthritis Progression in a Rat Model. Oxidative Med. Cell. Longev. 2019, 2019, 8574386. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.S.; Zhang, F.J.; Zeng, C.; Luo, W.; Xiao, W.F.; Gao, S.G.; Lei, G.H. Autophagy in osteoarthritis. Jt. Bone Spine 2016, 83, 143–148. [Google Scholar] [CrossRef]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef]
- Carames, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010, 62, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Feng, K.; Li, J.; Yu, D.; Fan, Q.; Tang, T.; Yao, X.; Wang, X. Curcumin Inhibits Apoptosis of Chondrocytes through Activation ERK1/2 Signaling Pathways Induced Autophagy. Nutrients 2017, 9, 414. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, J.; Yang, E.; Liang, B.; Ding, J.; Liang, J.; Xu, J. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.M.; Ahmad, I.; Haqqi, T.M. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic. Biol. Med. 2018, 116, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. = Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Gu, H.; Li, K.; Li, X.; Yu, X.; Wang, W.; Ding, L.; Liu, L. Oral Resveratrol Prevents Osteoarthritis Progression in C57BL/6J Mice Fed a High-Fat Diet. Nutrients 2016, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Bai, L. Resveratrol inhibits apoptosis by increase in the proportion of chondrocytes in the S phase of cell cycle in articular cartilage of ACLT plus Mmx rats. Saudi J. Biol. Sci. 2019, 26, 839–844. [Google Scholar] [CrossRef]
- Kang, D.G.; Lee, H.J.; Lee, C.J.; Park, J.S. Inhibition of the Expression of Matrix Metalloproteinases in Articular Chondrocytes by Resveratrol through Affecting Nuclear Factor-Kappa B Signaling Pathway. Biomol. Ther. 2018, 26, 560–567. [Google Scholar] [CrossRef]
- Qin, N.; Wei, L.; Li, W.; Yang, W.; Cai, L.; Qian, Z.; Wu, S. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J. Pharmacol. Sci. 2017, 134, 166–174. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nakahama, T.; Nguyen, C.H.; Tran, T.T.; Le, V.S.; Chu, H.H.; Kishimoto, T. Aryl hydrocarbon receptor antagonism and its role in rheumatoid arthritis. J. Exp. Pharmacol. 2015, 7, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.; Savouret, J.F.; Widerak, M.; Corvol, M.T.; Rannou, F. Resveratrol, Potential Therapeutic Interest in Joint Disorders: A Critical Narrative Review. Nutrients 2017, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Li, Y.; Liu, H.; Xiao, S.; Li, L.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabay, O.; Sanchez, C.; Dvir-Ginzberg, M.; Gagarina, V.; Zaal, K.J.; Song, Y.; He, X.H.; McBurney, M.W. Sirtuin 1 enzymatic activity is required for cartilage homeostasis in vivo in a mouse model. Arthritis Rheum. 2013, 65, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Liu, S.; Shu, H.; Crawford, J.; Xing, Y.; Tao, F. Resveratrol alleviates temporomandibular joint inflammatory pain by recovering disturbed gut microbiota. Brain Behav. Immun. 2020. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, D.; Lauritano, D.; Romano, A.; Salerno, C.; Minervini, G.; Minervini, G.; Gentile, E.; Serpico, R.; Lucchese, A. In Vivo Characterization of Oral Pemphigus Vulgaris by Optical Coherence Tomography. J. Biol. Regul. Homeost. Agents 2015, 29, 39–41. [Google Scholar] [PubMed]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [Green Version]
- Eng, Q.Y.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018, 210, 296–310. [Google Scholar] [CrossRef]
- Karatas, A.; Dagli, A.F.; Orhan, C.; Gencoglu, H.; Ozgen, M.; Sahin, N.; Sahin, K.; Koca, S.S. Epigallocatechin 3-gallate attenuates arthritis by regulating Nrf2, HO-1, and cytokine levels in an experimental arthritis model. Biotechnol. Appl. Biochem. 2019. [Google Scholar] [CrossRef]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, S.J.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model. Arthritis Res. Ther. 2014, 16, 508. [Google Scholar] [CrossRef] [Green Version]
- Minervini, G.; Romano, A.; Petruzzi, M.; Maio, C.; Serpico, R.; Lucchese, A.; Candotto, V.; Di Stasio, D. Telescopic overdenture on natural teeth: Prosthetic rehabilitation on (OFD) syndromic patient and a review on available literature. J. Biol. Regul. Homeost. Agents 2018, 32, 131–134. [Google Scholar]
- Singh, R.; Ahmed, S.; Malemud, C.J.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate selectively inhibits interleukin-1beta-induced activation of mitogen activated protein kinase subgroup c-Jun N-terminal kinase in human osteoarthritis chondrocytes. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2003, 21, 102–109. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahmed, S.; Islam, N.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: Suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum. 2002, 46, 2079–2086. [Google Scholar] [CrossRef]
- Huang, G.S.; Tseng, C.Y.; Lee, C.H.; Su, S.L.; Lee, H.S. Effects of (-)-epigallocatechin-3-gallate on cyclooxygenase 2, PGE(2), and IL-8 expression induced by IL-1beta in human synovial fibroblasts. Rheumatol. Int. 2010, 30, 1197–1203. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al-Shaya, O. Epigallocatechin-3-O-gallate modulates global microRNA expression in interleukin-1beta-stimulated human osteoarthritis chondrocytes: Potential role of EGCG on negative co-regulation of microRNA-140-3p and ADAMTS5. Eur. J. Nutr. 2018, 57, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Rasheed, Z.; Ramamurthy, S.; Anbazhagan, A.N.; Voss, F.R.; Haqqi, T.M. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010, 62, 1361–1371. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al-Shobaili, H.A. Epigallocatechin-3-O-gallate up-regulates microRNA-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes. Arthritis Rheum. 2016, 20, 2241–2248. [Google Scholar] [CrossRef] [Green Version]
- Tipton, D.A.; Christian, J.; Blumer, A. Effects of cranberry components on IL-1beta-stimulated production of IL-6, IL-8 and VEGF by human TMJ synovial fibroblasts. Arch. Oral Biol. 2016, 68, 88–96. [Google Scholar] [CrossRef]
- Figueira, M.E.; Camara, M.B.; Direito, R.; Rocha, J.; Serra, A.T.; Duarte, C.M.; Fernandes, A.; Freitas, M.; Fernandes, E.; Marques, M.C.; et al. Chemical characterization of a red raspberry fruit extract and evaluation of its pharmacological effects in experimental models of acute inflammation and collagen-induced arthritis. Food Funct. 2014, 5, 3241–3251. [Google Scholar] [CrossRef]
- Figueira, M.E.; Oliveira, M.; Direito, R.; Rocha, J.; Alves, P.; Serra, A.T.; Duarte, C.; Bronze, R.; Fernandes, A.; Brites, D.; et al. Protective effects of a blueberry extract in acute inflammation and collagen-induced arthritis in the rat. Biomed. Pharm. 2016, 83, 1191–1202. [Google Scholar] [CrossRef]
- Schell, J.; Scofield, R.H.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, D.; Romano, A.; Paparella, R.S.; Gentile, C.; Serpico, R.; Minervini, G.; Candotto, V.; Laino, L. How social media meet patients questions: YouTube review for mouth sores in children. J. Biol. Regul. Homeost. Agents 2018, 32, 117–121. [Google Scholar] [PubMed]
- Di Stasio, D.; Romano, A.N.; Paparella, R.S.; Gentile, C.; Minervini, G.; Serpico, R.; Candotto, V.; Laino, L. How social media meet patients questions: YouTube review for children oral thrush. J. Biol. Regul. Homeost. Agents 2018, 32, 101–106. [Google Scholar] [PubMed]
- Uttra, A.M.; Shahzad, M.; Shabbir, A.; Jahan, S.; Bukhari, I.A.; Assiri, A.M. Ribes orientale: A novel therapeutic approach targeting rheumatoid arthritis with reference to pro-inflammatory cytokines, inflammatory enzymes and anti-inflammatory cytokines. J. Ethnopharmacol. 2019, 237, 92–107. [Google Scholar] [CrossRef]
- Funk, J.L.; Oyarzo, J.N.; Frye, J.B.; Chen, G.; Lantz, R.C.; Jolad, S.D.; Solyom, A.M.; Timmermann, B.N. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J. Nat. Prod. 2006, 69, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.Y. The spice for joint inflammation: Anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des. Dev. Ther. 2016, 10, 3029–3042. [Google Scholar] [CrossRef] [Green Version]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Rahimnia, A.R.; Panahi, Y.; Alishiri, G.; Sharafi, M.; Sahebkar, A. Impact of Supplementation with Curcuminoids on Systemic Inflammation in Patients with Knee Osteoarthritis: Findings from a Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2015, 65, 521–525. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complementary Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Product-evaluation registry of Meriva(R), a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010, 52, 55–62. [Google Scholar]
- Zheng, Z.; Sun, Y.; Liu, Z.; Zhang, M.; Li, C.; Cai, H. The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des. Dev. Ther. 2015, 9, 4931–4942. [Google Scholar] [CrossRef] [Green Version]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Resveratrol Supplementation Reduces Pain and Inflammation in Knee Osteoarthritis Patients Treated with Meloxicam: A Randomized Placebo-Controlled Study. J. Med. Food 2018. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiao, L.; Yu, C.; Jin, P.; Qin, D.; Xu, Y.; Yin, J.; Liu, Z.; Du, Q. Enhanced Antiarthritic Efficacy by Nanoparticles of (-)-Epigallocatechin Gallate-Glucosamine-Casein. J. Agric. Food Chem. 2019, 67, 6476–6486. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, U.; Fichna, J.; Gorlach, S. Enhancement of anticancer potential of polyphenols by covalent modifications. Biochem. Pharmacol. 2016, 109, 1–13. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [Green Version]
- Milincic, D.D.; Popovic, D.A.; Levic, S.M.; Kostic, A.Z.; Tesic, Z.L.; Nedovic, V.A.; Pesic, M.B. Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef] [Green Version]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; Garcia-Gonzalez, C.A.; Landin, M.; Aquino, R.P. Technologies and Formulation Design of Polysaccharide-Based Hydrogels for Drug Delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhang, J.N.; Gan, Y.H.; Zhou, Y.H. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 2015, 94, 666–673. [Google Scholar] [CrossRef]
- Hersh, E.V.; Balasubramaniam, R.; Pinto, A. Pharmacologic management of temporomandibular disorders. Oral Maxillofac. Surg. Clin. N. Am. 2008, 20, 197–210. [Google Scholar] [CrossRef]
- Manfredini, D. A better definition of counselling strategies is needed to define effectiveness in temporomandibular disorders management. Evid. Based Dent. 2013, 14, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Della Vella, F.; Raimondo, E.; Minervini, G.; Buljubasic, M.; Ogodescu, A.; Sinescu, C.; Serpico, R. Early Childhood Oral Health Impact Scale (ECOHIS): Literature review and Italian validation. Int. J. Dent. Hyg. 2020. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, R.F.; Ferreira, M.A.; Barbosa, G.A.; Calderon, P.S. Counselling and self-management therapies for temporomandibular disorders: A systematic review. J. Oral Rehabil. 2013, 40, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Sojka, A.; Stelcer, B.; Roy, M.; Mojs, E.; Prylinski, M. Is there a relationship between psychological factors and TMD? Brain Behav. 2019, 9, e01360. [Google Scholar] [CrossRef] [Green Version]
- Porporatti, A.L.; Costa, Y.M.; Reus, J.C.; Stuginski-Barbosa, J.; Conti, P.C.R.; Velly, A.M.; De Luca Canto, G. Placebo and nocebo response magnitude on temporomandibular disorder-related pain: A systematic review and meta-analysis. J. Oral Rehabil. 2019, 46, 862–882. [Google Scholar] [CrossRef]
- Serakinci, N.; Savtekin, G. Modeling Mesenchymal Stem Cells in TMJ Rheumatoid Arthritis and Osteoarthritis Therapy. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 205–210. [Google Scholar] [CrossRef]
- Zhang, S.; Yap, A.U.; Toh, W.S. Stem Cells for Temporomandibular Joint Repair and Regeneration. Stem Cell Rev. Rep. 2015, 11, 728–742. [Google Scholar] [CrossRef]
- Cui, D.; Li, H.; Xu, X.; Ye, L.; Zhou, X.; Zheng, L.; Zhou, Y. Mesenchymal Stem Cells for Cartilage Regeneration of TMJ Osteoarthritis. Stem Cells Int. 2017, 2017, 5979741. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Natarajan, V.; Madhan, B.; Tiku, M.L. Intra-Articular Injections of Polyphenols Protect Articular Cartilage from Inflammation-Induced Degradation: Suggesting a Potential Role in Cartilage Therapeutics. PloS ONE 2015, 10, e0127165. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moccia, S.; Nucci, L.; Spagnuolo, C.; d’Apuzzo, F.; Piancino, M.G.; Minervini, G. Polyphenols as Potential Agents in the Management of Temporomandibular Disorders. Appl. Sci. 2020, 10, 5305. https://0-doi-org.brum.beds.ac.uk/10.3390/app10155305
Moccia S, Nucci L, Spagnuolo C, d’Apuzzo F, Piancino MG, Minervini G. Polyphenols as Potential Agents in the Management of Temporomandibular Disorders. Applied Sciences. 2020; 10(15):5305. https://0-doi-org.brum.beds.ac.uk/10.3390/app10155305
Chicago/Turabian StyleMoccia, Stefania, Ludovica Nucci, Carmela Spagnuolo, Fabrizia d’Apuzzo, Maria Grazia Piancino, and Giuseppe Minervini. 2020. "Polyphenols as Potential Agents in the Management of Temporomandibular Disorders" Applied Sciences 10, no. 15: 5305. https://0-doi-org.brum.beds.ac.uk/10.3390/app10155305