Self-Assembled Three-Dimensional Microporous rGO/PNT/Fe3O4 Hydrogel Sorbent for Magnetic Preconcentration of Multi-Residue Insecticides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
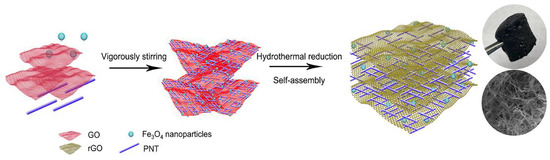
2.2. Synthesis of the Sorbent Materials
2.3. Instrument
2.4. Sample Preparation
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Investigation of 3D-rGOPFH
3.1.1. Characterization of 3D-rGOPFH
3.1.2. Selection of 3D-rGOPFH
3.1.3. Comparison of the Extraction Efficiency of 3D-rGOPFH with Different Sorbents
3.2. Optimization of Extraction Conditions
3.3. Analytical Performance
3.4. The Reusability and Batch Difference of 3D-rGOPFH
3.5. Comparison with Other Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masia, A.; Suarez-Varela, M.M.; Llopis-Gonzalez, A.; Pico, Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: A review. Anal. Chim. Acta 2016, 936, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Dell’Oro, D.; Casamassima, F.; Gesualdo, G.; Iammarino, M.; Mambelli, P.; Nardelli, V. Determination of pyrethroids in chicken egg samples: Development and validation of a confirmatory analytical method by gas chromatography/mass spectrometry. Int. J. Food Sci. Technol. 2014, 49, 1391–1400. [Google Scholar] [CrossRef]
- Nedaei, M.; Salehpour, A.R.; Mozaffari, S.; Yousefi, S.M.; Yousefi, S.R. Determination of organophosphorus pesticides by gas chromatography with mass spectrometry using a large-volume injection technique after magnetic extraction. J. Sep. Sci. 2014, 37, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhu, X.; Wang, J.; Zhao, E.; He, M.; Chen, L.; Yu, P. Combination of dispersive solid-phase extraction and salting-out homogeneous liquid-liquid extraction for the determination of organophosphorus pesticides in cereal grains. J. Sep. Sci. 2014, 37, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.; Ma, W.; Guo, Z.; Li, X.; Song, S.; Tang, H.; Li, X.; Zhang, Q. Development of precise GC-EI-MS method to determine the residual fipronil and its metabolites in chicken egg. Food Chem. 2019, 281, 85–90. [Google Scholar] [CrossRef]
- Li, J.-W.; Wang, Y.-L.; Yan, S.; Li, X.-J.; Pan, S.-Y. Molecularly imprinted calixarene fiber for solid-phase microextraction of four organophosphorous pesticides in fruits. Food Chem. 2016, 192, 260–267. [Google Scholar] [CrossRef]
- Cacho, J.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. In situ ionic liquid dispersive liquid-liquid microextraction coupled to gas chromatography-mass spectrometry for the determination of organophosphorus pesticides. J. Chromatogr. A 2018, 1559, 95–101. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Q.; Wu, C.; Wang, Z. Application of dispersion-solidification liquid-liquid microextraction for the determination of triazole fungicides in environmental water samples by high-performance liquid chromatography. J. Hazard. Mater. 2011, 185, 71–76. [Google Scholar] [CrossRef]
- Hou, X.; Tang, S.; Wang, J. Recent advances and applications of graphene-based extraction materials in food safety. TrAC Trends Anal. Chem. 2019, 119, 115603. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, C.; Guo, L.; Ling, L.; Li, M.; Li, H.; Guo, X. Rapidly quantitative analysis of γ-glutamyltranspeptidase activity in the lysate and blood via a rational design of the molecular probe by matrix-assisted laser desorption ionization mass spectrometry. Talanta 2019, 205, 120141. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zang, X.; Chang, Q.; Wang, C.; Wang, Z. A graphene-coated magnetic nanocomposite for the enrichment of fourteen pesticides in tomato and rape samples prior to their determination by gas chromatography-mass spectrometry. Anal. Methods 2014, 6, 253–260. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Wu, Q.; Wang, C.; Zang, X.; Wang, Z. The use of graphene-based magnetic nanoparticles as adsorbent for the extraction of triazole fungicides from environmental water. J. Sep. Sci. 2012, 35, 2266–2272. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Xing, M.; Zhang, J. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef]
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene as a new sorbent in analytical chemistry. TrAC Trends Anal. Chem. 2013, 51, 33–43. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Shi, Y.P. Magnetic polyethyleneimine functionalized reduced graphene oxide as a novel magnetic solid-phase extraction adsorbent for the determination of polar acidic herbicides in rice. Anal. Chim. Acta 2017, 949, 23–34. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, M.; Zhu, L.; Chen, H.; Liu, X.; Lu, C. Facile synthesis of amine-functional reduced graphene oxides as modified quick, easy, cheap, effective, rugged and safe adsorbent for multi-pesticide residues analysis of tea. J. Chromatogr. A 2018, 1531, 22–31. [Google Scholar] [CrossRef]
- Wu, Z.S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Mullen, K. 3D nitrogen-doped graphene aerogel-supported Fe3O4 nanoparticles as efficient electrocatalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef]
- Mahpishanian, S.; Sereshti, H. Three-dimensional graphene aerogel-supported iron oxide nanoparticles as an efficient adsorbent for magnetic solid phase extraction of organophosphorus pesticide residues in fruit juices followed by gas chromatographic determination. J. Chromatogr. A 2016, 1443, 43–53. [Google Scholar] [CrossRef]
- Tang, B.; Wang, S.; Zhang, J.; Wang, Z.; He, Y.; Huang, W. Three-dimensional graphene monolith-based composite: Superiority in properties and applications. Int. Mater. Rev. 2017, 63, 204–225. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Tkaczewska, J.; Dordevic, D.; Jancikova, S.; Kulawik, P.; Milosavljevic, V.; Dolezelikova, K.; Smerkova, K.; Svec, P. Nanocomposite Furcellaran Films—The Influence of Nanofillers on Functional Properties of Furcellaran Films and Effect on Linseed Oil Preservation. Polymers 2019, 11, 2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Chen, Y.; Li, H.; Tian, R.; Liu, H. Facile Fabrication of Three-Dimensional Lightweight RGO/PPy Nanotube/Fe3O4 Aerogel with Excellent Electromagnetic Wave Absorption Properties. ACS Omega 2018, 3, 5735–5743. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.J.; Lancas, F.M.; Queiroz, M.E. Determination of fluoxetine and norfluoxetine enantiomers in human plasma by polypyrrole-coated capillary in-tube solid-phase microextraction coupled with liquid chromatography-fluorescence detection. J. Chromatogr. A 2009, 1216, 8590–8597. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, H.L.; Ma, S.Y.; Zhang, J.; Zhang, Z.Q. Solidification of a Switchable Solvent-Based QuEChERS Method for Detection of 16 Pesticides in Some Fruits and Vegetables. J. Agric. Food. Chem. 2019, 67, 8045–8052. [Google Scholar] [CrossRef]
- Kittirattanapiboon, K.; Krisnangkura, K. Separation of acylglycerols, FAME and FFA in biodiesel by size exclusion chromatography. Eur. J. Lipid Sci. Technol. 2008, 110, 422–427. [Google Scholar] [CrossRef]
- Mao, X.; Yan, A.; Wan, Y.; Luo, D.; Yang, H. Dispersive solid-phase extraction using microporous sorbent UiO-66 coupled to gas chromatography-tandem mass spectrometry: A QuEChERS-type method for the determination of organophosphorus pesticide residues in edible vegetable oils without matrix interference. J. Agric. Food. Chem. 2019, 67, 1760–1770. [Google Scholar]
- Wu, J.Y.; Zhang, H.X.; Peng, X.T. Rapid determination of organophosphorus pesticides in edible vegetable oils by direct microextraction using magnetic mesoporous silica microspheres. Sep. Sci. Plus 2020, 3, 158–166. [Google Scholar] [CrossRef]
- Ying, S.; Zheng, W.; Li, B.; She, X.; Huang, H.; Li, L.; Huang, Z.; Huang, Y.; Liu, Z.; Yu, X. Facile fabrication of elastic conducting polypyrrole nanotube aerogels. Synth. Met. 2016, 218, 50–55. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Sun, S.; Zhang, X.; Duan, L.; Ge, X.; Lü, W. Self-assembled three-dimensional graphene/Fe3O4 hydrogel for efficient pollutant adsorption and electromagnetic wave absorption. Mater. Res. Bull. 2016, 73, 401–408. [Google Scholar] [CrossRef]
- Cong, H.; Ren, X.; Wang, P.; Yu, S. Macroscopic Multifunctional Graphene-Based Hydrogels and Aerogels by a Metal Ion Induced Self-Assembly Process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, G.; Feng, C.; Wang, C.; Wang, Z. Preparation of a graphene-based magnetic nanocomposite for the extraction of carbamate pesticides from environmental water samples. J. Chromatogr. A 2011, 1218, 7936–7942. [Google Scholar] [CrossRef]
- European Commission. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed, Supersedes SANTE/11945/2017; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 2012, 101, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Hii, T.M.; Basheer, C.; Lee, H.K. Commercial polymeric fiber as sorbent for solid-phase microextraction combined with high-performance liquid chromatography for the determination of polycyclic aromatic hydrocarbons in water. J. Chromatogr. A 2009, 1216, 7520–7526. [Google Scholar] [CrossRef] [PubMed]
- Nodeh, H.R.; Sereshti, H.; Gaikani, H.; Kamboh, M.A.; Afsharsaveh, Z. Magnetic graphene coated inorganic-organic hybrid nanocomposite for enhanced preconcentration of selected pesticides in tomato and grape. J. Chromatogr. A 2017, 1509, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Sereshti, H.; Afsharsaveh, Z.; Gaikani, H.; Nodeh, H.R. Electroless-coated magnetic three-dimensional graphene with silver nanoparticles used for the determination of pesticides in fruit samples. J. Sep. Sci. 2018, 41, 1567–1575. [Google Scholar] [CrossRef]
- Gao, L.; Piao, C.; Chen, L. Determination of Acephate in Vegetables by Magnetic Molecularly Imprinted Polymer Isolation Coupled with High-Performance Liquid Chromatography. Anal. Lett. 2014, 48, 752–765. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, Y.; Yu, X.; Gao, Y.; Zhang, L.; Wang, Y.; Zhang, H.; Song, D. Liquid-solid extraction coupled with magnetic solid-phase extraction for determination of pyrethroid residues in vegetable samples by ultra fast liquid chromatography. Talanta 2013, 114, 167–175. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Shi, Y.P. Magnetic graphene solid-phase extraction for the determination of carbamate pesticides in tomatoes coupled with high performance liquid chromatography. Talanta 2015, 141, 212–219. [Google Scholar] [CrossRef]




| Analyte | Regression Curves | Linearity (ng g−1) | R2 | LODs (ng g−1) | LOQs (ng g−1) | MRLs (ng g−1) | RSD | |
|---|---|---|---|---|---|---|---|---|
| Intraday (n = 5) | Inter-Day (n = 15) | |||||||
| Isocarbophos | Y = 17,453X + 4380 | 5–100 | 0.9993 | 0.03 | 5 | 50 | 4.5 | 5.1 |
| Quinalphos | Y = 162,933X − 43,204 | 5–100 | 0.9998 | 0.009 | 5 | - | 3.6 | 4.5 |
| Phorate | Y = 23,107X + 9314 | 5–100 | 0.9975 | 0.009 | 5 | 10 | 4.6 | 7.0 |
| Chlorpyrifos | Y = 91,978X − 27,323 | 5–100 | 0.9986 | 0.009 | 5 | 10–1000 | 2.5 | 4.5 |
| Phosalone | Y = 202,703X + 34,570 | 5–100 | 0.9995 | 0.01 | 5 | 1000 | 6.8 | 8.8 |
| Pirimor | Y = 42,473X + 37,167 | 5–100 | 0.9996 | 0.01 | 5 | 10–5000 | 3.3 | 5.2 |
| Carbaryl | Y = 39,737X + 39,214 | 5–100 | 0.9990 | 0.01 | 5 | 20–5000 | 2.8 | 6.4 |
| Myclobutanil | Y = 637,361X − 4792 | 5–100 | 0.9972 | 0.006 | 5 | 50–3000 | 3.4 | 3.7 |
| Diniconazole | Y = 162,443X – 37,706 | 5–100 | 0.9991 | 0.006 | 5 | 50 | 5.5 | 5.2 |
| Lambda-cyhalothrin | Y = 111,654X – 53,779 | 5–100 | 0.9992 | 0.03 | 5 | 10–5000 | 7.1 | 3.6 |
| Bifenthrin | Y = 698,826X – 193,428 | 5–100 | 0.9993 | 0.01 | 5 | 50–4000 | 2.6 | 3.5 |
| 2, 4′-DDT | Y = 121,476X – 22,140 | 5–100 | 0.9992 | 0.01 | 5 | 50–200 | 4.8 | 4.1 |
| Mirex | Y = 239,134X – 137,874 | 5–100 | 0.9998 | 0.01 | 5 | 10 | 3.0 | 4.2 |
| Analytes | Spiked (ng g−1) | Tomato | Cucumber | Pakchoi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Found (ng g−1) | Recovery (%) | RSD (%) | Found (ng g−1) | Recovery (%) | RSD (%) | Found (ng g−1) | Recovery (%) | RSD (%) | ||
| Isocarbophos | 0 | ND | ND | ND | ||||||
| 5 | 5.3 ± 0.3 | 105.3 | 6.5 | 4.8 ± 0.2 | 96.4 | 5.0 | 5.2 ± 0.3 | 104.3 | 5.8 | |
| 25 | 24.6 ± 0.9 | 98.5 | 3.8 | 23.7 ± 1.1 | 94.6 | 4.5 | 26.5 ± 1.0 | 106.1 | 3.7 | |
| 50 | 50.8 ± 2.6 | 101.6 | 5.1 | 51.7 ± 1.9 | 103.4 | 3.9 | 46.7 ± 2.8 | 93.3 | 5.9 | |
| Quinalphos | 0 | ND | ND | ND | ||||||
| 5 | 4.4 ± 0.2 | 87.7 | 3.6 | 4.8 ± 0.3 | 95.7 | 5.6 | 5.2 ± 0.2 | 104.2 | 4.5 | |
| 25 | 23.7 ± 1.1 | 94.6 | 4.8 | 22.6 ± 1.4 | 90.2 | 6.3 | 25.7 ± 1.0 | 103.0 | 3.7 | |
| 50 | 49.2 ± 2.3 | 98.3 | 4.7 | 48.3 ± 2.3 | 96.5 | 4.7 | 46.3 ± 3.1 | 92.6 | 6.7 | |
| Phorate | 0 | ND | ND | ND | ||||||
| 5 | 4.9 ± 0.6 | 97.1 | 11.2 | 4.0 ± 0.4 | 79.2 | 9.8 | 4.9 ± 0.4 | 82.3 | 8.0 | |
| 25 | 24.4 ± 1.8 | 97.7 | 7.2 | 24.6 ± 1.6 | 98.3 | 6.4 | 26.8 ± 1.6 | 107.0 | 5.8 | |
| 50 | 50.7 ± 2.0 | 101.4 | 4.0 | 47.3 ± 1.6 | 94.5 | 3.4 | 46.2 ± 1.5 | 92.4 | 3.2 | |
| Chlorpyrifos | 0 | ND | ND | ND | ||||||
| 5 | 5.1 ± 0.2 | 101.9 | 4.2 | 5.3 ± 0.4 | 105.6 | 6.8 | 5.2 ± 0.2 | 104.3 | 4.5 | |
| 25 | 24.1 ± 0.9 | 96.4 | 3.8 | 25.6 ± 1.7 | 102.5 | 6.7 | 24.1 ± 0.9 | 96.5 | 3.7 | |
| 50 | 48.3 ± 1.3 | 96.5 | 2.7 | 46.8 ± 2.6 | 93.6 | 5.5 | 47.9 ± 1.9 | 95.8 | 3.9 | |
| Phosalone | 0 | ND | ND | ND | ||||||
| 5 | 4.9 ± 0.4 | 97.2 | 8.5 | 5.1 ± 0.2 | 102.8 | 3.6 | 4.8 ± 0.2 | 96.7 | 4.1 | |
| 25 | 23.8 ± 0.9 | 95.1 | 3.6 | 23.6 ± 0.6 | 94.6 | 2.5 | 22.6 ± 0.9 | 90.3 | 3.8 | |
| 50 | 47.3 ± 2.5 | 94.6 | 5.4 | 48.3 ± 1.2 | 96.6 | 2.4 | 46.4 ± 1.3 | 92.9 | 2.7 | |
| Pirimor | 0 | ND | ND | ND | ||||||
| 5 | 5.5 ± 0.3 | 109.4 | 4.8 | 5.0 ± 0.3 | 100.5 | 6.2 | 5.2 ± 0.1 | 104.5 | 2.8 | |
| 25 | 25.3 ± 1.4 | 101.3 | 5.6 | 24.6 ± 1.0 | 98.4 | 4.1 | 24.7 ± 0.9 | 98.6 | 3.7 | |
| 50 | 47.6 ± 1.6 | 95.2 | 3.3 | 52.4 ± 1.6 | 104.7 | 3.0 | 50.1 ± 2.6 | 100.2 | 5.1 | |
| Carbaryl | 0 | ND | ND | ND | ||||||
| 5 | 5.4 ± 0.5 | 107.7 | 9.8 | 5.4 ± 0.2 | 108.7 | 4.2 | 4.7 ± 0.3 | 94.5 | 6.4 | |
| 25 | 24.3 ± 2.6 | 97.2 | 10.9 | 24.6 ± 1.2 | 98.4 | 4.8 | 23.5 ± 1.3 | 94.0 | 5.5 | |
| 50 | 48.7 ± 2.8 | 97.4 | 5.7 | 52.8 ± 3.6 | 105.6 | 6.8 | 49.4 ± 3.7 | 98.7 | 7.6 | |
| Myclobutanil | 0 | ND | ND | ND | ||||||
| 5 | 4.8 ± 0.3 | 95.3 | 6.5 | 5.0 ± 0.2 | 100.5 | 3.2 | 5.0 ± 0.3 | 100.7 | 5.3 | |
| 25 | 24.2 ± 1.3 | 96.9 | 5.4 | 24.8 ± 0.8 | 99.2 | 5.3 | 24.7 ± 1.9 | 98.7 | 7.7 | |
| 50 | 50.6 ± 1.6 | 101.1 | 3.1 | 49.6 ± 1.4 | 99.1 | 2.8 | 49.3 ± 1.2 | 98.5 | 2.5 | |
| Diniconazole | 0 | ND | ND | ND | ||||||
| 5 | 5.2 ± 0.2 | 103.1 | 3.5 | 4.3 ± 0.2 | 85.2 | 5.1 | 5.2 ± 0.3 | 104.9 | 6.5 | |
| 25 | 23.9 ± 0.7 | 95.5 | 2.8 | 24.3 ± 0.5 | 97.3 | 1.9 | 23.9 ± 1.8 | 95.4 | 7.6 | |
| 50 | 51.9 ± 3.6 | 103.8 | 6.9 | 48.7 ± 3.0 | 97.3 | 6.2 | 52.7 ± 0.9 | 105.3 | 1.7 | |
| Lambda-cyhalothrin | 0 | ND | ND | 4.9 ± 0.4 | ||||||
| 5 | 4.4 ± 0.2 | 88.8 | 3.8 | 5.0 ± 0.2 | 99.0 | 3.6 | 10.4 ± 0.4 | 109.0 | 4.3 | |
| 25 | 26.5 ± 1.7 | 105.9 | 6.4 | 26.2 ± 0.7 | 104.8 | 2.8 | 29.5 ± 0.7 | 98.4 | 2.5 | |
| 50 | 47.6 ± 1.8 | 95.1 | 3.7 | 53.9 ± 2.4 | 107.8 | 4.5 | 53.9 ± 1.8 | 98.0 | 3.4 | |
| Bifenthrin | 0 | ND | 4.7 ± 0.2 | ND | ||||||
| 5 | 5.1 ± 0.3 | 102.5 | 5.8 | 9.6 ± 0.6 | 98.0 | 5.8 | 5.3 ± 0.2 | 106.2 | 4.5 | |
| 25 | 25.5 ± 1.5 | 101.8 | 5.9 | 30.8 ± 0.8 | 104.4 | 2.6 | 24.2 ± 1.2 | 96.8 | 5.1 | |
| 50 | 49.8 ± 2.1 | 99.7 | 4.2 | 53.6 ± 2.0 | 97.8 | 3.8 | 48.6 ± 1.3 | 97.2 | 2.6 | |
| 2, 4′-DDT | 0 | ND | ND | ND | ||||||
| 5 | 4.7 ± 0.3 | 93.5 | 7.3 | 5.1 ± 0.2 | 102.6 | 3.8 | 5.2 ± 0.4 | 104.7 | 8.3 | |
| 25 | 22.5 ± 1.1 | 90.1 | 5.1 | 26.8 ± 1.6 | 107.2 | 5.8 | 24.7 ± 0.9 | 98.7 | 3.5 | |
| 50 | 45.6 ± 1.8 | 91.2 | 4.0 | 46.2 ± 3.7 | 92.4 | 8.0 | 53.3 ± 2.6 | 106.7 | 4.8 | |
| Mirex | 0 | ND | ND | ND | ||||||
| 5 | 4.4 ± 0.4 | 87.3 | 8.1 | 4.8 ± 0.3 | 95.3 | 7.2 | 4.9 ± 0.2 | 98.4 | 4.3 | |
| 25 | 23.5 ± 1.5 | 94.0 | 6.4 | 22.9 ± 1.3 | 91.4 | 5.5 | 26.4 ± 1.4 | 105.6 | 5.2 | |
| 50 | 51.9 ± 2.9 | 103.7 | 5.6 | 52.7 ± 2.7 | 105.4 | 5.1 | 52.4 ± 1.7 | 104.7 | 3.2 | |
| Method | Analyte | Linearity (ng g−1) | LOD (ng g−1) | Recovery (%) | Matrix Effect | Nitrogen Blowing and Reconstitution | Desorption Times | Refs |
|---|---|---|---|---|---|---|---|---|
| MSPE-GC-μECD | 3 insecticides | 1–20 | 0.23–0.3 | 82–113 | not reported | yes | 1 | [36] |
| MSPE-GC-μECD | 3 insecticides | 0.1–5 | 0.07–0.13 | 72.8–109.6 | not reported | yes | 1 | [37] |
| MMIP-HPLC-UV | 1 acephate | 10–5000 | 0.1 | 89.2–93.4 | not reported | yes | 3 | [38] |
| MSPE-HPLC-UV | 6 pyrethroids | 5–500 | 0.63–1.2 | 76–99.5 | not reported | yes | 1 | [39] |
| MSPE-HPLC-DAD | 6 carbamates | 5–200 | 0.58–2.06 | 90.34–101.98 | not reported | yes | 3 | [40] |
| MSPE-GC-MS/MS | 5 OPPs, 2 OCPs, 2 carbamates, 2 pyrethroids, and 2 triazoles | 0.1–100 | 0.006–0.03 | 79.2–109.4 | no | no | 1 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Li, X.; Li, M.; Li, X.; Li, X.; Li, S.; Zhang, Q.; Li, H. Self-Assembled Three-Dimensional Microporous rGO/PNT/Fe3O4 Hydrogel Sorbent for Magnetic Preconcentration of Multi-Residue Insecticides. Appl. Sci. 2020, 10, 5665. https://0-doi-org.brum.beds.ac.uk/10.3390/app10165665
Wang S, Li X, Li M, Li X, Li X, Li S, Zhang Q, Li H. Self-Assembled Three-Dimensional Microporous rGO/PNT/Fe3O4 Hydrogel Sorbent for Magnetic Preconcentration of Multi-Residue Insecticides. Applied Sciences. 2020; 10(16):5665. https://0-doi-org.brum.beds.ac.uk/10.3390/app10165665
Chicago/Turabian StyleWang, Sheng, Xiuqin Li, Ming Li, Xianjiang Li, Xiaomin Li, Shuangqing Li, Qinghe Zhang, and Hongmei Li. 2020. "Self-Assembled Three-Dimensional Microporous rGO/PNT/Fe3O4 Hydrogel Sorbent for Magnetic Preconcentration of Multi-Residue Insecticides" Applied Sciences 10, no. 16: 5665. https://0-doi-org.brum.beds.ac.uk/10.3390/app10165665