The Applicability of Total Color Difference ΔE for Determining the Blooming Time in Longissimus Lumborum and Semimembranosus Muscles from Holstein-Friesian Bulls at Different Ageing Times
Abstract
:Featured Application
Abstract
1. Introduction
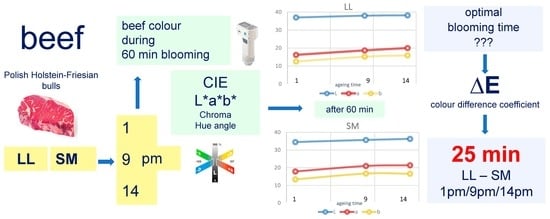
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Font-i-Furnols, M.; Guerrero, L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef]
- Purslow, P.P.; Warner, R.D.; Clarke, F.M.; Hughes, J.M. Variations in meat colour due to factors other than myoglobin chemistry; a synthesis of recent findings (invited review). Meat Sci. 2020, 159, 107941. [Google Scholar] [CrossRef]
- Holman, B.W.B.; van de Ven, R.J.; Mao, Y.; Coombs, C.E.O.; Hopkins, D.L. Using instrumental (CIE and reflectance) measures to predict consumers’ acceptance of beef colour. Meat Sci. 2017, 127, 57–62. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Šuput, D.Z.; Lazić, L.V.; Lević, B.L.; Pezo, L.L.; Tomović, M.V.; Hromiš, M.N. Effect of specific packaging conditions on myoglobin and meat color. Food Feed Res. 2013, 40, 1–10. [Google Scholar]
- Carpenter, C.E.; Cornforth, D.P.; Whittier, D. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 2001, 57, 359–363. [Google Scholar] [CrossRef]
- Salueña, B.H.; Gamasa, C.S.; Rubial, J.M.D.; Odriozola, C.A. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 1078–1089. [Google Scholar] [CrossRef] [Green Version]
- Canto, A.C.; Costa-Lima, B.R.; Suman, S.P.; Monteiro, M.L.G.; Viana, F.M.; Salim, A.P.A.; Nair, M.N.; Silva, T.J.P.; Conte-Junior, C.A. Color attributes and oxidative stability of longissimus lumborum and psoas major muscles from Nellore bulls. Meat Sci. 2016, 121, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Holman, B.W.B.; Kerr, M.J.; Morris, S.; Hopkins, D.L. The identification of dark cutting beef carcasses in Australia, using Nix Pro Color Sensor™ colour measures, and their relationship to bolar blade, striploin and topside quality traits. Meat Sci. 2019, 148, 50–54. [Google Scholar] [CrossRef]
- Suman, S.P.; Hunt, M.C.; Nair, M.N.; Rentfrow, G. Improving beef color stability: Practical strategies and underlying mechanisms. Meat Sci. 2014, 98, 490–504. [Google Scholar] [CrossRef]
- Van Rooyen, L.A.; Allen, P.; Crawley, S.M.; O’Connor, D.I. The effect of carbon monoxide pretreatment exposure time on the colour stability and quality attributes of vacuum packaged beef steaks. Meat Sci. 2017, 129, 74–80. [Google Scholar] [CrossRef]
- AMSA. Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012. [Google Scholar]
- Mancini, R.A.; Ramanathan, R. Effects of postmortem storage time on color and mitochondria in beef. Meat Sci. 2014, 98, 65–70. [Google Scholar] [CrossRef]
- Salim, A.P.A.A.; Suman, S.P.; Canto, A.C.V.C.S.; Costa-Lima, B.R.C.; Viana, F.M.; Monteiro, M.L.G.; Silva, T.J.P.; Conte-Junior, C.A. Muscle-specific color stability in fresh beef from grain-finished Bos indicus cattle. Asian Austral. J. Anim. 2019, 32, 1036–1043. [Google Scholar] [CrossRef] [Green Version]
- Beriain, M.J.; Goñi, M.V.; Indurain, G.; Sarriés, M.V.; Insausti, K. Predicting Longissimus dorsi myoglobin oxidation in aged beef based on early post-mortem colour measurements on the carcass as a colour stability index. Meat Sci. 2009, 81, 439–445. [Google Scholar] [CrossRef]
- Rentfrow, G.; Linville, M.L.; Stahl, C.A.; Olson, K.C.; Berg, E.P. The effects of the antioxidant lipoic acid on beef longissimus bloom time. J. Anim. Sci. 2004, 82, 3034–3037. [Google Scholar] [CrossRef]
- Lindahl, G. Colour stability of steaks from large beef cuts aged under vacuum or high oxygen modified atmosphere. Meat Sci. 2011, 87, 428–435. [Google Scholar] [CrossRef]
- Cierach, M.; Niedźwiedź, J. Effects of three lighting intensities during display on discolouration of beef semitendinosus muscle. Eur. Food Res. Technol. 2014, 239, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Mancini, R.A.; Belskie, K.; Suman, S.P.; Ramanathan, R. Muscle-specific mitochondrial functionality and its influence on fresh beef color stability. J. Food Sci. 2018, 83, 2077–2082. [Google Scholar] [CrossRef]
- Salim, A.P.A.A.; Suman, S.P.; Canto, A.C.V.C.S.; Costa-Lima, B.R.C.; Viana, F.M.; Monteiro, M.L.G.; Silva, T.J.P.; Conte-Junior, C.A. Influence of muscle source on proximate composition, texture profile and protein oxidation of beef from grain-finished Bos indicus cattle. Ciência Rural 2019, 49, 1–6. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, G.D.; Jeong, J.Y.; Hur, S.J.; Joo, S.T. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010, 86, 456–461. [Google Scholar] [CrossRef]
- Lee, M.S.; Apple, J.K.; Yancey, J.W.S.; Sawyer, J.T.; Johnson, Z.B. Influence of vacuum-aging period on bloom development of the beef gluteus medius from top sirloin butts. Meat Sci. 2008, 80, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Apple, J.K.; Yancey, J.W.S.; Sawyer, J.T.; Johnson, Z.B. Influence of wet-aging on bloom development in the longissimus thoracis. Meat Sci. 2008, 80, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Kilgannon, A.K.; Holman, B.W.B.; Mawson, J.C.; Campbell, M.; Collins, D.; Hopkins, D.L. The effect of different temperature-time combinations when ageing beef: Sensory quality traits and microbial loads. Meat Sci. 2019, 150, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Utama, D.; Lee, C.; Park, Y.; Jang, A.; Lee, S. Comparison of meat quality, fatty acid composition and aroma volatiles of Chikso and Hanwoo beef. Asian Austral. J. Anim. 2018, 31, 1500–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CIE. Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms; Commission Internationale de l’Eclairage: Paris, France, 1978. [Google Scholar]
- Belew, J.B.; Brooks, J.C.; McKenna, D.R.; Savell, J.W. Warner-Bratzler shear evaluations of 40 bovine muscles. Meat Sci. 2004, 64, 507–512. [Google Scholar] [CrossRef]
- Warner, R.D.; Kearney, G.; Hopkins, D.L.; Jacob, R.H. Retail colour stability of lamb meat is influenced by breed type, muscle, packaging and iron concentration. Meat Sci. 2019, 129, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska-Kapituła, M.; Tkacz, K.; Nogalski, Z.; Karpińska-Tymoszczyk, M.; Draszanowska, A.; Pietrzak-Fiećko, R.; Purwin, C.; Lipiński, K. Addition of herbal extracts to the Holstein-Friesian bulls’ diet changes the quality of beef. Meat Sci. 2018, 145, 163–170. [Google Scholar] [CrossRef]
- AMSA. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat, 2nd ed.; American Meat Science Association: Champaign, IL, USA, 2015. [Google Scholar]
- Wyrwisz, J.; Moczkowska, M.; Kurek, M.; Stelmasiak, A.; Półtorak, A.; Wierzbicka, A. Influence of 21 days of vacuum-aging on color, bloom development, and WBSF of beef semimembranosus. Meat Sci. 2016, 122, 48–54. [Google Scholar] [CrossRef]
- Domaradzki, P.; Litwińczuk, Z.; Florek, M.; Żółkiewski, P. Effect of ageing on the physicochemical properties of musculus longissimus lumborum of young bulls of five breeds. Med. Wet. 2017, 73, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, T.I.R.C.; Hopkins, D.L.; Ramos, E.M.; Almeida, A.K.; Geesink, G. Ageing-freezing/thaw process affects blooming time and myoglobin forms of lamb meat during retail display. Meat Sci. 2019, 153, 19–25. [Google Scholar] [CrossRef]
- Gajaweera, C.; Chung, K.Y.; Lee, S.H.; Wijayananda, H.I.; Kwon, E.G.; Kim, H.J.; Cho, S.H.; Lee, S.H. Assessment of carcass and meat quality of longissimus thoracis and semimembranosus muscles of Hanwoo with Korean beef grading standards. Meat Sci. 2010, 160, 107944. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rooyen, L.A.; Allen, P.; O’Connor, D.I. Effect of muscle type and CO-pretreatment combinations on the colour stability, protein oxidation and shelf-life of vacuum packaged beef steaks. Meat Sci. 2018, 145, 407–414. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.R.; Mies, P.D.; Baird, B.E.; Pfeiffer, K.D.; Ellebracht, J.W.; Savell, J.W. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 2005, 70, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Insausti, K.; Beriain, M.J.; Purroy, A.; Alberti, P.; Lizaso, L.; Hernandez, B. Colour stability of beef from different Spanish native cattle breeds stored under vacuum and modified atmosphere. Meat Sci. 1999, 53, 241–249. [Google Scholar] [CrossRef]
| Ageing | Muscles | |||
|---|---|---|---|---|
| LL | SM | |||
| ΔE0 | ΔExy | ΔE0 | ΔExy | |
| 1-day | ||||
| 0 min | - | - | - | - |
| 5 min | 1.93 ± 0.24 b | 1.93 ± 0.24 a | 2.80 ± 0.24 b | 2.80 ± 0.24 a |
| 10 min | 2.17 ± 0.3 b | 1.69 ± 0.18 a | 3.71 ± 0.28 a | 2.15 ± 0.17 b |
| 15 min | 2.58 ± 0.26 a,b | 1.33 ± 0.21 c | 4.20 ± 0.23 a | 1.30 ± 0.15 c |
| 20 min | 2.92 ± 0.24 a,b | 1.29 ± 0.19 c | 4.44 ± 0.26 a | 1.46 ± 0.17 c |
| 25 min | 3.2 ± 0.4 a | 1.52 ± 0.18 b | 4.37 ± 0.29 a | 1.67 ± 0.18 c |
| 30 min | 3.8 ± 0.4 a | 1.72 ± 0.29 a | 4.5 ± 0.3 a | 1.27 ± 0.10 c |
| 35 min | 3.6 ± 0.3 a | 1.8 ± 0.3 a | 4.4 ± 0.3 a | 1.39 ± 0.17 c |
| 40 min | 3.8 ± 0.4 a | 1.80 ± 0.29 a | 3.9 ± 0.4 a | 1.51 ± 0.15 c |
| 45 min | 3.8 ± 0.4 a | 1.7 ± 0.3 a | 4.1 ± 0.4 a | 1.58 ± 0.14 c |
| 50 min | 3.9 ± 0.4 a | 1.79 ± 0.23 a | 4.1 ± 0.3 a | 1.42 ± 0.15 c |
| 55 min | 3.6 ± 0.4 a | 1.48 ± 0.16 b,c | 3.75 ± 0.26 a | 1.21 ± 0.13 c |
| 60 min | 3. 6 ± 0.3 a | 1.22 ± 0.13 c | 3.9 ± 0.3 a | 1.51 ± 0.15 c |
| 9-days | ||||
| 0 min | - | - | - | - |
| 5 min | 1.83 ± 0.23 c | 1.83 ± 0.23 a | 2.55 ± 0.22 c | 2.55 ± 0.27 a |
| 10 min | 3.60 ± 0.26 b | 1.80 ± 0.26 a | 3.8 ± 0.3 b | 1.75 ± 0.19 b |
| 15 min | 4.6 ± 0.3 b | 1.58 ± 0.27 a | 4.74 ± 0.15 a | 1.19 ± 0.08 c |
| 20 min | 5.8 ± 0.3 a | 1.48 ± 0.22 a,b | 4.9 ± 0.4 a | 1.58 ± 0.28 b,c |
| 25 min | 6.5 ± 0.7 a | 1.51 ± 0.17 a | 5.48 ± 0.27 a | 1.50 ± 0.15 b,c |
| 30 min | 7.4 ± 0.6 a | 1.50 ± 0.12 a | 5.5 ± 0.3 a | 1.58 ± 0.12 b,c |
| 35 min | 7.3 ± 0.5 a | 1.48 ± 0.16 a,b | 5.6 ± 0.4 a | 1.66 ± 0.16 b,c |
| 40 min | 7.1 ± 0.7 a | 1.52 ± 0.16 a | 5.2 ± 0.3 a | 1.62 ± 0.16 b,c |
| 45 min | 7.0 ± 0.7 a | 1.76 ± 0.14 a | 5.5 ± 0.4 a | 1.39 ± 0.14 b,c |
| 50 min | 7.1 ± 0.7 a | 1.37 ± 0.13 b | 5.3 ± 0.4 a | 1.27 ± 0.13 b,c |
| 55 min | 7.0 ± 0.6 a | 1.22 ± 0.15 c | 5.7 ± 0.3 a | 1.64 ± 0.15 b,c |
| 60 min | 6.8 ± 0.6 a | 1.39 ± 0.14 b | 5.5 ± 0.3 a | 1.39 ± 0.14 b,c |
| 14-days | ||||
| 0 min | - | - | - | - |
| 5 min | 1.86 ± 0.21 d | 1.86 ± 0.21 a | 2.39 ± 0.20 c | 2.39 ± 0.16 a |
| 10 min | 3.5 ± 0.3 c | 1.62 ± 0.20 a | 3.54 ± 0.21 b | 2.08 ± 0.19 a,b |
| 15 min | 3.8 ± 0.3 c | 1.61 ± 0.18 a | 3.69 ± 0.24 b | 1.79 ± 0.08 b,c |
| 20 min | 5.2 ± 0.4 b | 1.28 ± 0.17 b | 5.1 ± 0.4 a | 1.69 ± 0.21 b,c |
| 25 min | 6.2 ± 0.5 a | 1.56 ± 0.21 a | 5.26 ± 0.19 a | 1.54 ± 0.13 c,d |
| 30 min | 6.1 ± 0.5 a | 1.12 ± 0.18 b,c | 5.31 ± 0.25 a | 1.35 ± 0.10 d |
| 35 min | 6.2 ± 0.5 a | 1.45 ± 0.20 a | 5.20 ± 0.24 a | 1.32 ± 0.16 d |
| 40 min | 6.4 ± 0.5 a | 1.40 ± 0.12 a | 5.39 ± 0.25 a | 1.31 ± 0.16 d |
| 45 min | 6.5 ± 0.5 a | 1.18 ± 0.15 b,c | 5.4 ± 0.4 a | 1.20 ± 0.11 d |
| 50 min | 7.1 ± 0.4 a | 1.57 ± 0.16 a | 5.9 ± 0.3 a | 1.26 ± 0.15 d |
| 55 min | 7.3 ± 0.4 a | 1.01 ± 0.12 c | 5.9 ± 0.3 a | 1.13 ± 0.11 d |
| 60 min | 6.9 ± 0.4 a | 0.96 ± 0.14 c | 5.8 ± 0.4 a | 0.92 ± 0.10 d |
| Parameter | Muscles n = 72 | Ageing (A) n = 48 | Significance (p Value) | |||||
|---|---|---|---|---|---|---|---|---|
| LL | SM | 1 Day | 9 Days | 14 Days | M | A | MxA | |
| Lightness, L * | 37.22 ± 0.24 a | 35.6 ± 0.3 b | 35.1 ± 0.3 B | 36.8 ± 0.4 A | 37.3 ± 0.3 A | 0.001 | 0.001 | NS |
| Redness, a * | 17.94 ± 0.21 b | 19.93 ± 0.24 a | 17.25 ± 0.22 B | 19.70 ± 0.26 A | 19.91 ± 0.29 A | 0.001 | 0.001 | NS |
| Yellowness, b * | 13.98 ± 0.25 b | 15.66 ± 0.25 a | 12.84 ± 0.22 B | 15.80 ± 0.22 A | 15.88 ± 0.26 A | 0.001 | 0.001 | 0.031 |
| Chroma, C * | 22.8 ± 0.3 b | 25.4 ± 0.3 a | 21.5 ± 0.3 B | 25.3 ± 0.3 A | 25.5 ± 0.4 A | 0.001 | 0.001 | NS |
| Hue angle, H * | 37.8 ± 0.26 a | 37.8 ± 0.21 a | 36.59 ± 0.24 B | 38.54 ± 0.21 A | 38.74 ± 0.27 A | NS | 0.001 | 0.012 |
| Ageing (A) | LL | SM | ||||
|---|---|---|---|---|---|---|
| 1 Day | 9 Days | 14 Days | 1 Day | 9 Days | 14 Days | |
| Lightness, L * | 35.9 ± 0.4 b | 37.9 ± 0.4 a | 37.7 ± 0.4 a | 34.3 ± 0.3 b | 35.7 ± 0.6 a | 36.7 ± 0.5 a |
| Redness, a * | 16.30 ± 0.18 b | 18.61 ± 0.29 a | 19.02 ± 0.24 a | 18.21 ± 0.23 b | 20.78 ± 0.23 a | 20.8 ± 0.5 a |
| Yellowness, b * | 11.78 ± 0.15 b | 14.81 ± 0.21 a | 15.44 ± 0.21 a | 13.89 ± 0.23 b | 16.80 ± 0.17 a | 16.3 ± 0.5 a |
| Chroma, C * | 20.12 ± 0.21 b | 23.8 ± 0.3 a | 24.5 ± 0.3 a | 22.9 ± 0.3 b | 26.73 ± 0.25 a | 25.4 ± 0.6 a |
| Hue angle, H * | 35.9 ± 0.3 b | 38.5 ± 0.3 a | 39.2 ± 0.23 a | 37.31± 0.28 b | 38.97 ± 0.26 a | 38.4 ± 0.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkacz, K.; Modzelewska-Kapituła, M.; Więk, A.; Nogalski, Z. The Applicability of Total Color Difference ΔE for Determining the Blooming Time in Longissimus Lumborum and Semimembranosus Muscles from Holstein-Friesian Bulls at Different Ageing Times. Appl. Sci. 2020, 10, 8215. https://0-doi-org.brum.beds.ac.uk/10.3390/app10228215
Tkacz K, Modzelewska-Kapituła M, Więk A, Nogalski Z. The Applicability of Total Color Difference ΔE for Determining the Blooming Time in Longissimus Lumborum and Semimembranosus Muscles from Holstein-Friesian Bulls at Different Ageing Times. Applied Sciences. 2020; 10(22):8215. https://0-doi-org.brum.beds.ac.uk/10.3390/app10228215
Chicago/Turabian StyleTkacz, Katarzyna, Monika Modzelewska-Kapituła, Adam Więk, and Zenon Nogalski. 2020. "The Applicability of Total Color Difference ΔE for Determining the Blooming Time in Longissimus Lumborum and Semimembranosus Muscles from Holstein-Friesian Bulls at Different Ageing Times" Applied Sciences 10, no. 22: 8215. https://0-doi-org.brum.beds.ac.uk/10.3390/app10228215