Postharvest UV-B and Photoperiod with Blue + Red LEDs as Strategies to Stimulate Carotenogenesis in Bell Peppers
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
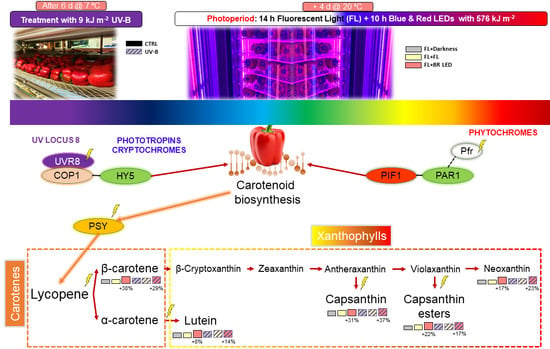
2.2. Postharvest Treatments and Light Photoperiod
- D: darkness, as it is usually performed.
- FL: fluorescent lighting with lower energy (280 kJ m−2) to save costs provided by fluorescent lamps (OSRAM DULUX L 36W/840, Munich, Germany) with broad white spectrum and 8 W m−2, simulating the conventional storage conditions measured by us for this study in several Spanish supermarkets during June–July 2020.
- BR LED: a combination of blue + red LEDs (LEDMurcia S.L., Murcia, Spain) with 576 kJ m−2. LED lamps were applied with a simultaneous combination (1:1) of blue (peak at 450 nm) and red (peak at 660 nm). This combination was chosen due to our previous preliminary test based on previous findings by Martínez-Zamora et al. [25] and Pennisi et al. [26].
2.3. Physicochemical and Sensory Quality Determination
2.4. Extraction and Determination of Bioactive Compounds
2.5. Extraction and Determination of Carotenoids
2.6. Data Analysis
3. Results
3.1. Physicochemical Quality Changes throughout Shelf Life
3.2. Bioactive Compound Content and Total Antioxidant Capacity
3.3. Carotenoid Biosynthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349. [Google Scholar] [CrossRef] [Green Version]
- Hassan, N.M.; Yusof, N.A.; Yahaya, A.F.; Rozali, N.N.M.; Othman, R. Carotenoids of Capsicum Fruits: Pigment Profile and Health-Promoting Functional Attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Molina, F.D.; Valle-Guadarrama, S.; Guerra-Ramírez, D.; Arévalo-Galarza, M.D.L.; Pérez-Grajales, M.; Artés-Hernández, F. Quality attributes and antioxidant properties of Serrano chili peppers (Capsicum annuum L.) affected by thermal conditions postharvest. Int. Food Res. J. 2019, 26, 1889–1898. [Google Scholar]
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, Polyphenols and Antioxidant Activities of Capsicum annuum: Comparative Study of the Effect of Ripening Stage and Cooking Methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penella, C.; Calatayud, A. Pepper Crop under Climate Change: Grafting as an Environmental Friendly Strategy. Clim. Resilient Agric. Strateg. Perspect. 2018. [Google Scholar] [CrossRef] [Green Version]
- MAPA. Superficies y Producciones de Cultivos 2019. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (accessed on 2 March 2021).
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Controlled Abiotic Stresses Revisited: From Homeostasis through Hormesis to Extreme Stresses and the Impact on Nutraceuticals and Quality during Pre- and Postharvest Applications in Horticultural Crops. J. Agric. Food Chem. 2020, 68, 1877–1879. [Google Scholar] [CrossRef] [PubMed]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]
- Guidi, L.; Brunetti, C.; Fini, A.; Agati, G.; Ferrini, F.; Gori, A.; Tattini, M. UV radiation promotes flavonoid biosynthesis, while negatively affecting the biosynthesis and the de-epoxidation of xanthophylls: Consequence for photoprotection? Environ. Exp. Bot. 2016, 127, 14–25. [Google Scholar] [CrossRef]
- Jenkins, G.I. Signal Transduction in Responses to UV-B Radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef] [PubMed]
- León-Chan, R.G.; López-Meyer, M.; Osuna-Enciso, T.; Sañudo-Barajas, J.A.; Heredia, J.B.; León-Félix, J. Low temperature and ultraviolet-B radiation affect chlorophyll content and induce the accumulation of UV-B-absorbing and antioxidant compounds in bell pepper (Capsicum annuum) plants. Environ. Exp. Bot. 2017, 139. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, H.; Niu, G.; Gu, M. Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum Basilicum) Plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef] [Green Version]
- Schreiner, M.; Martínez-Abaigar, J.; Glaab, J.; Jansen, M. UV-B Induced Secondary Plant Metabolites. Opt. Photon. 2014, 9, 34–37. [Google Scholar] [CrossRef]
- Woltering, E.J.; Witkowska, I.M. Effects of pre- and postharvest lighting on quality and shelf life of fresh-cut lettuce. Acta Hortic. 2016. [Google Scholar] [CrossRef]
- Jin, P.; Yao, D.; Xu, F.; Wang, H.; Zheng, Y. Effect of light on quality and bioactive compounds in postharvest broccoli florets. Food Chem. 2015, 172, 705–709. [Google Scholar] [CrossRef]
- Pintos, F.M.; Hasperué, J.H.; Vicente, A.R.; Rodoni, L.M. Role of white light intensity and photoperiod during retail in broccoli shelf-life. Postharvest Biol. Technol. 2020, 163, 111121. [Google Scholar] [CrossRef]
- Loi, M.; Liuzzi, V.C.; Fanelli, F.; De Leonardis, S.; Maria Creanza, T.; Ancona, N.; Paciolla, C.; Mulè, G. Effect of different light-emitting diode (LED) irradiation on the shelf life and phytonutrient content of broccoli (Brassica oleracea L. var. italica). Food Chem. 2019, 283, 206–214. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Rodoni, L.M.; Guardianelli, L.M.; Chaves, A.R.; Martínez, G.A. Use of LED light for Brussels sprouts postharvest conservation. Sci. Hortic. 2016, 213, 281–286. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Crepaldi, A.; Fernández, J.A.; Orsini, F.; Artés-Hernández, F. Postharvest LED lighting: Effect of red, blue and far red on quality of minimally processed broccoli sprouts. J. Sci. Food Agric. 2021, 101, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ambrocio, A.; Guerrero-Beltrán, J.A.; Aparicio-Fernández, X.; Ávila-Sosa, R.; Hernández-Carranza, P.; Cid-Pérez, S.; Ochoa-Velasco, C.E. Effect of blue and ultraviolet-C light irradiation on bioactive compounds and antioxidant capacity of habanero pepper (Capsicum chinense) during refrigeration storage. Postharvest Biol. Technol. 2018, 135, 19–26. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Use of postharvest UV-B and UV-C radiation treatments to revalorize broccoli byproducts and edible florets. Innov. Food Sci. Emerg. Technol. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Gómez, P.A.; Artés, F. Amelioration Effect of LED Lighting in the Bioactive Compounds Synthesis during Carrot Sprouting. Agronomy 2021, 11, 304. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Torres-Sánchez, R.; Martínez-Zafra, M.T.; Castillejo, N.; Guillamón-Frutos, A.; Artés-Hernández, F. Real-Time Monitoring System for Shelf Life Estimation of Fruit and Vegetables. Sensors 2020, 20, 1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Organization for Standardization (ISO) 8586. Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. Available online: https://www.iso.org/standard/45352.html (accessed on 5 February 2021).
- Castillejo, N.; Martínez-Hernández, G.B.; Monaco, K.; Gómez, P.A.; Aguayo, E.; Artés, F.; Artés-Hernández, F. Preservation of bioactive compounds of a green vegetable smoothie using short time-high temperature mild thermal treatment. Food Sci. Technol. Int. 2017, 23, 46–60. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, G.; Orsini, F.; Castillejo, N.; Gómez, P.A.; Crepaldi, A.; Fernández, J.A.; Egea-Gilabert, C.; Artés-Hernández, F.; Gianquinto, G. Spectral composition from led lighting during storage affects nutraceuticals and safety attributes of fresh-cut red chard (Beta vulgaris) and rocket (Diplotaxis tenuifolia) leaves. Postharvest Biol. Technol. 2021, 175, 111500. [Google Scholar] [CrossRef]
- Kasim, M.U.; Kasim, R. The effects of ultraviolet B (UV-B) irradiation on color quality and decay rate of Capia pepper during postharvest storage. Food Sci. Technol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Villaseñor-Aguilar, M.J.; Bravo-Sánchez, M.G.; Padilla-Medina, J.A.; Vázquez-Vera, J.L.; Guevara-González, R.G.; García-Rodríguez, F.J.; Barranco-Gutiérrez, A.I. A Maturity Estimation of Bell Pepper (Capsicum annuum L.) by Artificial Vision System for Quality Control. Appl. Sci. 2020, 10, 5097. [Google Scholar] [CrossRef]
- Maroga, G.M.; Soundy, P.; Sivakumar, D. Different Postharvest Responses of Fresh-Cut Sweet Peppers Related to Quality and Antioxidant and Phenylalanine Ammonia Lyase Activities during Exposure to Light-Emitting Diode Treatments. Foods 2019, 8, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicente, A.R.; Pineda, C.; Lemoine, L.; Civello, P.M.; Martinez, G.A.; Chaves, A.R. UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol. Technol. 2005. [Google Scholar] [CrossRef]
- Cuvi, M.J.A.; Vicente, A.R.; Concellón, A.; Chaves, A.R. Changes in red pepper antioxidants as affected by UV-C treatments and storage at chilling temperatures. LWT Food Sci. Technol. 2011, 44, 1666–1671. [Google Scholar] [CrossRef]
- Del Gómez-García, M.R.; Ochoa-Alejo, N. Biochemistry and Molecular Biology of Carotenoid Biosynthesis in Chili Peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.L.; Li, L.; Shah, S.N.M.; Gong, Z.H. The relationship between red fruit colour formation and key genes of capsanthin biosynthesis pathway in Capsicum annuum. Biol. Plant. 2015. [Google Scholar] [CrossRef]
- Llorente, B.; Martinez-Garcia, J.F.; Stange, C.; Rodriguez-Concepcion, M. Illuminating colors: Regulation of carotenoid biosynthesis and accumulation by light. Curr. Opin. Plant Biol. 2017, 37, 49–55. [Google Scholar] [CrossRef]
- Pola, W.; Sugaya, S.; Photchanachai, S. Color Development and Phytochemical Changes in Mature Green Chili (Capsicum annuum L.) Exposed to Red and Blue Light-Emitting Diodes. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef]
- Llewellyn, C.A.; Airs, R.L.; Farnham, G.; Greig, C. Synthesis, Regulation and Degradation of Carotenoids under Low Level UV-B Radiation in the Filamentous Cyanobacterium Chlorogloeopsis fritschii PCC 6912. Front. Microbiol. 2020, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Jiang, C.Q.; Yan, Y.F.; Liu, B.R.; Zu, C.L. Effect of increased UV-B radiation on carotenoid accumulation and total antioxidant capacity in tobacco (Nicotiana tabacum L.) leaves. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef] [PubMed]





| Quality Attribute | Value at Harvest | ||
|---|---|---|---|
| Weight (g) | 198.9 ± 26.5 |   | |
| Calibre (mm) | Equatorial | 87.6 ± 7.1 | |
| Longitudinal | 87.5 ± 8.6 | ||
| Thickness | 5.9 ± 0.6 | ||
| Colour | L* | 33.3 ± 1.9 | |
| a* | 26.8 ± 5.7 | ||
| b* | 19.5 ± 3.1 | ||
| Chroma | 33.3 ± 5.8 | ||
| Hue angle | 36.6 ± 6.0 | ||
| T | P 14 h + 10 h | t | Weight Losses (1) | Firmness (2) | ΔE | TSS (3) | TA (4) | TSS/TA | |
|---|---|---|---|---|---|---|---|---|---|
| At harvest | 0 d | - | 19.8 ± 7.3 | - | 7.5 ± 0.4 | 0.35 ± 0.02 | 21.8 ± 0.7 | ||
| After 6 d at 7 °C | CTRL | - | 6 d | 0.5 ± 0.1 | 19.7 ± 4.5 | 8.4 ± 6.1 | 7.6 ± 0.3 | 0.35 ± 0.03 | 21.7 ± 1.1 |
| UV-B | - | 0.5 ± 0.1 | 17.2 ± 3.6 | 5.9 ± 3.9 | 7.6 ± 0.3 | 0.31 ± 0.01 | 24.7 ± 1.6 | ||
| After a retail sale period at 20 °C | CTRL | FL + D | +2 d | 1.1 ± 0.2 | 17.2 ± 2.6 | 6.2 ± 2.6 | 7.8 ± 0.2 | 0.30 ± 0.02 | 26.2 ± 1.1 |
| +3 d | 2.5 ± 0.5 | 13.8 ± 3.3 | 6.4 ± 2.6 | 7.5 ± 0.1 | 0.28 ± 0.02 | 26.5 ± 1.2 | |||
| +4 d | 3.7 ± 0.7 | 10.1 ± 2.0 | 6.3 ± 2.2 | 8.1 ± 0.3 | 0.32 ± 0.01 | 25.6 ± 0.2 | |||
| FL + FL | +2 d | 1.3 ± 0.3 | 16.7 ± 3.0 | 7.4 ± 5.8 | 7.9 ± 0.2 | 0.36 ± 0.02 | 21.8 ± 0.7 | ||
| +3 d | 2.8 ± 0.6 | 13.7 ± 3.0 | 7.7 ± 4.6 | 7.6 ± 0.3 | 0.31 ± 0.01 | 24.4 ± 0.6 | |||
| +4 d | 4.0 ± 0.8 | 9.9 ± 2.2 | 7.4 ± 5.7 | 7.8 ± 0.3 | 0.28 ± 0.05 | 28.7 ± 0.6 | |||
| FL + BR LED | +2 d | 1.5 ± 0.2 | 15.2 ± 3.2 | 6.3 ± 3.5 | 7.7 ± 0.2 | 0.30 ± 0.01 | 25.3 ± 1.2 | ||
| +3 d | 3.1 ± 0.5 | 13.7 ± 3.4 | 6.0 ± 2.4 | 7.6 ± 0.2 | 0.31 ± 0.02 | 24.3 ± 1.4 | |||
| +4 d | 4.6 ± 0.7 | 8.1 ± 2.1 | 6.2 ± 2.6 | 7.7 ± 0.1 | 0.31 ± 0.01 | 25.1 ± 1.1 | |||
| UV-B | FL + D | +2 d | 1.2 ± 0.3 | 16.7 ± 4.0 | 6.1 ± 2.7 | 7.7 ± 0.2 | 0.32 ± 0.01 | 23.7 ± 0.8 | |
| +3 d | 2.5 ± 0.6 | 14.4 ± 3.2 | 6.6 ± 2.8 | 7.5 ± 0.1 | 0.30 ± 0.01 | 25.2 ± 1.2 | |||
| +4 d | 3.7 ± 0.9 | 9.7 ± 2.6 | 6.2 ± 2.3 | 7.8 ± 0.2 | 0.30 ± 0.01 | 26.4 ± 0.1 | |||
| FL + FL | +2 d | 1.3 ± 0.2 | 17.4 ± 3.3 | 8.5 ± 5.6 | 7.6 ± 0.5 | 0.31 ± 0.02 | 24.7 ± 0.7 | ||
| +3 d | 2.7 ± 0.4 | 12.2 ± 2.9 | 8.1 ± 5.4 | 7.6 ± 0.1 | 0.29 ± 0.02 | 26.4 ± 2.1 | |||
| +4 d | 4.0 ± 0.5 | 9.1 ± 1.8 | 6.8 ± 4.7 | 7.7 ± 0.3 | 0.30 ± 0.01 | 25.7 ± 1.0 | |||
| FL + BR LED | +2 d | 1.5 ± 0.2 | 14.5 ± 2.5 | 7.1 ± 3.4 | 7.5 ± 0.3 | 0.27 ± 0.03 | 27.8 ± 1.7 | ||
| +3 d | 3.1 ± 0.4 | 11.2 ± 2.5 | 6.8 ± 4.1 | 7.8 ± 0.2 | 0.33 ± 0.06 | 24.1 ± 0.5 | |||
| +4 d | 4.7 ± 0.6 | 8.4 ± 1.8 | 6.5 ± 2.5 | 8.1 ± 0.2 | 0.31 ± 0.00 | 25.7 ± 0.9 | |||
| T | n.s. | n.s. | n.s. | (0.042) * | (0.004) ** | n.s. | |||
| P | (0.139) *** | (0.758) *** | (1.039) * | n.s. | n.s. | n.s. | |||
| t | (0.139) *** | (0.758) *** | n.s. | (0.052) *** | (0.005) *** | (0.477) *** | |||
| T × P | n.s. | n.s. | n.s. | (0.074) *** | (0.008) *** | (0.675) *** | |||
| T × t | n.s. | n.s. | n.s. | (0.074) *** | (0.008) *** | (0.675) ** | |||
| P × t | (0.241) ** | n.s. | n.s. | (0.090) *** | (0.009) *** | (0.827) *** | |||
| T × P × t | n.s. | n.s. | n.s. | (0.127) *** | (0.013) *** | (1.169) *** | |||
| T | P (14 h + 10 h) | t | TPC | TFC | TAC | |
|---|---|---|---|---|---|---|
| At harvest | 0 d | 2.12 ± 0.11 | 0.17 ± 0.04 | 1.57 ± 0.10 | ||
| After 6 d at 7 °C | CTRL | - | 6 d | 1.81 ± 0.14 | 0.13 ± 0.02 | 1.64 ± 0.07 |
| UV-B | - | 1.62 ± 0.14 | 0.16 ± 0.04 | 1.19 ± 0.08 | ||
| After a retail sale period at 20 °C | CTRL | FL + D | +2 d | 1.51 ± 0.08 | 0.16 ± 0.02 | 1.09 ± 0.12 |
| +3 d | 1.79 ± 0.12 | 0.17 ± 0.02 | 1.26 ± 0.26 | |||
| +4 d | 1.83 ± 0.17 | 0.21 ± 0.04 | 1.10 ± 0.15 | |||
| FL + FL | +2 d | 1.69 ± 0.24 | 0.17 ± 0.04 | 1.17 ± 0.11 | ||
| +3 d | 1.56 ± 0.14 | 0.18 ± 0.02 | 1.24 ± 0.16 | |||
| +4 d | 1.80 ± 0.00 | 0.20 ± 0.01 | 1.43 ± 0.06 | |||
| FL + BR LED | +2 d | 2.12 ± 0.09 | 0.20 ± 0.02 | 1.28 ± 0.13 | ||
| +3 d | 1.87 ± 0.24 | 0.17 ± 0.04 | 1.43 ± 0.16 | |||
| +4 d | 1.96 ± 0.10 | 0.19 ± 0.01 | 1.36 ± 0.26 | |||
| UV-B | FL + D | +2 d | 1.56 ± 0.11 | 0.15 ± 0.02 | 1.10 ± 0.05 | |
| +3 d | 1.81 ± 0.17 | 0.17 ± 0.00 | 1.39 ± 0.05 | |||
| +4 d | 1.76 ± 0.04 | 0.19 ± 0.05 | 1.28 ± 0.08 | |||
| FL + FL | +2 d | 1.69 ± 0.22 | 0.17 ± 0.04 | 1.25 ± 0.17 | ||
| +3 d | 1.78 ± 0.12 | 0.17 ± 0.03 | 1.29 ± 0.12 | |||
| +4d | 1.74 ± 0.12 | 0.18 ± 0.03 | 1.62 ± 0.06 | |||
| FL + BR LED | +2 d | 1.74 ± 0.02 | 0.17 ± 0.01 | 1.24 ± 0.26 | ||
| +3 d | 1.90 ± 0.15 | 0.18 ± 0.02 | 1.48 ± 0.12 | |||
| +4 d | 1.86 ± 0.16 | 0.18 ± 0.01 | 1.28 ± 0.11 | |||
| T | n.s. | n.s. | n.s. | |||
| P | (0.097) *** | n.s. | (0.092) * | |||
| t | n.s. | (0.019) * | (0.092) ** | |||
| T × P | n.s. | n.s. | n.s. | |||
| T × t | n.s. | n.s. | n.s. | |||
| P × t | n.s. | n.s. | (0.160) * | |||
| T × P × t | n.s. | n.s. | n.s. | |||
| T | P (14 h + 10 h) | t | All-Trans-Neoxanthin | All-Trans-Lutein | Capsanthin | Capsanthin Laurate | 13-Cis-β-Carotene | All-Trans-β-Carotene | 9-Cis-β-Carotene | Capsanthin Myristate | Capsanthin Esters | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At harvest | 0 d | 9.9 ± 0.7 | 2.3 ± 0.3 | 54.6 ± 4.8 | 7.8 ± 0.3 | 19.0 ± 0.9 | 35.2 ± 0.6 | 6.2 ± 0.2 | 7.7 ± 0.1 | 77.5 ± 2.7 | ||
| After 6 d at 7 °C | CTRL | - | 6 d | 9.6 ± 1.8 | 2.2 ± 0.3 | 39.5 ± 0.8 | 8.2 ± 0.0 | 15.3 ± 0.5 | 33.5 ± 1.6 | 6.8 ± 0.9 | 10.5 ± 1.1 | 80.2 ± 6.9 |
| UV-B | - | 10.8 ± 0.2 | 2.2 ± 0.3 | 48.0 ± 4.5 A | 11.3 ± 0.4 A | 19.6 ± 0.8 A | 36.6 ± 1.6 | 7.2 ± 0.6 | 10.4 ± 1.1 | 102.2 ± 7.2 A | ||
| After a retail sale period at 20 °C | CTRL | FL+D | +2 d | 10.5 ± 0.2 | 2.4 ± 0.1 | 54.7 ± 0.7 | 9.7 ± 0.7 | 18.4 ± 1.3 | 33.9 ± 3.1 | 7.8 ± 0.1 | 8.5 ± 0.8 | 82.7 ± 3.3 |
| +3 d | 14.0 ± 0.2 | 2.8 ± 0.0 | 51.9 ± 0.0 | 10.0 ± 0.3 | 16.9 ± 0.7 | 30.9 ± 0.7 | 6.6 ± 0.4 | 8.1 ± 0.5 | 76.5 ± 2.9 | |||
| +4 d | 15.3 ± 1.2 | 3.1 ± 0.2 | 78.1 ± 3.4 | 9.9 ± 0.8 | 17.2 ± 0.7 | 28.8 ± 0.4 | 8.4 ± 0.2 | 8.4 ± 0.1 | 75.9 ± 7.7 | |||
| FL + FL | +2 d | 10.1 ± 0.3 | 2.5 ± 0.2 | 58.5 ± 5.8 | 8.7 ± 0.4 | 17.9 ± 0.4 | 31.6 ± 1.7 | 7.5 ± 0.1 | 7.4 ± 0.5 | 80.0 ± 2.4 | ||
| +3 d | 14.2 ± 1.0 | 2.7 ± 0.1 | 52.0 ± 2.3 | 10.4 ± 0.5 | 19.6 ± 1.0 | 31.3 ± 1.4 | 6.2 ± 0.1 | 8.0 ± 0.2 | 94.9 ± 2.6 | |||
| +4 d | 15.8 ± 1.0 | 2.9 ± 0.1 | 84.6 ± 2.4 | 10.5 ± 0.7 | 20.0 ± 1.0 | 34.2 ± 1.9 | 8.3 ± 0.4 | 9.4 ± 0.3 | 81.7 ± 2.3 | |||
| FL + BR LED | +2 d | 14.1 ± 1.3 | 2.8 ± 0.2 | 70.3 ± 4.5 | 13.2 ± 0.0 | 24.5 ± 1.8 | 43.8 ± 2.9 | 9.8 ± 0.4 | 10.5 ± 0.0 | 108.1 ± 7.2 | ||
| +3 d | 14.4 ± 1.8 | 2.7 ± 0.2 | 70.5 ± 5.6 | 9.8 ± 0.4 | 24.0 ± 2.2 | 32.3 ± 1.7 | 9.4 ± 0.6 | 9.3 ± 0.7 | 90.6 ± 8.8 | |||
| +4 d | 18.2 ± 0.7 | 3.5 ± 0.2 | 102.4 ± 4.3 | 12.7 ± 1.1 | 24.6 ± 1.3 | 42.2 ± 2.7 | 9.4 ± 0.5 | 9.8 ± 0.4 | 88.6 ± 2.5 | |||
| UV-B | FL + D | +2 d | 9.6 ± 0.4 | 2.2 ± 0.0 | 69.2 ± 0.6 | 9.9 ± 0.9 | 18.3 ± 0.5 | 34.3 ± 2.8 | 7.1 ± 0.5 | 8.6 ± 0.5 | 83.8 ± 5.6 | |
| +3 d | 14.2 ± 0.2 | 2.8 ± 0.1 | 67.1 ± 1.0 | 10.7 ± 0.4 | 19.3 ± 0.9 | 35.7 ± 2.1 | 7.7 ± 1.0 | 10.4 ± 0.7 | 81.6 ± 12.3 | |||
| +4 d | 17.7 ± 0.5 | 3.4 ± 0.0 | 78.7 ± 2.1 | 12.1 ± 0.5 | 23.7 ± 1.4 | 41.5 ± 2.4 | 9.8 ± 0.8 | 11.0 ± 0.9 | 90.7 ± 8.2 | |||
| FL + FL | +2 d | 9.9 ± 0.3 | 2.7 ± 0.1 | 67.8 ± 2.1 | 10.3 ± 0.2 | 18.5 ± 0.4 | 36.6 ± 1.9 | 7.2 ± 0.5 | 10.8 ± 0.5 | 91.1 ± 6.6 | ||
| +3 d | 13.9 ± 0.9 | 2.8 ± 0.2 | 68.5 ± 3.6 | 9.8 ± 1.1 | 18.0 ± 0.2 | 33.7 ± 3.2 | 7.6 ± 0.3 | 9.3 ± 0.8 | 77.2 ± 6.3 | |||
| +4 d | 17.2 ± 0.9 | 3.3 ± 0.0 | 87.6 ± 6.3 | 12.8 ± 0.8 | 23.8 ± 0.9 | 43.2 ± 1.6 | 8.5 ± 1.0 | 9.5 ± 0.3 | 88.8 ± 5.9 | |||
| FL + BR LED | +2 d | 14.6 ± 1.1 | 2.8 ± 0.1 | 73.6 ± 3.9 | 11.4 ± 1.1 | 20.4 ± 1.6 | 37.5 ± 2.6 | 8.4 ± 0.3 | 10.0 ± 0.7 | 87.3 ± 5.4 | ||
| +3 d | 16.5 ± 0.7 | 3.1 ± 0.2 | 81.1 ± 3.9 | 12.7 ± 1.3 | 21.3 ± 0.3 | 38.8 ± 2.5 | 8.3 ± 0.7 | 11.1 ± 0.7 | 92.8 ± 9.1 | |||
| +4 d | 18.1 ± 0.2 | 3.5 ± 0.0 | 98.3 ± 4.3 | 13.3 ± 0.5 | 26.1 ± 0.1 | 45.9 ± 1.3 | 10.0 ± 0.4 | 11.5 ± 1.2 | 97.1 ± 6.3 | |||
| T | (0.461) * | (0.079) *** | (2.017) *** | (0.401) *** | (0.602) * | (1.204) *** | n.s. | (0.343) *** | n.s. | |||
| P | (0.564) *** | (0.097) *** | (2.470) *** | (0.491) *** | (0.737) *** | (1.474) *** | (0.360) *** | (0.420) *** | (4.370) *** | |||
| t | (0.564) *** | (0.097) *** | (2.470) *** | (0.491) *** | (0.737) *** | (1.474) *** | (0.360) *** | (0.420) * | n.s. | |||
| T × P | n.s. | n.s. | (3.493) * | n.s. | (1.042) *** | (2.085) ** | (0.509) ** | n.s. | n.s. | |||
| T × t | n.s. | n.s. | (3.493) *** | (0.694) * | (1.042) *** | (2.085) *** | (0.509) *** | n.s. | (6.179) ** | |||
| P × t | (0.978) *** | (0.167) * | (4.278) ** | (0.851) * | n.s. | (2.553) * | n.s. | n.s. | n.s. | |||
| T × P × t | (1.382) * | (0.237) * | n.s. | (1.203) *** | n.s. | (3.611) ** | (0.881) * | (1.030) *** | (10.703) *** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and Photoperiod with Blue + Red LEDs as Strategies to Stimulate Carotenogenesis in Bell Peppers. Appl. Sci. 2021, 11, 3736. https://0-doi-org.brum.beds.ac.uk/10.3390/app11093736
Martínez-Zamora L, Castillejo N, Artés-Hernández F. Postharvest UV-B and Photoperiod with Blue + Red LEDs as Strategies to Stimulate Carotenogenesis in Bell Peppers. Applied Sciences. 2021; 11(9):3736. https://0-doi-org.brum.beds.ac.uk/10.3390/app11093736
Chicago/Turabian StyleMartínez-Zamora, Lorena, Noelia Castillejo, and Francisco Artés-Hernández. 2021. "Postharvest UV-B and Photoperiod with Blue + Red LEDs as Strategies to Stimulate Carotenogenesis in Bell Peppers" Applied Sciences 11, no. 9: 3736. https://0-doi-org.brum.beds.ac.uk/10.3390/app11093736