1. Introduction
Hop (Humulus lupulus) is a plant usually used as a fundamental ingredient in the brewing industry.
Inflorescences of the female
H. lupulus plant are rich in essential oils, resins, and polyphenolic compounds, which exert a preserving action on the beer and are responsible for its typical flavors and aromas [
1]. Moreover, in recent years, pharmaceutical applications of hops have been investigated due to their anti-inflammatory, antiseptic, antidiuretic, hypnotic, and stomachic properties [
2].
Furthermore, its sedative effect has been exploited in food supplements for the treatment of sleep disorders [
3]. As regards the application in the brewing field, resinous compounds, such as α- and β-acids, give a bitter taste to beer and may protect the beer against lactic acid bacteria (genera of
Lactobacillus and
Pediococcus) and contribute to the improvement of its foam stability [
4].
Moreover, essential oils are responsible for the specific aroma, tannins play a part in the clarification of beer by precipitating proteins during boiling, and hop polyphenol compounds, which represent almost 4% of the total hop dry mass, have been identified as important bioactive compounds with strong antioxidant and preserving functions [
5,
6]. During the boiling phase of a brewing process, α-acids are isomerized into iso-α-acids, (isohumulone, isocohumulone, and isoadhumulone), which play an active role in the antibacterial preservative action of hops, are more soluble in water, and are present in the finished beer in larger quantities than their non-isomerized precursors, providing its distinctive bitter flavor and aroma.
α-acids and β-acids are susceptible to oxygen and degrade rapidly during hop storage [
7]. The effects of oxidized hops on the bitterness quality of beer are controversial; Algazzali and Shellhammer [
8] suggested that, although iso-α-acids were confirmed to be more bitter than oxidized α- and β-acids, the latter resulted to be bitter enough to have a potentially significant impact on beer bitterness.
Several hop products are available for commercial use, such as hop powders or pellets, enriched hop powders or pellets, hop oils, hop extracts, and isomerized hop extracts [
9].
Over the years, hop extracts have been used to replace hop powders or pellets in brewing, ensuring the following advantages: better bitterness control between different batches owing to their greater homogeneity, improved hop utilization due to the use of concentrated extracts, improved stability during the storage, which allows the preservation of excess production from one season to the next, and reduced transport and storage costs [
10]. Hop extracts consist of a resinous fraction, responsible for the bitter taste of the beer and a volatile fraction that influences the aroma [
11].
The resinous fraction represents 20% of the dried cones and is mainly formed by α-acids (humulone and its congeners, such as cohumulone, adhumulone, prehumulone, and posthumulone) and β-acids (mainly lupulone and its congeners, such as colupulone, adlupulone, prelupulone and postlupulone). The volatile fraction is instead composed of terpenes, such as myrcene, α-humulene, and β-caryophyllene, and its content in dried hops can vary from 0.1% to 2% [
11]. Moreover, the use of pre-isomerized extracts, such as replacements for conventional hop extracts, guarantee reduced hopping costs without affecting the sensory characteristics of a beer, as confirmed by Mitter and Cocuzza [
12]. These extracts allow brewers to significantly reduce boiling times, which results in the reduction of energy costs by maintaining flavor stability. Sometimes, they can be added to the wort after fermentation to adjust the bitterness of a finished beer. Steam distillation and organic solvent extraction are the most widespread extraction methods used for the recovery of oils from vegetable matrices.
While the first technique requires high operative temperatures which could reduce the quality of the final extracts, organic solvents are toxic, flammable, require expensive safety measures, and always involve subsequent separation processes [
10]. Moreover, the presence of solvent residues in the final products requires additional purification steps that are time-consuming and increase the total process cost [
13]. In both cases, the real disadvantage is the alteration of the extract composition [
14]. Supercritical CO
2 (sc-CO
2) extraction represents an optimal alternative to traditional techniques; it uses a non-toxic solvent with high solvating power and is easily removable from both the extract and the exhausted matrix. The main features distinguishing CO
2 extracts from conventional extracts are the lack of traces of undesired organic solvents and the absence of expensive purification treatments. Moreover, it allows an improvement in the flexibility of the process parameters [
15] and in-process selectivity, ensuring high extraction rates and extracts with high bitterness power [
16,
17].
In particular, a relatively high recovery of volatile compounds is obtained at lower temperatures, whilst elevated pressures and temperatures favor the high recovery of bitter acids and resinous compounds [
13].
Recently, several innovative extraction techniques have been proposed, such as microwave and ultrasound systems, to increase the extraction efficiency of bioactive compounds in hop cones using water, ethanol, and their mixture as food-grade solvents [
18]. Santarelli et al. [
19] investigated the effectiveness of innovative ultrasound-assisted and high hydrostatic pressure extraction methods: the recovery of polyphenols and carotenoids from hop and the antiradical capacity of the resulting extracts were evaluated and compared with those of conventional extractions. In this work, the use of novel hop extracts in brewing, obtained in short times without excessively high costs, was investigated. An innovative patented extraction process [
20] using hydrofluorocarbon (HFC) Norflurane (1,1,1,2- tetrafluoro ethane) as a solvent in subcritical conditions was proposed. The properties of this refrigerant fluid as an extracting solvent for the treatment of vegetable matrices were already investigated; this compound guarantees the recovery of bioactive compounds, such as lycopene and other carotenoids [
21], or value-added oils from wet or dried agri-food wastes, such as spent coffee grounds and waste bilberry seeds [
22,
23].
Moreover, despite the high environmental impact due to its Global Warming Potential (GWP) value of 1400, its convenience in terms of safety, low risk, low toxicity, and low plant and operating costs was confirmed by Lapkin et al. [
24], which compared hydrofluorocarbon extraction techniques with traditional and sc-CO
2 processes.
The problems linked to its contribution as a greenhouse gas can be significantly overcome by a closed extraction system in which the solvent is continuously regenerated and recirculated. Moreover, the use of hydrofluorocarbon solvents in subcritical conditions allows overcoming the need for a drying pre-treatment of the solid matrices [
22] and the use of suitable co-solvents aimed at improving the recovery of polar molecules of interest [
25], indispensable for other extraction techniques, including sc-CO
2 process.
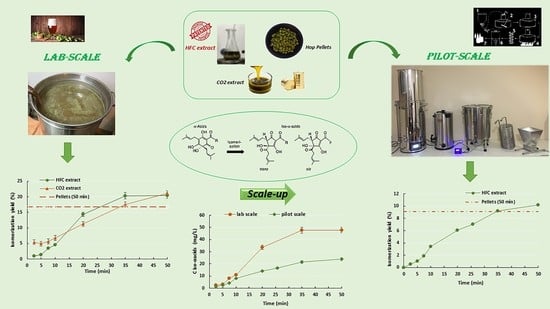
To verify the potential of this novel extraction process, the total extraction yield and α-acid recovery were determined and compared with those reported in the literature and obtained with a supercritical CO2 process. The kinetics of the α-acid isomerization during boiling were investigated on a laboratory scale using hop pellets, commercial CO2 extracts, and HFC extracts. Moreover, the use of HFC extracts was tested in a pilot-scale brewing apparatus, and also in this case, the resulting isomerization rates were compared with those obtained by the traditional procedure with hop pellets to confirm the potential application of these novel extracts in the brewing field.
2. Materials and Methods
2.1. Raw Materials and Reagents
Dried and pelletized hop pellets (Herkules variety) cultivated and harvested in the Bavarian region of Hallertau in 2017 were provided by Hopsteiner Company (Mainburg, Germany).
Hopsteiner also supplied a commercial sc-CO2 extract coming from the same crop batch. Diethyl ether (purity grade ≥ 99.8%), hydrochloric acid (purity grade ≥ 37%), orthophosphoric acid (purity grade 99%), acetonitrile (purity grade ≥ 99.9%), and methanol (purity grade ≥ 99.9%) were purchased from Sigma Aldrich (Milan, Italy). 2,2,4-trimethylpentane (purity grade ≥ 99.9%) and Norflurane were supplied by Lab-Scan Analytical Sciences and LGC Standards PROMOCHEM (Milan, Italy), respectively.
2.2. Pilot Apparatus for Beer Production Process
The mill used for grinding malt grains consists of knurled steel rollers arranged parallel to each other and manually operated. The distance between the rollers was set to about 1.5 mm.
The system has a hopper with a maximum capacity of 2.5 kg malt. The mashing, lautering, sparging, and boiling phases are carried out in a single vessel. It consists of an external cylindrical vessel equipped with a non-hermetic closing device, which is initially filled with water.
It is equipped with a PLC unit that allows the adjustment of the liquid temperature, the thermal power supplied by the heating bottom, and the duration of the operation.
A cylindrical jacket is inserted within the external vessel and is characterized by a perforated bottom grid on which the ground malt is placed. The size of the grid mesh allows the passage of the liquid and retains the solid during the lautering and sparging phases.
A central hole with ¾ inch diameter allows the placement of a tube that works as a liquid weir.
A recirculation pump provides the ability to recycle the hot liquid from the bottom of the external vessel to the top of the internal cylinder, allowing its percolation through the malt. Through a suitable valve on the recycling pipe, it is possible to carry out an appropriate liquid flow rate regulation.
At the end of the mashing phase, the internal cylinder is raised and positioned on appropriate supports provided by the manufacturer to rinse the exhausted threshers and allow the liquid drainage. The water used for sparging (15 L) is previously heated at a temperature of 80 °C in a second vessel and equipped with an adjustable thermostat, where its pH is adjusted with phosphoric acid to a value of 5.5. At the end of this operation, the internal cylinder is removed, and the resulting liquid is heated to proceed with boiling. After 60 min of boiling, a cooling coil is inserted into the vessel and rapid cooling of the wort is obtained. The whirlpool technique is used to remove the trub from the bitter and clarified must. The external vessel is equipped with a valve positioned on the bottom through which it is possible to drain the liquid. A further stage of filtration of the must is ensured by a bag filter so that it is possible to send to the fermenter a liquid with the lowest possible content of suspended materials.
The fermenter is a cylindrical container with a capacity of 50 L.
It is characterized by a conical bottom and is equipped with a temperature control system.
This system consists of an external coating of polymeric material to reduce heat exchange and a coil immersed in the must to be fermented, suitably installed on the hermetically sealed lid of the vessel.
A control unit allows the regulation of the refrigerant fluid flow rate to maintain the set point temperature (20 °C). Preliminary oxygenation of the must is carried out by “splashing” the liquid while loading the fermenter and is followed by immediate yeast inoculation.
All the units described are composed of AISI 304 steel. In
Figure 1, the main components of the pilot plant described are shown. A forced carbonation system was used, based on a commercial system known as SodaStream
®. It allows immediate carbonation by regulating the quantity of carbon dioxide dissolved in the finished drink without having to wait for long times owing to the awakening of the yeasts and the completion of their metabolic processes.
2.3. HFC Extraction
HFC extracts were obtained using a lab-scale extraction system based on a patented process [
20] described by Colucci Cante et al. [
21,
22,
23]. Briefly, it consisted of:
(a) repeated percolation of liquid Norflurane through the extraction bed (8–10 bar); (b) regeneration of the solvent by means of depressurization in an expansion chamber and the recovery of the extracts at the bottom (4 bar); and (c) subsequent recompression and recirculation of the solvent in liquid form to the extraction reactor. According to the compressor capacity, the maximum solvent flow rate (100 mL/min) was recirculated in the system; at each extraction cycle, recompressed R134a was used to provide the evaporation heat exchanged in the expansion chamber, while part of the undissipated compression work determined the inlet temperature into the extraction chamber (35 °C).
In
Table 1, the main operating conditions adopted during the extraction process are summarized.
Samples were collected at specific times and the recovery efficiencies of extracts (η
e) and α-acids (η
α-acids) were determined for each of them, according to Equations (1) and (2):
where m
e, m
p, m
α, and m
αi indicate the amounts by weight of hop extract, pellets, and extracted α-acids and total α-acids initially contained in the hop, respectively.
2.4. Preliminary Hop Characterization
Hop pellet characterization was carried out by determining the total α-acid content through a preliminary extraction procedure aimed at obtaining α-acid extracts to be analysed by the HPLC method as described in Salanta et al. [
26]. Finely ground pellets (10 g) were placed into a 250 mL flask and added to 20 mL of methanol, 100 mL of diethyl ether, and 40 mL of 0.1 M HCL solution. The resulting mixture was shaken for 40 min and left to stand at 20 °C for 10 min to allow the phase separation. The hop extract was collected and homogenized by stirring.
Afterwards, 5 mL of extract were placed in a 50 mL volumetric flask, filled up to volume with methanol, and gently mixed. Then, the solution was filtered using 0.45 µm microfilters and was injected into a High-Pressure Liquid Chromatography (HPLC) system for the α- and iso-α-acid analysis described below.
2.5. Isomerization Trials of α-acids
2.5.1. Laboratory Scale Trials
Isomerization trials were performed using three different extracts: (a) traditional extract, obtained from hop pellets directly introduced into the wort, (b) sc-CO
2 extract, and (c) HFC extract, as reported in
Table 2. A total of 1 L of wort with a sugar content of 12.5° Brix was produced in the pilot plant previously described. The product was brought to a boil at ambient pressure using an electric laboratory stove equipped with a thermostat for regulating the temperature; once the boiling temperature was reached, the source of α-acids (pellets, CO
2 extract, or HFC extract) was added.
The isomerization process was conducted according to an APA beer recipe which consists of a 50 min boiling of the wort after the addition of hop pellets in a ratio of 1:1 with the volume of liquid. For this reason, a pellet mass of 1 g was chosen to be added to 1 L wort, corresponding to an initial α-acid content of approximately 24 g. During trials I2 and I3, CO
2 extract and HFC extract, respectively, were added in such quantities as to guarantee the same initial amount of α-acids, as specified in
Table 2. Seven withdrawals were conducted during the boiling (after 2.5 min (P
1), 5 min (P
2), 7.5 min (P
3), 10 min (P
4), 20 min (P
5), 35 min (P
6), and 50 min (P
7) of the process) to describe the isomerization kinetics when each of the three extracts was used.
Each sample was placed in a test tube equipped with an airtight cap and suddenly cooled to prevent further progress of the isomerization reaction.
2.5.2. Pilot Scale Trials Using HFC Extract
The use of the HFC extract on a larger scale was investigated and its isomerization behavior was compared with that observed during the tests carried out at a laboratory scale.
A total of 25 L of must with a sugar content of 12.5° Brix were prepared. The HFC extract was added during the boiling phase in an amount that ensured the same α-acid mass to liquid volume ratio as that used for the laboratory scale trials (approximately 0.24 g
α-acids/L), as shown in
Table 2.
Samples were collected during boiling at the same times both in the lab-scale tests and in the pilot-scale tests and the total iso-α-acid amount was determined as the per cent efficiency of isomerization, ηiso, was evaluated with respect to the total α-acid amount present in the liquid at the beginning of the isomerization trials, according to Equation (3):
2.6. Analytical Methods
α- and iso-α-acid concentrations in the extracts were measured by HPLC, Agilent Technologies Series 1100, equipped with a Waters 2487 Dual λ Absorbance Detector UV-Vis.
The column used was a Phenomenex Synergi 4μ Fusion-RP 80 (4 μm, 250 × 4.6 mm).
Two eluents were fed in an isocratic regime at a flow rate of 1.5 mL/min: A, 100% water acidified to a pH of 2.8 using orthophosphoric acid, and B, 100% acetonitrile. A and B were eluted with a volume ratio of 48:52. The detection wavelength was set at 314 and 270 nm and runs of 50 min and 20 min were carried out for α- and iso-α-acids, respectively. Before HPLC analysis, the extracts were pre-treated: 10 mL of extract were mixed with 0.5 mL of phosphoric acid and 10 mL of trimethylpentane; after stirring for 1 min, 2 mL of methanol were added; the mixture was sonicated at room temperature for 15 min and then centrifuged at 2000 rpm for 5 min; the supernatant was collected and 20 µL were injected into the HPLC system. International Calibration Extract (ICE-3) and International Calibration Standard (ICS-I3), provided by The American Society of Brewing Chemists, were used as external standards for α- and iso-α-acid determination, respectively.
2.7. Statistical Analysis
Statistical analysis was carried out using Microsoft Excel 2016®.
Extraction tests and analysis were performed in triplicate and mean values and standard deviations (n = 3) were calculated. Student’s t-test was used to assess the statistical significance of each experimental data, setting a significance level, p, equal to 0.05.
3. Results and Discussion
Hop Extraction Using HFC System
The overall content of α-acids in pellets, CO
2 extract, and HFC extract resulted to be equal to 0.234 g/g
pellets, 0.513 g/g
CO2 extract, and 0.597 g/g
HFC extract, respectively, as previously reported in
Table 2. In
Figure 2, the recovery efficiency of hop extracts, evaluated during extraction runs of 180 min with the HFC system, is reported.
As shown in
Figure 2, a rapid increase in the extraction yield was observed during the first 20 min of process followed by a slightly decreasing trend for the extraction rate and the achievement of a maximum extract recovery of approximately 19% after 120 min of process.
The extraction system used in this work allowed operation at moderate temperature and pressure conditions (9 bar, 35 °C) and provided extraction performances higher than those reported by some authors in the literature by sc-CO
2 techniques. In Zeković et al. [
27], hop extraction was carried out using sc-CO
2 and extraction yields of 9.79% and 13.65% were reached by an operating temperature of 40 °C and pressures of 15 MPa and 30 MPa, respectively, in 150 min of process.
Furthermore, Formato et al. [
28] reported even lower yield values: a recovery of approximately 3% was obtained after an extraction time of 250 min, through two different extraction techniques: sc-CO
2 extraction with and without using ethanol as co-solvent, and cyclically pressurized solid–liquid extraction performed by a Naviglio extractor [
29].
The maximum α-acid yield obtained in this work through the innovative HFC extraction system (approximately 50.5%) was comparable with the maximum efficiency of 50.7% reached by Bizaj et al. [
30] using sc-CO
2 at 150 bar and 20 °C. Moreover, higher yields were obtained only by stronger pressures with different solvents at various densities, such as propane, sulfur hexafluoride, and dimethyl ether. Furthermore, comparable extraction yields in α-acids were obtained by Ntourtoglou et al. [
31], which carried out a pulsed-field extraction treatment on methanolic hop extracts. Additionally, in this case, HFC technology results in a less complex and expensive technique in addition to ensuring a final solvent-free extract.
As shown in
Figure 3, the α-acids concentration profile, c
α-acids, found in samples progressively collected during the process time, remained approximately constant (about 0.6 g/g
extract). As the extraction proceeded, hop extracts equally concentrated in α-acids were obtained due to their similar extraction kinetics.
Figure 4 shows a comparison between the isomerization performances obtained with hop pellets, sc-CO
2 extract, and HFC extract during lab-scale isomerization trials I1, I2, and I3, respectively. Pellets and CO
2 extract showed an initial iso-α-acid concentration equal to approximately 10 mg/L while HFC extract contained a negligible initial iso-α-acid amount.
Despite this, HFC extract provided a higher isomerization rate during boiling than that observed using CO2 extract. Furthermore, both CO2 and HFC extracts showed a higher final iso-α-acid content than that reached using pellets after 50 min.
During the first 15 min of process, a higher increase in total iso-α-acids concentration (ciso-α-acids) was observed when pellets and CO2 extracts were added into the boiling wort (trials I1 and I2, respectively) in comparison with the iso-α-acid amount determined by adding HFC extract.
However, for longer times, HFC extract provided the highest production rate of iso- α-acids, with a final concentration of 47.550 ± 0.637 mg/L, reached after 35 min of boiling process.
In
Figure 5, the isomerization yields, η
iso, evaluated over the process time with sc-CO
2 and HFC extracts, were compared with the maximum isomerization efficiency of approximately 16.73%, reached by pellet addition (trial I1) after 50 min of boiling.
A maximum isomerization yield of 20.32% was achieved after 35 min of process using HFC extracts. This result could be further improved, as suggested by Jaskula et al. [
32], which studied the effect of optimizing pH, ionic strength, wort gravity, hopping rate, and metal ion catalysts on isomerization performances.
Assuming as reference value the maximum amount of iso-α-acids reached using traditional pellets (trial I1), it was evident that both sc-CO2 extract and HFC extract allowed the achievement of this value in shorter process times (approximately 34 min and 25 min, respectively) than those commonly used for the traditional process (50 min).
As the addition of the HFC extract allowed to considerably reduce the treatment times, its implementation into a pilot-scale process was studied to confirm this behavior.
Samples collected during the pilot-scale trial I4 showed lower iso-α-acid concentrations than those measured in lab-scale withdrawals (trial I3), as shown in
Figure 6.
However, the same isomerization yield of approximately 9%, obtained after 50 min using the traditional procedure (hop pellets), was reached with the HFC system after a shorter time of about 35 min, as observed in
Figure 7.
It can be assumed that this discrepancy was essentially attributable to structural differences in the experimental systems used to conduct the laboratory- and pilot-scale tests.
In both cases, the mixing was essentially due to natural convection phenomena. The different reactor geometry, the use of a completely different heat adduction system, and a greater liquid head in the pilot-scale apparatus affected the heat and mass transfer processes and allowed the presence of temperature and concentration gradients with a significant impact on the α-acid solubility, influenced by pH and temperature [
32], and the resulting isomerization performances. Therefore, less efficient agitation and wall adhesion conditions inside the pilot vessel could have negatively affected the conversion of α-acids into iso-α-acids, leading to isomerization yields lower than those reached during the lab-scale experimentations.
However, the isomerization kinetics resulted to be higher than those observed during the conventional boiling with pellets.
4. Conclusions
The aim of this work was to investigate the addition of hop extracts obtained through an innovative extraction technique using a hydrofluorocarbon solvent, to a beer wort prepared in a pilot brewing plant. The proposed HFC extraction system allowed to operate at moderate temperature and pressure conditions and provided extraction performances higher than those reported by some authors in the literature using sc-CO2. An extraction efficiency of approximately 19.8% was reached using Norflurane after 180 min of process, with a final α-acid concentration of 0.6 g/g extract.
Moreover, the α-acid isomerization kinetics were investigated using a small-scale experimental system and compared with those related to sc-CO2 extracts and traditional hop pellets added to the wort during boiling. HFC extract provided the highest production rate of iso-α-acids, with a final concentration of 47.550 ± 0.637 mg/L, reached after 35 min of the boiling process.
Moreover, both sc-CO2 and HFC extracts allowed the achievement of the maximum iso-α-acids amount reached using pellets in shorter process times, approximately 34 min and 25 min, respectively, than those commonly used with the traditional process (50 min).
When HFC extracts were implemented in the pilot-scale process, lower iso-α-acid concentrations than those measured in the lab-scale samples were observed, probably due to structural differences between the laboratory and pilot experimental systems and a subsequent less efficient agitation in the pilot vessel. Nevertheless, the isomerization yield obtained in 50 min using the traditional procedure was achieved by HFC extracts after 35 min, confirming the possibility of considerably reducing the conventional treatment times used during brewing processes.