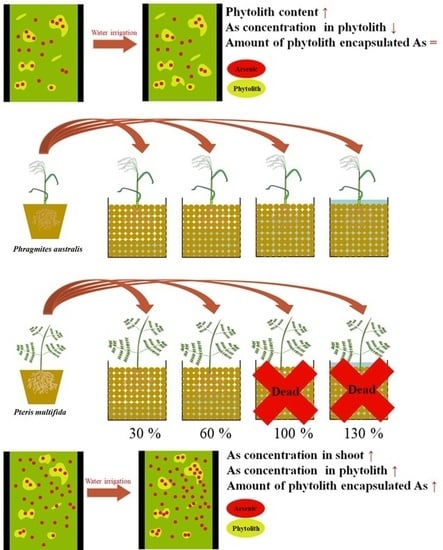
Effect of Soil Water Contents on Arsenic Accumulation in Phytoliths of Pteris multifida and Phragmites australis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Analysis
2.2.1. Soil Analysis
2.2.2. Plant Analysis
2.2.3. Phytolith Extraction and Analysis
2.2.4. Arsenic Accumulation Ratio
2.3. Statistical Analysis
3. Results
3.1. Soil Characteristics
3.2. Plant Water Condition and Phytoliths
3.3. Concentration and Amount of As
3.4. Accumulation of As from Soil to Phytolith
4. Discussion
4.1. Soil Characteristics
4.2. Plant Water Condition and Phytolith
4.3. Concentration of As in Plant
4.4. Amount of As in Plant
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. J. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, R.D.; Singh, R.P.; Dwivedi, S.; Goutam, D.; Shri, M.; Trivedi, P.K.; Chakrabarty, D. Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol. Eng. 2013, 52, 96–103. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, V.; Kumar, R. Distribution of phytoliths in plants: A review. Geol. Ecol. Landsc. 2019, 3, 123–148. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Liu, H.; Strömberg, C.A.; Yang, X.; Zhang, X. Phytolith carbon sequestration in global terrestrial biomes. Sci. Total Environ. 2017, 603, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Delplace, G.; Schreck, E.; Pokrovsky, O.S.; Zouiten, C.; Blondet, I.; Darrozes, J.; Viers, J. Accumulation of heavy metals in phytoliths from reeds growing on mining environments in Southern Europe. Sci. Total Environ. 2020, 712, 135595. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, Y.; Jin, Q.; Li, F. Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ. Sci. Nano 2020, 7, 162–171. [Google Scholar] [CrossRef]
- Fernandes-Horn, H.M.; Sampaio, R.A.; Horn, A.H.; de Oliveira, E.S.A.; Lepsch, I.F.; Bilal, E. Use of Si-Phytoliths in depollution of mining areas in the Cerrado-Caatinga region, MG, Brazil. Int. J. Geomate 2016, 11, 2216–2221. [Google Scholar]
- Nguyen, T.N.; Nguyen, M.N.; McNamara, M.; Dultz, S.; Meharg, A.; Nguyen, V.T. Encapsulation of lead in rice phytoliths as a possible pollutant source in paddy soils. Environ. Exp. Bot. 2019, 162, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.N.; Dam, T.T.N.; Nguyen, A.T.Q.; Nguyen, A.M.; Nguyen, L.N.; Duong, L.T.; Dang, Q.T.; Tran, T.T. Arsenic in rice straw phytoliths: Encapsulation and release properties. Appl. Geochem. 2021, 127, 104907. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Nguyen, A.T.; Dultz, S.; Tsubota, T.; Duong, L.T.; Nguyen, A.M.; Pham, N.T. Thermal induced changes of rice straw phytolith in relation to arsenic release: A perspective of rice straw arsenic under open burning. J. Environ. Manag. 2022, 304, 114294. [Google Scholar] [CrossRef] [PubMed]
- de Melo Farnezi, M.M.; de Barros Silva, E.; Lopes Dos Santos, L.; Silva, A.C.; Grazziotti, P.H.; Prochnow, J.T.; Pereira, I.M.; Fontan, I.D.C.I. Potential of grasses in phytolith production in soils contaminated with cadmium. Plants 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.; Li, Z.; Zou, B.; Peng, S. Pteris multifida Poir., a new arsenic hyperaccumulator: Characteristics and potential. Int. J. Environ. Pollut. 2005, 23, 388–396. [Google Scholar] [CrossRef]
- Han, J.H.; Kwon, H.J.; Lee, C.H. Effect of Arsenic Types in Soil on Growth and Arsenic Accumulation of Pteris multifida. Korean J. Plant Resour. 2014, 27, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Seo, J.Y.; Oh, W.K.; Sung, S.H. Anti-neuroinflammatory ent-kaurane diterpenoids from Pteris multifida roots. Molecules 2017, 22, 27. [Google Scholar] [CrossRef] [Green Version]
- Sundue, M. Silica bodies and their systematic implications in Pteridaceae (Pteridophyta). Bot. J. Linn. Soc. 2009, 161, 422–435. [Google Scholar] [CrossRef]
- Madella, M.; Jones, M.K.; Echlin, P.; Powers-Jones, A.; Moore, M. Plant water availability and analytical microscopy of phytoliths: Implications for ancient irrigation in arid zones. Quat. Int. 2009, 193, 32–40. [Google Scholar] [CrossRef]
- Rosen, A.M.; Weiner, S. Identifying ancient irrigation: A new method using opaline phytoliths from emmer wheat. J. Archaeol. Sci. 1994, 21, 125–132. [Google Scholar] [CrossRef]
- Wan, X.M.; Lei, M.; Chen, T.B.; Yang, J.X.; Liu, H.T.; Chen, Y. Role of transpiration in arsenic accumulation of hyperaccumulator Pteris vittata L. Environ. Sci. Pollut. Res. 2015, 22, 16631–16639. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Maah, M.J.; Yusoff, I. Evaluation of natural phytoremediation process occurring at ex-tin mining catchment. Chiang Mai J. Sci. 2013, 40, 198–213. [Google Scholar]
- Praveen, A.; Mehrotra, S.; Singh, N. Rice planted along with accumulators in arsenic amended plots reduced arsenic uptake in grains and shoots. Chemosphere 2017, 184, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Praveen, A.; Mehrotra, S.; Singh, N. Mixed plantation of wheat and accumulators in arsenic contaminated plots: A novel way to reduce the uptake of arsenic in wheat and load on antioxidative defence of plant. Ecotoxicol. Environ. Saf. 2019, 182, 109462. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Jamil, S.; Srivastava, P.K.; Tripathi, R.D.; Sharma, Y.K.; Singh, N. Feasibility Study of Phragmites karka and Christella dentata Grown in West Bengal as Arsenic Accumulator. Int. J. Phytoremediat. 2015, 17, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, S.H.; Kim, J.G. Assessment of fraction and mobility of arsenic in soil near the mine Waste Dam. Sustainability 2020, 12, 1480. [Google Scholar] [CrossRef] [Green Version]
- Madyiwa, S.; Chimbari, M.J.; Schutte, F. Lead and cadmium interactions in Cynodon nlemfuensis and sandy soil subjected to treated wastewater application under greenhouse conditions. Phys. Chem. Earth 2004, 29, 1043–1048. [Google Scholar] [CrossRef]
- Vengadaramana, A.; Jashothan, P.T.J. Effect of organic fertilizers on the water holding capacity of soil in different terrains of Jaffna peninsula in Sri Lanka. J. Nat. Prod. Plant Resour. 2012, 2, 500–503. [Google Scholar]
- NIAST. Method of SOIL and Plant Analysis; National Institute of Agricultural Science and Technology, Rural Development Administration: Suwon, Korea, 2000.
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Part 3 Chemical Methods, 5.3.; Wiley: New York, NY, USA, 1996; pp. 961–1010. [Google Scholar]
- Imaizumi, K.; Yoshida, S. Edaphological studies on silicon supplying power of paddy fields. Bull. Natl. Inst. Agric. Sci. 1958, 8, 261–304. [Google Scholar]
- Tighe, M.; Lockwood, P.; Wilson, S.; Lisle, L. Comparison of digestion methods for ICP-OES analysis of a wide range of analytes in heavy metal contaminated soil samples with specific reference to arsenic and antimony. Commun. Soil Sci. Plant Anal. 2004, 35, 1369–1385. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Gulz, P.A.; Gupta, S.K.; Schulin, R. Arsenic accumulation of common plants from contaminated soils. Plant Soil 2005, 272, 337–347. [Google Scholar] [CrossRef]
- Li, Z.; Song, Z.; Cornelis, J.T. Impact of rice cultivar and organ on elemental composition of phytoliths and the release of bio-available silicon. Front. Plant Sci. 2014, 5, 529. [Google Scholar] [CrossRef] [PubMed]
- Parr, J.F.; Lentfer, C.J.; Boyd, W.E. A comparative analysis of wet and dry ashing techniques for the extraction of phytoliths from plant material. J. Archaeol. Sci. 2001, 28, 875–886. [Google Scholar] [CrossRef]
- Onken, B.M.; Adriano, D.C. Arsenic Availability in Soil with Time under Saturated and Subsaturated Conditions. Soil Sci. Soc. Am. J. 1997, 61, 746–752. [Google Scholar] [CrossRef]
- Ascar, L.; Ahumada, I.; Richter, P. Influence of redox potential (Eh) on the availability of arsenic species in soils and soils amended with biosolid. Chemosphere 2008, 72, 1548–1552. [Google Scholar] [CrossRef]
- Meharg, A.A. Arsenic in rice–understanding a new disaster for South-East Asia. Trends Plant Sci. 2004, 9, 415–417. [Google Scholar] [CrossRef]
- Meharg, A.A.; Jardine, L. Arsenite transport into paddy rice (Oryza sativa) roots. New Phytol. 2003, 157, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syu, C.H.; Huang, C.C.; Jiang, P.Y.; Lee, C.H.; Lee, D.Y. Arsenic accumulation and speciation in rice grains influenced by arsenic phytotoxicity and rice genotypes grown in arsenic-elevated paddy soils. J. Hazard. Mater. 2015, 286, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef]
- Teuling, A.J.; Uijlenhoet, R.; Hupert, F.; Troch, P.A. Impact of plant water uptake strategy on soil moisture and evapotranspiration dynamics during drydown. Geophys. Res. Lett. 2006, 33, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Katz, O.; Lev-Yadun, S.; Pua Bar, K. Plasticity and variability in the patterns of phytolith formation in Asteraceae species along a large rainfall gradient in Israel. Flora Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 438–444. [Google Scholar] [CrossRef]
- Webb, E.A.; Longstaffe, F.J. The relationship between phytolith- and plant-water δ 18O values in grasses. Geochim. Cosmochim. Acta 2003, 67, 1437–1449. [Google Scholar] [CrossRef]
- Jenkins, E.; Jamjoum, K.; Nuimat, S.; Stafford, R.; Nortcliff, S.; Mithen, S. Identifying ancient water availability through phytolith analysis: An experimental approach. J. Archaeol. Sci. 2016, 73, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Meunier, J.D.; Barboni, D.; Anwar-ul-Haq, M.; Levard, C.; Chaurand, P.; Vidal, V.; Grauby, O.; Huc, R.; Laffont-Schwob, I.; Rabier, J.; et al. Effect of phytoliths for mitigating water stress in durum wheat. New Phytol. 2017, 215, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Ghassemzadeh, F.; Yousefzadeh, H.; Arbab-Zavar, M.H. Arsenic phytoremediation by Phragmites australis: Green technology. Int. J. Environ. Stud. 2008, 65, 587–594. [Google Scholar] [CrossRef]
- Ghassemzadeh, F.; Yousefzadeh, H.; Arbab-Zavar, M.H. Removing arsenic and antimony by Phragmites australis: Rhizofiltration technology. J. Appl. Sci. 2008, 8, 1668–1675. [Google Scholar] [CrossRef] [Green Version]
- Štrbac, S.; Šajnović, A.; Kašanin, G.M.; Vasić, N.; Dojčinović, B.; Simonović, P.; Jovančićević, B. Metals in sediment and phragmites Australis (common reed) from tisza river, Serbia. Appl. Ecol. Environ. Res. 2014, 12, 105–122. [Google Scholar] [CrossRef]
- Castaldi, P.; Silvetti, M.; Manzano, R.; Brundu, G.; Roggero, P.P.; Garau, G. Mutual effect of Phragmites australis, Arundo donax and immobilization agents on arsenic and trace metals phytostabilization in polluted soils. Geoderma 2018, 314, 63–72. [Google Scholar] [CrossRef]
- Lee, S.H.; Ji, W.; Lee, W.S.; Koo, N.; Koh, I.H.; Kim, M.S.; Park, J.S. Influence of amendments and aided phytostabilization on metal availability and mobility in Pb, Zn mine tailings. J. Environ. Manag. 2014, 139, 15–21. [Google Scholar] [CrossRef]
- Prica, M.; Andrejić, G.; Šinžar-Sekulić, J.; Rakić, T.; Dželetović, Ž. Bioaccumulation of heavy metals in common reed (Phragmites australis) growing spontaneously on highly contaminated mine tailing ponds in Serbia and potential use of this species in phytoremediation. Bot. Serb. 2019, 43, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Gardner, W.R.; Ehlig, C.F. The influence of soil water on transpiration by plants. J. Geophys. Res. 1963, 68, 5719–5724. [Google Scholar] [CrossRef]
- Sakamaki, Y.; Ino, Y. Tubers and rhizome fragments as propagules: Competence for vegetative reproduction in Equisetum arvense. J. Plant Res. 2006, 119, 677–683. [Google Scholar] [CrossRef] [PubMed]



| Treatments | Results | ||||||
|---|---|---|---|---|---|---|---|
| Plant Species | Soil Water Content a | Soil Available As b (mg kg−1) | Soil Available Si c (mg kg−1) | Soil pH | |||
| Control d | 237.45 | d | 203.43 | c | 8.51 | c | |
| Pteris multifida | 30% | 256.03 | bc | 220.50 | abc | 8.63 | abc |
| 60% | 258.77 | b | 213.64 | bc | 8.58 | bc | |
| Phragmites australis | 30% | 249.86 | c | 228.76 | ab | 8.71 | a |
| 60% | 255.88 | bc | 233.74 | a | 8.63 | abc | |
| 100% | 284.58 | a | 223.59 | ab | 8.67 | ab | |
| 130% | 280.67 | a | 216.93 | abc | 8.58 | bc | |
| Treatments | Results | ||||||
|---|---|---|---|---|---|---|---|
| Plant Species | Soil Water Content a | Phytolith Content (mg kg−1) | Water Content in Plant Shoot (%) | Water Potential in Plant Leaf (bar) | Water Content in Plant Root (%) | ||
| Pteris multifida | 30% | 114,157.51 a | 64.41 a | −11.47 | cd | 70.49 | c |
| 60% | 104,516.49 a | 69.78 a | −7.00 | a | 72.06 | c | |
| Phragmites australis | 30% | 58,642.31 b | 69.61 a | −13.60 | d | 79.36 | b |
| 60% | 137,370.92 a | 67.78 a | −10.73 | c | 84.32 | a | |
| 100% | 116,530.55 a | 68.28 a | −10.40 | bc | 85.08 | a | |
| 130% | 103,573.27 a | 69.15 a | −8.87 | ab | 85.78 | a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, H.-G.; Kim, M.-S.; Kim, J.-G. Effect of Soil Water Contents on Arsenic Accumulation in Phytoliths of Pteris multifida and Phragmites australis. Appl. Sci. 2022, 12, 12518. https://0-doi-org.brum.beds.ac.uk/10.3390/app122412518
Min H-G, Kim M-S, Kim J-G. Effect of Soil Water Contents on Arsenic Accumulation in Phytoliths of Pteris multifida and Phragmites australis. Applied Sciences. 2022; 12(24):12518. https://0-doi-org.brum.beds.ac.uk/10.3390/app122412518
Chicago/Turabian StyleMin, Hyun-Gi, Min-Suk Kim, and Jeong-Gyu Kim. 2022. "Effect of Soil Water Contents on Arsenic Accumulation in Phytoliths of Pteris multifida and Phragmites australis" Applied Sciences 12, no. 24: 12518. https://0-doi-org.brum.beds.ac.uk/10.3390/app122412518