Rich Dynamics of a General Producer–Grazer Interaction Model under Shared Multiple Resource Limitations
Abstract
:1. Introduction
2. Methods
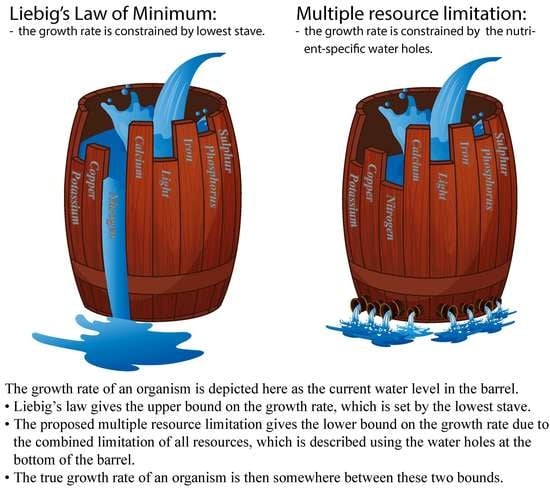
2.1. Formulation of the Multiple Resource Limitation
2.2. Producer–Grazer Model Formulation
- The producer–grazer system is closed. This means the total amounts of phosphorus and carbon are conserved at all times. Furthermore, P and C are present either in the grazer or in the producer (i.e., there are no free pools of P or C).
- The minimum P:C ratio for the producer is q.
- The P:C ratio of the grazer is fixed at .
- The grazer dies at a constant rate, d.
2.3. The LKE and the MRL Models
3. Results
3.1. Basic Biological Properties
3.2. Model Simulation and Comparison
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LLM | Liebig’s law of the minimum |
| MRL | Multiple resource limitation |
| C | Carbon |
| N | Nitrogen |
| P | Phosphorus |
Appendix A. Bifurcation for All Parameters
References
- Von Liebig, J. Die Organische Chemie in Ihrer Anwendung auf Agricultur und Physiologie; Taylor and Walton: London, UK, 1841. [Google Scholar]
- Gorban, A.N.; Pokidysheva, L.I.; Smirnova, E.V.; Tyukina, T.A. Law of the minimum paradoxes. Bull. Math. Biol. 2011, 73, 2013–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyasingh, P.D.; Sherman, R.E.; Prater, C.; Pulkkinen, K.; Ketola, T. Adaptation to a limiting element involves mitigation of multiple elemental imbalances. J. R. Soc. Interface 2023, 20, 20220472. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P.; Da Silva, J.F. The Chemistry of Evolution: The Development of Our Ecosystem; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Saito, M.A.; Goepfert, T.J.; Ritt, J.T. Some thoughts on the concept of colimitation: Three definitions and the importance of bioavailability. Limnol. Oceanogr. 2008, 53, 276–290. [Google Scholar] [CrossRef] [Green Version]
- Pahlow, M.; Oschlies, A. Chain model of phytoplankton P, N and light colimitation. Mar. Ecol. Prog. Ser. 2009, 376, 69–83. [Google Scholar] [CrossRef]
- Sperfeld, E.; Martin-Creuzburg, D.; Wacker, A. Multiple resource limitation theory applied to herbivorous consumers: Liebig’s minimum rule vs. interactive co-limitation. Ecol. Lett. 2012, 15, 142–150. [Google Scholar] [CrossRef]
- Sperfeld, E.; Raubenheimer, D.; Wacker, A. Bridging factorial and gradient concepts of resource co-limitation: Towards a general framework applied to consumers. Ecol. Lett. 2016, 19, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Cherif, M.; Loreau, M. Towards a more biologically realistic use of Droop’s equations to model growth under multiple nutrient limitation. Oikos 2010, 119, 897–907. [Google Scholar] [CrossRef]
- Loladze, I.; Kuang, Y.; Elser, J.J. Stoichiometry in producer–grazer systems: Linking energy flow with element cycling. Bull. Math. Biol. 2000, 62, 1137–1162. [Google Scholar] [CrossRef]
- Elser, J.J.; Loladze, I.; Peace, A.L.; Kuang, Y. Lotka re-loaded: Modeling trophic interactions under stoichiometric constraints. Ecol. Model. 2012, 245, 3–11. [Google Scholar] [CrossRef]
- Peace, A.; Zhao, Y.; Loladze, I.; Elser, J.J.; Kuang, Y. A stoichiometric producer–grazer model incorporating the effects of excess food-nutrient content on consumer dynamics. Math. Biosci. 2013, 244, 107–115. [Google Scholar] [CrossRef]
- Yamamichi, M.; Meunier, C.L.; Peace, A.; Prater, C.; Rúa, M.A. Rapid evolution of a consumer stoichiometric trait destabilizes consumer–producer dynamics. Oikos 2015, 124, 960–969. [Google Scholar] [CrossRef]
- Isanta-Navarro, J.; Prater, C.; Peoples, L.M.; Loladze, I.; Phan, T.; Jeyasingh, P.D.; Church, M.J.; Kuang, Y.; Elser, J.J. Revisiting the growth rate hypothesis: Towards a holistic stoichiometric understanding of growth. Ecol. Lett. 2022, 25, 2324–2339. [Google Scholar] [CrossRef]
- Li, S.H.J.; Li, Z.; Park, J.O.; King, C.G.; Rabinowitz, J.D.; Wingreen, N.S.; Gitai, Z. Escherichia coli translation strategies differ across carbon, nitrogen and phosphorus limitation conditions. Nat. Microbiol. 2018, 3, 939–947. [Google Scholar] [CrossRef]
- Iyer, S.; Le, D.; Park, B.R.; Kim, M. Distinct mechanisms coordinate transcription and translation under carbon and nitrogen starvation in Escherichia coli. Nat. Microbiol. 2018, 3, 741–748. [Google Scholar] [CrossRef]
- Phan, T.; He, C.; Loladze, I.; Prater, C.; Elser, J.; Kuang, Y. Dynamics and growth rate implications of ribosomes and mRNAs interaction in E. coli. Heliyon 2022, 8, e09820. [Google Scholar] [CrossRef]
- Borer, E.T.; Paseka, R.E.; Peace, A.; Asik, L.; Everett, R.; Frenken, T.; González, A.L.; Strauss, A.T.; Van de Waal, D.B.; White, L.A.; et al. Disease-mediated nutrient dynamics: Coupling host–pathogen interactions with ecosystem elements and energy. Ecol. Monogr. 2022, 92, e1510. [Google Scholar] [CrossRef]
- Phan, T.; Pell, B.; Kendig, A.E.; Borer, E.T.; Kuang, Y. Rich dynamics of a simple delay host-pathogen model of cell-to-cell infection for plant virus. Discret. Contin. Dyn. Syst. B 2021, 26, 515. [Google Scholar] [CrossRef]
- Kendig, A.E.; Borer, E.T.; Boak, E.N.; Picard, T.C.; Seabloom, E.W. Host nutrition mediates interactions between plant viruses, altering transmission and predicted disease spread. Ecology 2020, 101, e03155. [Google Scholar] [CrossRef]
- Pell, B.; Kendig, A.E.; Borer, E.T.; Kuang, Y. Modeling nutrient and disease dynamics in a plant-pathogen system. Math. Biosci. Eng. 2019, 16, 234–264. [Google Scholar] [CrossRef]
- Peace, A.; Wang, H.; Kuang, Y. Dynamics of a producer–grazer model incorporating the effects of excess food nutrient content on grazer’s growth. Bull. Math. Biol. 2014, 76, 2175–2197. [Google Scholar] [CrossRef]
- Kong, J.D.; Salceanu, P.; Wang, H. A stoichiometric organic matter decomposition model in a chemostat culture. J. Math. Biol. 2018, 76, 609–644. [Google Scholar] [CrossRef] [PubMed]
- Peace, A.; Wang, H. Compensatory foraging in stoichiometric producer–grazer models. Bull. Math. Biol. 2019, 81, 4932–4950. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wang, H. Competitive Exclusion and Coexistence in a Stoichiometric Chemostat Model. J. Dyn. Differ. Equ. 2022, 1–33. [Google Scholar] [CrossRef]
- Droop, M. The nutrient status of algal cells in continuous culture. J. Mar. Biol. Assoc. UK 1974, 54, 825–855. [Google Scholar] [CrossRef]
- Wang, H.; Garcia, P.V.; Ahmed, S.; Heggerud, C.M. Mathematical comparison and empirical review of the Monod and Droop forms for resource-based population dynamics. Ecol. Model. 2022, 466, 109887. [Google Scholar] [CrossRef]
- Pahlow, M.; Oschlies, A. Optimal allocation backs Droop’s cell-quota model. Mar. Ecol. Prog. Ser. 2013, 473, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Loladze, I. Iterative chemostat: A modelling framework linking biosynthesis to nutrient cycling on ecological and evolutionary time scales. Math. Biosci. Eng. 2019, 16, 990–1004. [Google Scholar] [CrossRef]
- Loladze, I.; Elser, J.J. The origins of the Redfield nitrogen-to-phosphorus ratio are in a homoeostatic protein-to-rRNA ratio. Ecol. Lett. 2011, 14, 244–250. [Google Scholar] [CrossRef]
- Kafri, M.; Metzl-Raz, E.; Jonas, F.; Barkai, N. Rethinking cell growth models. FEMS Yeast Res. 2016, 16, fow081. [Google Scholar] [CrossRef] [Green Version]
- Sterner, R.W.; Elser, J.J. Ecological stoichiometry. In Ecological Stoichiometry; Princeton University Press: Princeton, NJ, USA, 2017. [Google Scholar]
- Scott, M.; Gunderson, C.W.; Mateescu, E.M.; Zhang, Z.; Hwa, T. Interdependence of cell growth and gene expression: Origins and consequences. Science 2010, 330, 1099–1102. [Google Scholar] [CrossRef]
- Hsu, S.B.; Hwang, T.W.; Kuang, Y. Global analysis of the Michaelis–Menten-type ratio-dependent predator–prey system. J. Math. Biol. 2001, 42, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Gourley, S.A.; Kuang, Y. A stage structured predator–prey model and its dependence on maturation delay and death rate. J. Math. Biol. 2004, 49, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhao, Y.; Sang, B. Four limit cycles in a predator–prey system of Leslie type with generalized Holling type III functional response. Nonlinear Anal. Real World Appl. 2019, 50, 218–239. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Nagy, J.D.; Gilg, O.; Kuang, Y. The roles of predator maturation delay and functional response in determining the periodicity of predator–prey cycles. Math. Biosci. 2009, 221, 1–10. [Google Scholar] [CrossRef]
- Wu, C.; Balakrishnan, R.; Braniff, N.; Mori, M.; Manzanarez, G.; Zhang, Z.; Hwa, T. Cellular perception of growth rate and the mechanistic origin of bacterial growth law. Proc. Natl. Acad. Sci. USA 2022, 119, e2201585119. [Google Scholar] [CrossRef]
- Li, Z.; Liu, B.; Li, S.H.J.; King, C.G.; Gitai, Z.; Wingreen, N.S. Modeling microbial metabolic trade-offs in a chemostat. PLoS Comput. Biol. 2020, 16, e1008156. [Google Scholar] [CrossRef]
- Basan, M.; Honda, T.; Christodoulou, D.; Hörl, M.; Chang, Y.F.; Leoncini, E.; Mukherjee, A.; Okano, H.; Taylor, B.R.; Silverman, J.M.; et al. A universal trade-off between growth and lag in fluctuating environments. Nature 2020, 584, 470–474. [Google Scholar] [CrossRef]
- Guignard, M.S.; Leitch, A.R.; Acquisti, C.; Eizaguirre, C.; Elser, J.J.; Hessen, D.O.; Jeyasingh, P.D.; Neiman, M.; Richardson, A.E.; Soltis, P.S.; et al. Impacts of nitrogen and phosphorus: From genomes to natural ecosystems and agriculture. Front. Ecol. Evol. 2017, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Klausmeier, C.A.; Litchman, E.; Levin, S.A. Phytoplankton growth and stoichiometry under multiple nutrient limitation. Limnol. Oceanogr. 2004, 49, 1463–1470. [Google Scholar] [CrossRef] [Green Version]
- Packer, A.; Li, Y.; Andersen, T.; Hu, Q.; Kuang, Y.; Sommerfeld, M. Growth and neutral lipid synthesis in green microalgae: A mathematical model. Bioresour. Technol. 2011, 102, 111–117. [Google Scholar] [CrossRef]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef]
- Marquet, P.A.; Allen, A.P.; Brown, J.H.; Dunne, J.A.; Enquist, B.J.; Gillooly, J.F.; Gowaty, P.A.; Green, J.L.; Harte, J.; Hubbell, S.P.; et al. On theory in ecology. BioScience 2014, 64, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Kobayashi, K.; Loladze, I.; Zhu, J.; Jiang, Q.; Xu, X.; Liu, G.; Seneweera, S.; Ebi, K.L.; Drewnowski, A.; et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018, 4, eaaq1012. [Google Scholar] [CrossRef] [Green Version]
- Curtsdotter, A.; Banks, H.T.; Banks, J.E.; Jonsson, M.; Jonsson, T.; Laubmeier, A.N.; Traugott, M.; Bommarco, R. Ecosystem function in predator–prey food webs—Confronting dynamic models with empirical data. J. Anim. Ecol. 2019, 88, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Heggerud, C.M.; Wang, H.; Lewis, M.A. Transient dynamics of a stoichiometric cyanobacteria model via multiple-scale analysis. SIAM J. Appl. Math. 2020, 80, 1223–1246. [Google Scholar] [CrossRef]
- Peace, A.; Frost, P.C.; Wagner, N.D.; Danger, M.; Accolla, C.; Antczak, P.; Brooks, B.W.; Costello, D.M.; Everett, R.A.; Flores, K.B.; et al. Stoichiometric ecotoxicology for a multisubstance world. BioScience 2021, 71, 132–147. [Google Scholar] [CrossRef]
- Feng, Z.; DeAngelis, D.L. Mathematical Models of Plant-Herbivore Interactions; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Peace, A.; Poteat, M.D.; Wang, H. Somatic growth dilution of a toxicant in a predator–prey model under stoichiometric constraints. J. Theor. Biol. 2016, 407, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Allgeier, J.E.; Rosemond, A.D.; Layman, C.A. The frequency and magnitude of non-additive responses to multiple nutrient enrichment. J. Appl. Ecol. 2011, 48, 96–101. [Google Scholar] [CrossRef]
- Harpole, W.S.; Ngai, J.T.; Cleland, E.E.; Seabloom, E.W.; Borer, E.T.; Bracken, M.E.; Elser, J.J.; Gruner, D.S.; Hillebrand, H.; Shurin, J.B.; et al. Nutrient co-limitation of primary producer communities. Ecol. Lett. 2011, 14, 852–862. [Google Scholar] [CrossRef]
- Prater, C.; Bullard, J.E.; Osburn, C.L.; Martin, S.L.; Watts, M.J.; Anderson, N.J. Landscape controls on nutrient stoichiometry regulate lake primary production at the margin of the Greenland Ice Sheet. Ecosystems 2022, 25, 931–947. [Google Scholar] [CrossRef]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.; Mayntz, D.; Toft, S.; Clissold, F.J.; Hunt, J.; Raubenheimer, D.; Simpson, S.J. Optimal foraging for specific nutrients in predatory beetles. Proc. R. Soc. B Biol. Sci. 2012, 279, 2212–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacker, A.; Martin-Creuzburg, D. Biochemical nutrient requirements of the rotifer B rachionus calyciflorus: Co-limitation by sterols and amino acids. Funct. Ecol. 2012, 26, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.J.; Peace, A.L.; Kyle, M.; Wojewodzic, M.; McCrackin, M.L.; Andersen, T.; Hessen, D.O. Atmospheric nitrogen deposition is associated with elevated phosphorus limitation of lake zooplankton. Ecol. Lett. 2010, 13, 1256–1261. [Google Scholar] [CrossRef]
- Prater, C.; Wagner, N.D.; Frost, P.C. Effects of calcium and phosphorus limitation on the nutritional ecophysiology of D aphnia. Limnol. Oceanogr. 2016, 61, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Halvorson, H.M.; Sperfeld, E.; Evans-White, M.A. Quantity and quality limit detritivore growth: Mechanisms revealed by ecological stoichiometry and co-limitation theory. Ecology 2017, 98, 2995–3002. [Google Scholar] [CrossRef] [Green Version]
- Prater, C.; Wagner, N.D.; Frost, P.C. Seasonal effects of food quality and temperature on body stoichiometry, biochemistry, and biomass production in Daphnia populations. Limnol. Oceanogr. 2018, 63, 1727–1740. [Google Scholar] [CrossRef] [Green Version]
- Isanta Navarro, J.; Fromherz, M.; Dietz, M.; Zeis, B.; Schwarzenberger, A.; Martin-Creuzburg, D. Dietary polyunsaturated fatty acid supply improves Daphnia performance at fluctuating temperatures, simulating diel vertical migration. Freshw. Biol. 2019, 64, 1859–1866. [Google Scholar] [CrossRef]
- Isanta-Navarro, J.; Arnott, S.E.; Klauschies, T.; Martin-Creuzburg, D. Dietary lipid quality mediates salt tolerance of a freshwater keystone herbivore. Sci. Total. Environ. 2021, 769, 144657. [Google Scholar] [CrossRef]
- Laspoumaderes, C.; Meunier, C.L.; Magnin, A.; Berlinghof, J.; Elser, J.J.; Balseiro, E.; Torres, G.; Modenutti, B.; Tremblay, N.; Boersma, M. A common temperature dependence of nutritional demands in ectotherms. Ecol. Lett. 2022, 25, 2189–2202. [Google Scholar] [CrossRef]
- Jeyasingh, P.D.; Goos, J.M.; Thompson, S.K.; Godwin, C.M.; Cotner, J.B. Ecological stoichiometry beyond redfield: An ionomic perspective on elemental homeostasis. Front. Microbiol. 2017, 8, 722. [Google Scholar] [CrossRef] [Green Version]
- Jeyasingh, P.D.; Goos, J.M.; Lind, P.R.; Roy Chowdhury, P.; Sherman, R.E. Phosphorus supply shifts the quotas of multiple elements in algae and Daphnia: Ionomic basis of stoichiometric constraints. Ecol. Lett. 2020, 23, 1064–1072. [Google Scholar] [CrossRef]
- Ipek, Y.; Jeyasingh, P.D. Growth and ionomic responses of a freshwater cyanobacterium to supplies of nitrogen and iron. Harmful Algae 2021, 108, 102078. [Google Scholar] [CrossRef]
- Laubmeier, A.N.; Rebarber, R.; Tenhumberg, B. Towards understanding factors influencing the benefit of diversity in predator communities for prey suppression. Ecosphere 2020, 11, e03271. [Google Scholar] [CrossRef]
- Wootton, K.L.; Curtsdotter, A.; Jonsson, T.; Banks, H.; Bommarco, R.; Roslin, T.; Laubmeier, A.N. Beyond body size—New traits for new heights in trait-based modelling of predator–prey dynamics. PLoS ONE 2022, 17, e0251896. [Google Scholar] [CrossRef]
- Kuang, Y. Delay Differential Equations: With Applications in Population Dynamics; Academic Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Murray, J.D. Mathematical Biology II: Spatial Models and Biomedical Applications; Springer: New York, NY, USA, 2001; Volume 3. [Google Scholar]
- Kang, Y.; Sasmal, S.K.; Messan, K. A two-patch prey-predator model with predator dispersal driven by the predation strength. Math. Biosci. Eng. 2017, 14, 843. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.B.; Hwang, T.W.; Kuang, Y. Rich dynamics of a ratio-dependent one-prey two-predators model. J. Math. Biol. 2001, 43, 377–396. [Google Scholar] [CrossRef]
- Hsu, S.B.; Hwang, T.W.; Kuang, Y. A ratio-dependent food chain model and its applications to biological control. Math. Biosci. 2003, 181, 55–83. [Google Scholar] [CrossRef]
- Dickman, E.M.; Newell, J.M.; González, M.J.; Vanni, M.J. Light, nutrients, and food-chain length constrain planktonic energy transfer efficiency across multiple trophic levels. Proc. Natl. Acad. Sci. USA 2008, 105, 18408–18412. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.B.; Wang, F.B.; Zhao, X.Q. Competition for two essential resources with internal storage and periodic input. Differ. Integral Equ. 2016, 29, 601–630. [Google Scholar] [CrossRef]
- Ji, J.; Milne, R.; Wang, H. Stoichiometry and environmental change drive dynamical complexity and unpredictable switches in an intraguild predation model. J. Math. Biol. 2023, 86, 31. [Google Scholar] [CrossRef] [PubMed]
- Peace, A. Effects of light, nutrients, and food chain length on trophic efficiencies in simple stoichiometric aquatic food chain models. Ecol. Model. 2015, 312, 125–135. [Google Scholar] [CrossRef]
- Boersma, M.; Elser, J.J. Too much of a good thing: On stoichiometrically balanced diets and maximal growth. Ecology 2006, 87, 1325–1330. [Google Scholar] [CrossRef]





| Parameter | Value | Unit | |
|---|---|---|---|
| P | Total phosphorus | 0.025 | mg P L |
| Overall maximal growth rate | 1.2 | day | |
| K | Producer carrying capacity (light limited) | 0.5 | mg C L |
| q | Producer minimal P/C | 0.0038 | (mg P)/(mg C) |
| grazer constant P/C | 0.03 | (mg P)/(mg C) | |
| Theoretical maximum efficiency of biomass production | 0.8 | ||
| a | Maximum efficiency of biomass production (energy) | 8 | |
| m | Half-saturation of biomass production efficiency | 200 | (mg C)/(mg P) |
| c | Maximum ingestion rate of the grazer | 0.81 | day |
| k | Half-saturation of grazer ingestion response | 0.25 | mg C L |
| d | Grazer loss rate | 0.25 | day |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, T.; Elser, J.J.; Kuang, Y. Rich Dynamics of a General Producer–Grazer Interaction Model under Shared Multiple Resource Limitations. Appl. Sci. 2023, 13, 4150. https://0-doi-org.brum.beds.ac.uk/10.3390/app13074150
Phan T, Elser JJ, Kuang Y. Rich Dynamics of a General Producer–Grazer Interaction Model under Shared Multiple Resource Limitations. Applied Sciences. 2023; 13(7):4150. https://0-doi-org.brum.beds.ac.uk/10.3390/app13074150
Chicago/Turabian StylePhan, Tin, James J. Elser, and Yang Kuang. 2023. "Rich Dynamics of a General Producer–Grazer Interaction Model under Shared Multiple Resource Limitations" Applied Sciences 13, no. 7: 4150. https://0-doi-org.brum.beds.ac.uk/10.3390/app13074150