1. Introduction
The continuous complementary metal-oxide-semiconductor (CMOS) scaling is reaching the fundamental limits imposed by heat dissipation and short-channel effects. This will finally stop the increase of integration density and the ability of metal-oxide-semiconductor field-effect transistors (MOSFET) predicted by Moore’s law [
1,
2,
3,
4]. Huge demand has been thus created for high-performance semiconductor devices for future applications in memory and emerging portable/wearable electronic devices. Molecular technology has been aggressively pursued for its potential impact in nano-scale electronics since the early 1970s due to the inherent scalability and intrinsic properties [
5,
6,
7]. Molecular memory, among all of the new emerging candidates in recent years, is considered promising, particularly for the aim of reducing the size per cell and enhancing the memory speed, density and reliability [
8,
9,
10]. Molecular electronic devices are typically fabricated by forming a self-assembled monolayer (SAM) or multi-layer on different surfaces with very inexpensive and simple processing methods. Moreover, molecular memory works by the controlling of fewer electrons at the molecule scale and, therefore, has potential for low-power and ultra-high-density memory applications with a lower fabrication cost. Redox-active molecules have become attractive to replace the floating gate in conventional flash memory, due to their inherent and reliable redox behaviors. Typically, applying an oxidation voltage will cause electron loss in redox molecules; reversely, under a reduction voltage, electrons will be driven back to the molecules. Due to the oxidation and reduction of the redox centers, these redox molecules can exhibit distinct charged or discharged states, which can be deemed as logic on and off states, at different voltages with very fast write and erase speeds. It has been demonstrated that redox-active molecules attached to Si structures are stable and can endure more than 10
12 program/erase (P/E) cycles [
11]. This excellent performance is naturally derived from the intrinsic properties of the redox behavior in molecules. These advantageous properties of redox molecules make them very promising candidates for future applications in on-chip non-volatile memory, such as SRAM and dynamic random-access memory (DRAM). In addition, due to the scalability of molecules and the naturally-derived multiple redox states for robust charge storage, the molecular memory density can be much higher than the conventional memory devices.
Great efforts have been made to integrate molecules as the active component for future electronic devices. In this paper, we review the development of molecular electronics for non-volatile memory applications with a focus on redox-active molecules, meanwhile presenting further strategies to extend their applications in future low-power and ultra-high-density non-volatile memory applications.
2. Molecular Logic Switching Devices
Two fundamentally different approaches to molecular electronics are graphically termed “top-down” and “bottom-up”. Instead of top-down, which includes making nano-scale structures by machining and etching techniques, molecular electronics rely on the “bottom-up” approach, by taking advantage of molecule self-assembly. Bottom-up refers to building organic or inorganic structures by atom-by-atom or molecule-by-molecule techniques. In the past few decades, research on molecular electronics has been focusing more and more on the combination of top-down device fabrication (mainly lithography) with bottom-up molecule self-assembly.
The crossbar molecular circuit is one of the earliest logic forms of molecular memory arrays. The switching element is a metal/molecule/metal sandwich junction, wherein the molecules are located at the cross-section of two nanoscale metal wires (
Figure 1). The early demonstration of such junction molecular switching devices utilized molecules consisting of two mechanically-interlocked rings [
12,
13,
14,
15]. The molecular monolayers were deposited as a Langmuir–Blodgett film. The mechanical motion of these molecules is an activated redox process, and two stable and electrochemically-switchable states of the molecules form the logic on and off states. As shown in
Figure 1, the molecular switching devices can exhibit a ~10
2 on/off current ratio, but with limited endurance cycles. Based on such switching devices, an 8 × 8 crossbar has been built by the Hewlett-Packard research group [
16]. This approach has the advantage of architectural simplicity and the potential of high density via fabrication of highly dense nanowires. However, this approach has two major disadvantages, including a high rate of defective switching elements and the difficulty in controlling the metal/molecule interface. Even though a defect-tolerant architecture had been established earlier, the metal/molecule problems in such junction switching devices and crossbar circuits are still to be addressed [
17]. In later publications, it was reported that the electron transport phenomena in the metal/molecule/metal junction were molecule independent; instead, they were dominated by electrode reactions with molecules, and the intrinsic spacing of the energy levels might be modified [
18,
19,
20].
Another architecture utilizing metal/molecule/metal junction is the so-called nanocell molecular circuit, proposed by researchers at Rice University and Yale University [
21,
22,
23,
24]. A nanocell is a two-dimensional network of self-assembled metallic particles connected by molecules that show reprogrammable negative differential resistance (illustrated in
Figure 2) [
21,
22]. The nanocell is surrounded by a small number of lithographically-defined metal lines that provide input and output leads. The active component for a nanocell is also a metal/molecule/metal switch, and one pathway may include more than one molecular switch [
25,
26,
27]. The molecule pathways can be learned first by developing appropriate computation algorithms; then, the whole cell can be programmed to perform a particular function. Such a nanocell molecular circuit uses similar molecular switches as the crossbar molecular circuit, but the circuit architecture is quite different. Compared to the crossbar approach, the nanocell approach has the advantages of large-scale circuit fabrication and integration. However, the disadvantage is that it relies on complex programming algorithms. Meanwhile, both the nanocell and crossbar approaches may have the same difficulty in controlling the metal/molecule interface.
Figure 1.
(
Top) Atomic force microscopy image of a nanoscale cross-point molecular switching device with [
2] a rotaxane monolayer sandwiched between two metal line electrodes; (
Bottom) device resistance read at 0.2 V after multiple switching cycles. Reproduced with permission from [
14], © AIP Publishing LLC, 2003.
Figure 1.
(
Top) Atomic force microscopy image of a nanoscale cross-point molecular switching device with [
2] a rotaxane monolayer sandwiched between two metal line electrodes; (
Bottom) device resistance read at 0.2 V after multiple switching cycles. Reproduced with permission from [
14], © AIP Publishing LLC, 2003.
Figure 2.
SEM image of a nanocell after assembly of the Au nanowires and mononitro oligo(phenylene ethynylene) compound.
Top: five pairs of leads across the nanocell;
Bottom: higher magnification of the central portion of the nanocell with an attached Au nanowire, which is affixed via the derivative of mononitro oligo(phenylene ethynylene). Reproduced with permission from [
22], © American Chemical Society, 2003.
Figure 2.
SEM image of a nanocell after assembly of the Au nanowires and mononitro oligo(phenylene ethynylene) compound.
Top: five pairs of leads across the nanocell;
Bottom: higher magnification of the central portion of the nanocell with an attached Au nanowire, which is affixed via the derivative of mononitro oligo(phenylene ethynylene). Reproduced with permission from [
22], © American Chemical Society, 2003.
In addition to the metal/molecule/metal switching devices, molecules have also been tested as the channel material in a standard MOSFET structure [
28,
29,
30,
31]. As shown in
Figure 3, by replacing the high-k dielectric with an ultra-thin SAM of an organic active layer in a field-effect transistor (FET), the Infineon researchers have reported an organic field-effect transistor (OFET) with low operation voltage and low gate leakage current [
31]. The demonstrated OFETs operate with a supply voltage of less than 2 V, yet have gate currents lower than that of the traditional Si FET with the SiO
2 dielectric. However, the switching speed of the OFET is still not comparable to Si transistors, and the limited device lifetime remains as one of the major bottlenecks for further applications. Another disadvantage of such OFETs is the poor stability in a high temperature and volatile environment. Thus, the application of organic molecules in advanced silicon CMOS technology is still under question and requires further research efforts.
Figure 3.
Structure and electrical characteristics of a molecular FET. (
a) Structure of (18-phenoxyoctadecyl)trichlorosilane (PhO-OTS); (
b) structure of the organic semiconductor pentacene; (
c) cross-section of a pentacene organic field-effect transistor (OFET); (
d) output and transfer characteristics, with subthreshold region showing a subthreshold swing of 100 mV per decade [
31]. Reproduced with permission from [
31], © Nature Publishing Group, 2004.
Figure 3.
Structure and electrical characteristics of a molecular FET. (
a) Structure of (18-phenoxyoctadecyl)trichlorosilane (PhO-OTS); (
b) structure of the organic semiconductor pentacene; (
c) cross-section of a pentacene organic field-effect transistor (OFET); (
d) output and transfer characteristics, with subthreshold region showing a subthreshold swing of 100 mV per decade [
31]. Reproduced with permission from [
31], © Nature Publishing Group, 2004.
3. Redox-Active Molecule-Based Non-Volatile Memory
Appropriate modifications of the molecular structures and switching elements have been designed to change the switching kinetics and to enhance the performance for different memory applications. Different device platforms have been engineered to interface with the molecules, such as dielectrics, oxides, nanowires, carbon nanotubes, and so forth. A well-known method to achieve a memory effect is by employing redox-active elements. The redox mechanism involves electrons being transferred from one element to another, such that a donor element gives up an electron (oxidation) to an accepting element (reduction). This process usually requires an external stimulus for redox reactions, such as an electric field or change in temperature. Such redox-active molecule-based memory devices can show fast speed, low operation voltage and high reliability, due to the intrinsic redox behavior of the molecules. Therefore, it is very attractive for DRAM and flash memory device applications.
The research group at North Carolina State University proposed hybrid CMOS/molecular memory devices, in which the redox-active charge-storage molecules were incorporated into Si structures to generate a new class of electronic memory devices [
32,
33,
34,
35,
36]. These redox-active molecules, which can be designed to self-assemble on surfaces as monolayers, exhibit charge-storage states at discrete voltage levels. This approach can provide a smooth transition from CMOS technology to molecular electronics technology by integrating the vast infrastructure of developed CMOS technology with inherent molecular properties.
Figure 4.
Structure of redox-active molecules (
a) Fc-BzOH and (
b) Por-BzOH for memory applications; (
c) schematic of an electrolyte-molecule-Si capacitor with a simplified equivalent circuit. Reproduced with permission from [
36], © John Wiley and Sons, 2004.
Figure 4.
Structure of redox-active molecules (
a) Fc-BzOH and (
b) Por-BzOH for memory applications; (
c) schematic of an electrolyte-molecule-Si capacitor with a simplified equivalent circuit. Reproduced with permission from [
36], © John Wiley and Sons, 2004.
This approach focuses on developing hybrid molecular non-volatile memory with redox-active molecules as the active charge-storage medium. The redox-active molecule used in such molecular memory usually consists of a redox-active component, a linkage component and surface attachment groups.
Figure 4 shows two examples of such molecules with different redox-active components. Physically, the redox-active component acts as the charge-storage center, with both the linkage and the surface group acting as the insulator. From this standpoint, different redox-active component can be designed or synthesized for multiple redox states, thus for complex and high memory density. The linkage component can be engineered to tune the memory retention properties, while the surface attachment groups can be specifically designed for attachment on different surfaces via covalent bonds. The redox component, such as the Fe in 4-ferrocenylbenzyl alcohol (Fc-BzOH) and Zn in 5-(4-hydroxymethylphenyl)-10,15,20-trimesitylporphinatozinc(II) (Por-BzOH) (shown in
Figure 4), is the charge-storage medium, which can be in neutral and positively-charged states through losing electrons. The molecular components surrounding the redox center act as the barrier against charge loss. The electrons tunnel through the barrier during oxidation and reduction processes. The electrolyte/molecule interface acts as another barrier between the molecule and top gate electrode, which blocks the electron tunnel into the molecule from the electrolyte to reduce an oxidized molecule. The positive charges in the molecule can be reduced at a lower gate potential, wherein an electron tunnels back to the molecule from the Si substrate.
The SAM attachment of redox molecules to Si, SiO
2 or other surfaces was typically carried out by using chemical solution deposition [
35,
36,
37]. The attachment is characterized by using cyclic voltammetry (CyV) measurement, a current-voltage measurement at various voltage scan rates.
Figure 5a,b shows the CyV curves of electrolyte-molecule-oxide-Si (EMOS) structures with the Fc-BzOH and Por-BzOH redox molecules attached to SiO
2 for charge storage, respectively [
35,
37]. The CyV curves of both molecular structures demonstrate oxidation and reduction peaks. Fc-BzOH shows one pair of redox peaks, because the redox center Fe only demonstrates a neutral state and one positively-charged state. However, the Zn redox center in Por-BzOH exhibits a neutral state and two (mono- or di-) positively-charged states; therefore, two pairs of redox peaks were observed.
Figure 5.
Cyclic voltammetry of electrolyte-molecule-oxide-Si (EMOS) structures with (
a) the Fc-BzOH and (
b) the Por-BzOH redox molecule attached to SiO
2 at different voltage scan rates; capacitance-voltage (C-V) and conductance-voltage (G-V) curves of (
c) Fc-BzOH and (
d) Por-BzOH EMOS capacitors at 100 Hz. Reproduced with permission from [
35,
37], © AIP Publishing LLC, 2003, 2004.
Figure 5.
Cyclic voltammetry of electrolyte-molecule-oxide-Si (EMOS) structures with (
a) the Fc-BzOH and (
b) the Por-BzOH redox molecule attached to SiO
2 at different voltage scan rates; capacitance-voltage (C-V) and conductance-voltage (G-V) curves of (
c) Fc-BzOH and (
d) Por-BzOH EMOS capacitors at 100 Hz. Reproduced with permission from [
35,
37], © AIP Publishing LLC, 2003, 2004.
The capacitance-voltage (C-V) and conductance-voltage (G-V) characteristics also exhibited clear capacitance and conductance peaks associated with trapping and detrapping of charge in the molecules. Similarly, multiple peaks were observed with the Por-BzOH EMOS capacitor due to its two charged states. Interestingly, the two charged states within molecules, such as Por-BzOH, can be employed for a multi-bit storage application to further enhance the memory density. Despite using a redox molecule exhibiting multiple charged states, an alternative and perhaps more efficient strategy to achieve higher memory bits is afforded by simply mixing different redox-active molecules whose potentials are well separated. Fc-BzOH and Por-BzOH SAMs have been mixed on Si, and the mixed SAMs exhibit well-defined redox states [
36]. The mixed SAM approach is very attractive owing to the fact that this approach is synthetically far simpler than preparing a single molecule that exhibits three or more redox states. In addition, the potential of the redox charged states is more distinct than that of a single molecular redox center with multiple charged states. However, the disadvantage of this method is that the density of a given peak goes down [
38]. Nevertheless, this mixed SAM approach still paves the way for constructing multi-bit memory storage devices.
4. Solid-State Molecular Memory
The conventional polysilicon floating-gate flash memory has benefited the semiconductor memory technology for decades, due to its scalability, compatibility with CMOS process, and so forth. It relies on hot electron injection from the channel into the floating gate through a tunneling oxide layer. By attaching redox-active molecules to Si as the floating gate in the CMOS structure, one can further enhance the cell density and reduce the cell variations. The combination of top-down lithography and bottom-up molecule self-assembly processes renders a uniform charge density, and the monodisperse nature of the molecular orbitals leading to distinct energy levels can enable precise controlling of charged states [
39]. Moreover, with a solid-state insulating barrier deposited on both sides of the molecules, the possibility of orbital hybridization from the gate will be further lessened. Such an integration of redox-active molecules in a solid-state memory provides an efficient platform to study the molecules and devices with microelectronic characterization metrologies, which is unique in comparison with the liquid electrolyte-based electrochemical method.
The self-assembly process of the redox-active molecules to form a solid-state molecular memory enables not only high-quality attachment of molecules to semiconductor surfaces, but also uniform and high-density fabrication of low-cost devices and circuits. The disadvantages of molecular flash memory mainly lie in its reliability in volatile environments. Some redox-active molecules are instable or even do not function at temperatures higher than 200 °C due to evaporation or redox center reaction. Under volatile environments with oxygen or water, the molecule’s bond might be broken, leading to poor redox behavior. As a result, some specific conditions and environments need to be taken into account when integrating such redox molecules in CMOS devices and circuits. Future molecular technology requires advancement in both molecule properties and device integration processes.
The researchers from North Carolina State University reported a study on preventing contact metal penetration into the molecular layer in the metal-insulator-molecule-metal structure [
40]. Similar work demonstrating that redox properties of molecules can be preserved after thin film encapsulation, such as atomic layer deposition (ALD), initiated the investigation on high-performance solid-state molecular memory [
41]. The Cornell University research group reported a study on a metal-oxide-semiconductor (MOS) structure with molecules encapsulated in a high-k dielectric and functioning as the charge storage medium [
42,
43]. As shown in
Figure 6, molecules with different redox centers, which can show one and two charged states, have been studied for multi-bit memory. With the electrostatics determined by the alignment between the highest occupied or the lowest unoccupied molecular orbital (HOMO or LUMO, respectively) energy levels and the charge neutrality level of the dielectric, the asymmetric charge injection behavior in flat-band voltage shift (ΔV
FB)
vs. programming voltage measurement (
Figure 7) is due to the Fermi-level pinning between the molecules and the high-k dielectric [
44,
45,
46]. The three programmable molecular orbital states of the 5-(4-Carboxyphenyl)-10,15,20,-triphenyl-porphyrin-Co(II) (CoP) molecule have been experimentally observed and are attractive for low-variation multi-bit memory applications. However, the charge retention of this capacitor molecular memory is still far below the modern flash memory requirements. The engineering by the same group on the tunnel layer has greatly enhanced the electron retention and programming performance by forming an organic-inorganic tunnel barrier [
43]. Nevertheless, the endurance of this molecular memory (~10
4 cycles) is still quite limited; further engineering and improvement on the molecular orbitals and device structure are needed to achieve higher memory performance.
Recently, the NIST researchers have reported a similar capacitor-structure molecular non-volatile memory, but with enhanced charge retention and excellent endurance [
47]. The molecular memory uses an α-ferrocenylethanol redox molecule, which has a very simple structure and one redox center. As shown in
Figure 8, with the molecules attached to the SiO
2 tunnel oxide and encapsulated in the ALD Al
2O
3 dielectric, the solid-state molecular memory exhibited excellent memory behavior, such as a sufficient memory window, good retention and endurance. Even though only ~15% of the molecules were effectively involved in the redox processes after ALD, effective memory can be realized with sufficient charge density. These characteristics are attributed to the intrinsic redox behavior of the ferrocene molecule and the effective protection from the presence of the gate dielectric covering the molecule SAM. The molecular memory showed excellent endurance properties, with negligible memory window degradation after 10
9 P/E cycles, which is about 10,000-times better than that of the conventional floating gate memory (1 × 10
5 P/E cycles). However, the operation voltage of such molecular memory is still high, and the operation speed needs to be improved. Further study of the molecular memory depends on the engineering of redox-active molecules, such as molecules with multiple redox centers and a proper linker for multi-bit memory, and the device structure engineering to optimize the dielectric stack thickness and improve the molecule/dielectric interface. Such an exploration will be a significant breakthrough in the quest for charge-storage non-volatile memory and will enable the application of flash-like devices for future on-chip memory applications.
Figure 6.
Schematics of the metal-oxide-semiconductor (MOS) capacitor structure with (
a) ferrocenecarboxylic acid (FcCOOH) and (
b) CoP SAM attached to Si structures. Reproduced with permission from [
42], © IEEE, 2011.
Figure 6.
Schematics of the metal-oxide-semiconductor (MOS) capacitor structure with (
a) ferrocenecarboxylic acid (FcCOOH) and (
b) CoP SAM attached to Si structures. Reproduced with permission from [
42], © IEEE, 2011.
Figure 7.
High-frequency C-V and ΔV
FB as a function of programming voltage at 10 K and room temperature for the device with (
a,
b) FcCOOH and (
c,
d) CoP molecules. Reproduced with permission from [
42], © IEEE, 2011.
Figure 7.
High-frequency C-V and ΔV
FB as a function of programming voltage at 10 K and room temperature for the device with (
a,
b) FcCOOH and (
c,
d) CoP molecules. Reproduced with permission from [
42], © IEEE, 2011.
Figure 8.
(
a) Molecule structure of α-ferrocenylethanol and a schematic of the Metal-Al
2O
3-Ferrocene-Oxide-Silicon (MAFOS) capacitor memory structure; (
b) ΔV
FB of the molecular memory and control samples as a function of program/erase (P/-E) voltage; (
c) charge retention at room temperature; (
d) device endurance with different P/Epulse widths. Reproduced with permission from [
47], © AIP Publishing LLC, 2013.
Figure 8.
(
a) Molecule structure of α-ferrocenylethanol and a schematic of the Metal-Al
2O
3-Ferrocene-Oxide-Silicon (MAFOS) capacitor memory structure; (
b) ΔV
FB of the molecular memory and control samples as a function of program/erase (P/-E) voltage; (
c) charge retention at room temperature; (
d) device endurance with different P/Epulse widths. Reproduced with permission from [
47], © AIP Publishing LLC, 2013.
5. Nanowire/Nanotube-Based Molecular Memory
Semiconductor nanowire and nanotube MOSFETs have been regarded as the building blocks for nanoelectronics devices and circuits [
48,
49,
50]. Compared to planar devices based on bulk materials, the nanowires have smaller channels and a larger surface-to-volume ratio. Thus, they require less stored charges to induce the same memory window for the memory application. Moreover, the nanowire or nanotube channel can enable a gate-surrounding structure, allowing excellent electrostatic gate control. Integrating redox-active molecules with semiconductor nanowire or nanotube FETs for solid-state flash memory will not only improve the memory performance by utilizing the advantageous properties of redox-active molecules, but also will provide an efficient platform to study the redox-active molecules with microelectronics characterization metrologies.
Figure 9.
Nanowire-based non-volatile memory, with the nanowire surface functionalized with redox-active molecules. Logic on and off states are represented by different charged states. Reproduced with permission from [
51], © American Chemical Society, 2002.
Figure 9.
Nanowire-based non-volatile memory, with the nanowire surface functionalized with redox-active molecules. Logic on and off states are represented by different charged states. Reproduced with permission from [
51], © American Chemical Society, 2002.
In 2002, the researchers from Harvard University reported a molecular non-volatile memory based on a back-gated nanowire FET with the nanowire surface functionalized with cobalt phthalocyanine (CoPc) redox-active molecules (
Figure 9) [
51]. By modulating the channel conductance with back gate voltage, reversible switching between on and off states has been achieved, with the on/off ratio exceeding 10
4. The retention (>20 min) and endurance (>100 cycles) of the molecular memory device have outperformed the earlier junction molecular switching devices. Integrated arrays of independently-addressable molecular nanowire memory have also been characterized, which demonstrated another advantage of implementing nanowire structure memory in programmable logic arrays. The memory performance has been further improved with molecule and device engineering. In 2004, the University of Southern California research groups reported a back-gate nanowire FET-based molecular memory, which utilized In
2O
3 nanowires and a family of bis(terpyridine)-Fe(II) molecules for charge storage (
Figure 10) [
52,
53]. It was demonstrated that bis(terpyridine)-Fe(II) with a larger or more complex linkage component showed better retention, due to the increase in the tunneling barrier. In addition, by applying gate voltage pulses with different amplitudes, multilevels in memory cells can be illustrated by altering the population of the reduced/oxidized molecules [
53]. However, such multilevel memory realization depends on the gate voltage applied to oxidize/reduce a portion of molecules in SAM. Further cell scaling or increasing the number of memory levels will be extremely difficult, as the complicated top-down device fabrication and device structure will inevitably lead to significant device variation and a blurring of the multiple storage states. In addition, the demonstrated device performance is still far below the standards for on-chip memory applications. This is partly because of the back gate device structure, unclean steps in the nanowire FET device fabrication process and, most importantly, the absence of protection for redox-active molecules.
Figure 10.
Comparison of designs and operations between (
a) conventional silicon flash memory and (
b) molecular memory for multilevel nonvolatile data storage; (
c) comparison of multilevels in a two-bit silicon flash memory and molecular memory; (
d) molecular structure of Fe
2+-terpyridine compound. Reproduced with permission from [
53,
54], © AIP Publishing LLC, 2004,2007.
Figure 10.
Comparison of designs and operations between (
a) conventional silicon flash memory and (
b) molecular memory for multilevel nonvolatile data storage; (
c) comparison of multilevels in a two-bit silicon flash memory and molecular memory; (
d) molecular structure of Fe
2+-terpyridine compound. Reproduced with permission from [
53,
54], © AIP Publishing LLC, 2004,2007.
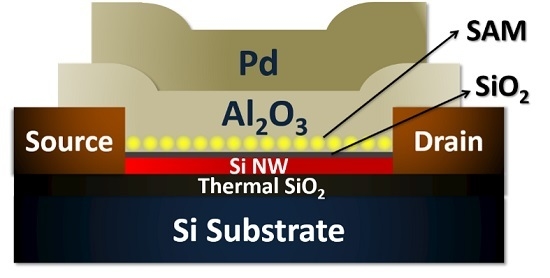
Recently, the NIST researchers have extended their work on the capacitor memory cell to a novel top-gated nanowire molecular flash memory based on Si nanowire FETs [
47,
55]. The memory cell consists of a self-aligned top-gated Si nanowire FET with α-ferrocenylethanol redox-active molecules attached to a Si nanowire surface (
Figure 11). The molecules are effectively protected by engineered top gate dielectrics. The injected charges are mainly located in the redox centers of molecules, with trace amount stored in the molecule/Al
2O
3 interface traps. The molecular flash memory cell exhibited fast P/E speed and excellent P/E endurance (>10
9 P/E cycles). These characteristics are attributed to both the intrinsic charging properties of redox-active molecules and the protection of the molecules obtained by the self-alignment fabrication enabling clean solid-molecule and dielectric interfaces. However, similar to their previous capacitor molecular memory, only ~20% of the molecules were effectively involved in the redox process. Further research efforts are required to increase the redox efficiency so as to lower the operation voltage and improve the operation speed. The memory density can be further enhanced by using redox molecules with multiple redox centers. Such a multi-bit memory concept is more reasonable and feasible than that by just modulating the voltage level, as the physically discrete charged states of the molecule can enable precise controlling of the charged states.
Figure 11.
(
Left) TEM image of the cross-section of a molecular flash memory device; (
Right) discrete charging behavior of the Ru
2 molecular flash memory and the endurance properties. Reproduced with permission from [
55], © American Chemical Society, 2015.
Figure 11.
(
Left) TEM image of the cross-section of a molecular flash memory device; (
Right) discrete charging behavior of the Ru
2 molecular flash memory and the endurance properties. Reproduced with permission from [
55], © American Chemical Society, 2015.
In addition to the traditional redox-active molecules, novel molecules and clusters have been synthesized and implemented in molecular memory. In 2014, Busche
et al. reported a study on molecular nanowire flash memory using core-shell polyoxometalate (POM) molecules for charge storage (
Figure 12) [
56]. The POM molecule functions as the switching component in the memory associated with the oxidation of selenite template at the cluster core and the reduction of the metal oxide cluster cage. The unique oxidation process of the selenite template at the cluster core was observed only after the initial excitation and cannot be recreated with consecutive operations, leading to a new type of “write-once-erase” memory. The flash memory with a 4-nm Si nanowire channel and POM for charge storage has shown excellent retention properties with no charge store decay after 336 h. With the increasing oxidation state of POM molecules, the on/off ratio can reach as high as 10
11 [
56]. Even though the operation voltage and the switching time of such molecular memory are still quite large, further engineering to shorten the distance from the control gate to the nanowire channel and to reduce the number of POMs can be expected to effectively lower the voltage and the write time into the sub-picosecond region.
Figure 12.
Image of the nanowire flash memory device and the drain current behavior with an applied voltage to the control gate. (
a) Cross-sectional image (left) of the memory device and the top view (right) of a device with a 5-nm Si nanowire channel and side control gate; measurements of the logarithmic (
b) and linear (
c) drain current
versus gate voltage at 0.5 V source-drain voltage: before deposition of the polyoxometalates (POMs) (green dashes), after the deposition of the POMs (orange dashes), after a −20 V pulse (blue line) and after a 120 V pulse (red line). Reproduced with permission from [
56], © American Chemical Society, 2014.
Figure 12.
Image of the nanowire flash memory device and the drain current behavior with an applied voltage to the control gate. (
a) Cross-sectional image (left) of the memory device and the top view (right) of a device with a 5-nm Si nanowire channel and side control gate; measurements of the logarithmic (
b) and linear (
c) drain current
versus gate voltage at 0.5 V source-drain voltage: before deposition of the polyoxometalates (POMs) (green dashes), after the deposition of the POMs (orange dashes), after a −20 V pulse (blue line) and after a 120 V pulse (red line). Reproduced with permission from [
56], © American Chemical Society, 2014.
As illustrated by the above experimental work, the performance of molecular flash memory depends on not only the redox behavior of the molecules, but also the preparation of the transistor platform and the integration of the molecules in the memory structure. While the memory endurance is mainly determined by the mechanism of the charge storage, further improving the memory performance, such as the memory density, speed and power, requires optimized engineering on the transistor platform structure and an enhanced molecule/semiconductor interface. For example, a high quality tunneling oxide layer in a molecular flash memory with less molecule/dielectric interface traps can significantly improve the memory retention and lower the operation voltage. The semiconductor nanowire FETs have been shown to be promising building blocks to create new functional molecular memory devices. A vast array of other device platforms, such as Si surfaces, metal oxide and carbon nanotubes, can be implemented for molecular memory if an appropriate modification can be designed to interface the molecules with such platforms. Therefore, the integration of redox-active molecules as the active component in a molecular flash memory is very attractive, because it will leverage the advantages afforded by the redox-active molecules with the vast infrastructure of current semiconductor technology.