Low-Cost Temperature Logger for a Polymerase Chain Reaction Thermal Cycler
Abstract
:1. Introduction
2. Materials and Methods
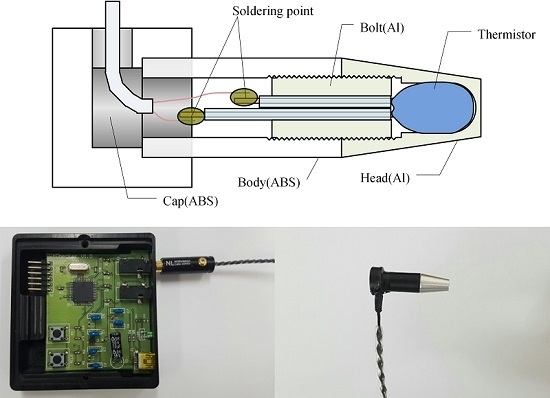
2.1. Probe and Local System Implementation
2.2. Selection of the Thermistor Resistance
2.3. Software Implementation
2.4. Long Term Precision Test
3. Results
3.1. Step Response Test Results for the Self-Heating Effect
3.2. Long Term Stability Test Results
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verdoy, D.; Barrenetxea, Z.; Berganzo, J.; Agirregabiria, M.; Ruano-López, J.M.; Marimón, J.M.; Olabarría, G. A novel Real Time micro PCR based Point-of-Care device for Salmonella detection in human clinical samples. Biosens. Bioelectron. 2012, 32, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kodzius, R.; Xiao, K.; Qin, J.; Wen, W. Fast detection of genetic information by an optimized PCR in an interchangeable chip. Biomed. Microdevices 2012, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Tung, S. Development and applications of portable biosensors. J. Lab. Autom. 2015, 20, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jing, W.; Zheng, L.; Liu, S.; Wu, W.; Sui, G. A continuous-flow high-throughput microfluidic device for airborne bacteria PCR detection. Lab Chip 2014, 14, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Volpatti, L.R.; Yetisen, A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Young, H.K.; Yang, I.; Bae, Y.S.; Park, S.R. Performance evaluation of thermal cyclers for PCR in a rapid cycling condition. Biotechniques 2008, 44, 495–505. [Google Scholar]
- Saunders, G.C.; Dukes, J.; Parkes, H.C.; Cornett, J.H. Interlaboratory study on thermal cycler performance in controlled PCR and random amplified polymorphic DNA analyses. Clin. Chem. 2001, 47, 47–55. [Google Scholar] [PubMed]
- Schoder, D.; Schmalwieser, A.; Schauberger, G.; Kuhn, M.; Hoorfar, J.; Wagner, M. Physical characteristics of six new thermocyclers. Clin. Chem. 2003, 49, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Kim, Y.-H.; Byun, J.-Y.; Park, S.-R. Use of multiplex polymerase chain reactions to indicate the accuracy of the annealing temperature of thermal cycling. Anal. Biochem. 2005, 338, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Xu, B.; Fu, R.; Li, D. Real time PCR on disposable PDMS chip with a miniaturized thermal cycler. Biomed. Microdevices 2005, 7, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Xu, B.; Li, D. Miniature real time PCR on chip with multi-channel fiber optical fluorescence detection module. Biomed. Microdevices 2007, 9, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Rpbert, L.; Grossman, J.W.F. Temperature Verification for Polymerase Chain Reaction Systems. U.S. Patent 5,224,778 A, 6 July 1993. [Google Scholar]
- Brothers, B.; Christianson, W.; Hunter, L.; Hunter, M.; Sitler, C.; Stauder, B. Portable Electronic Thermometer and Method of Temperature Measurement. U.S. Patent 3,822,598 A, 9 July 1974. [Google Scholar]
- Thermal Cycler Temperature Verification System. Available online: https://tools.thermofisher.com/content/sfs/manuals/cms_070449.pdf (accessed on 20 October 2016).
- 1 Channel Temperature Verification Kit. Available online: http://www.alphatechnics.com/precision-thermometry/1-channel-temperature-verification-kit/ (accessed on 20 October 2016).
- Knieriem, A.S.; Quinn, D.E.; Wawro, J.T.; Lane, J. Sealed Probe Chamber for Thermometry Apparatus. U.S. Patent 6,827,488 B2, 7 December 2004. [Google Scholar]
- On-Site Temperature Verification Service. Available online: https://www.thermofisher.com/us/en/home/products-and-services/services/instrument-qualification-services/compliance-and-validation/on-site-temperature-verification.html (accessed on 20 October 2016).
- Thermal Validation Services. Available online: http://www.bio-rad.com/en-ch/product/thermal-validation-services (accessed on 20 October 2016).
- Nicolae, M.; Lucaci, L.; Moise, I. Embedding Android devices in automation systems. In Proceedings of the 2013 IEEE 19th International Symposium Design Technology Electronic Packaging (SIITME), Galati, Romania, 24–27 October 2013; pp. 215–218.
- Drumea, A. Control of industrial systems using android-based devices. In Proceedings of the 36th International Spring Seminar on Electronics Technology, Alba Iulia, Romania, 8–12 May 2013; pp. 405–408.
- Diab, M.O.; Brome, R.A.M.; Dichari, M.; Moslem, B. The smartphone accessory heart rate monitor. In Proceedings of the International Conference on Computer Medical Application (ICCMA), Sousse, Tunisia, 20–22 January 2013.
- Kim, J.D.; Park, C.Y.; Yeon, J.; Kim, Y.S.; Song, H.J. Development of PCR controller and smart-phone application based on bluetooth communication. Int. J. Multimed. Ubiquitous Eng. 2013, 8, 223–230. [Google Scholar] [CrossRef]
- Driftcon Operations Manual. Available online: http://cyclertest.com/documentation/driftcon/manual.aspx (accessed on 20 October 2016).
- Sapoff, M.; Oppenheim, R.M. Theory and application of self-heated thermistors. Proc. IEEE 1963, 51, 1292–1305. [Google Scholar] [CrossRef]
- Park, C.; Kim, J.; Kim, J.; Kim, Y.; Song, H.; Kim, J. Buffer-less system for thermistor temperature measurement. In Proceedings of the 2012 IEEE International Conference on ICT Convergence (ICTC), Jeju, Korea, 15–17 October 2012; Volume 2012, pp. 240–242.
- Chen, C. Evaluation of resistance-temperature calibration equations for NTC thermistors. Meas. J. Int. Meas. Confed. 2009, 42, 1103–1111. [Google Scholar] [CrossRef]
- Kim, J.-D.; Jeong, D.-H.; Song, H.-J.; Kim, Y.-S.; Park, C.-Y. Efficient calibration tool for thermistor. Sens. Mater. 2015, 27, 593–598. [Google Scholar]




| Thermistor Resistance | 50 (°C) | 60 (°C) | 72 (°C) | 95 (°C) |
|---|---|---|---|---|
| 10 kΩ | 0.2 | 0.2 | 0.2 | 0.3 |
| 100 kΩ | 0.0 | 0.0 | 0.0 | 0.0 |
| Bare or Probe | 50 (°C) | 60 (°C) | 72 (°C) | 95 (°C) |
|---|---|---|---|---|
| Bare thermistor | 0.2 | 0.2 | 0.2 | 0.3 |
| Probe | 0.0 | 0.0 | 0.0 | 0.0 |
| True Temperature | Probe ID | 55 (°C) | 85 (°C) |
|---|---|---|---|
| 1st test (14 March 2016) | Probe 1 | 55 | 85.1 |
| Probe 2 | 55 | 85 | |
| Probe 3 | 55 | 85 | |
| Probe 4 | 55 | 85 | |
| 2nd test (17 March 2016) | Probe 1 | 55 | 85.1 |
| Probe 2 | 55.1 | 85 | |
| Probe 3 | 55 | 85 | |
| Probe 4 | 55 | 85 | |
| 3nd test (22 March 2016) | Probe 1 | 55 | 85 |
| Probe 2 | 55.1 | 85 | |
| Probe 3 | 55.1 | 85 | |
| Probe 4 | 55 | 85 | |
| 4th test (28 March 2016) | Probe 1 | 55 | 85 |
| Probe 2 | 55.1 | 85 | |
| Probe 3 | 55 | 84.9 | |
| Probe 4 | 55 | 85 | |
| 5th test (30 March 2016) | Probe 1 | 55 | 85 |
| Probe 2 | 55.1 | 85 | |
| Probe 3 | 55 | 85 | |
| Probe 4 | 55 | 85 | |
| 6th test (10 May 2016) | Probe 1 | 54.9 | 85 |
| Probe 2 | 55 | 85 | |
| Probe 3 | 55 | 85 | |
| Probe 4 | 55 | 85.1 | |
| 7th test (20 October 2016) | Probe 1 | 55 | 85 |
| Probe 2 | 55.1 | 84.9 | |
| Probe 3 | 55.1 | 85 | |
| Probe 4 | 55 | 85 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-Y.; Cho, J.-H.; Kim, Y.-S.; Song, H.-J.; Kim, J.-D. Low-Cost Temperature Logger for a Polymerase Chain Reaction Thermal Cycler. Appl. Sci. 2016, 6, 328. https://0-doi-org.brum.beds.ac.uk/10.3390/app6110328
Park C-Y, Cho J-H, Kim Y-S, Song H-J, Kim J-D. Low-Cost Temperature Logger for a Polymerase Chain Reaction Thermal Cycler. Applied Sciences. 2016; 6(11):328. https://0-doi-org.brum.beds.ac.uk/10.3390/app6110328
Chicago/Turabian StylePark, Chan-Young, Jae-Hyeon Cho, Yu-Seop Kim, Hye-Jeong Song, and Jong-Dae Kim. 2016. "Low-Cost Temperature Logger for a Polymerase Chain Reaction Thermal Cycler" Applied Sciences 6, no. 11: 328. https://0-doi-org.brum.beds.ac.uk/10.3390/app6110328