Effects of Ultrasonication on the Conformational, Microstructural, and Antioxidant Properties of Konjac Glucomannan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composition Analysis of KGM
2.3. Ultrasonic Degradation of KGM
2.4. Analysis of MW and Conformation Parameters
2.5. Rheological Measurement
2.6. Fourier-Transform Infrared Spectroscopy and Scanning Electronic Microscope Observation
2.7. Antioxidant Activity Assays
3. Results and Discussion
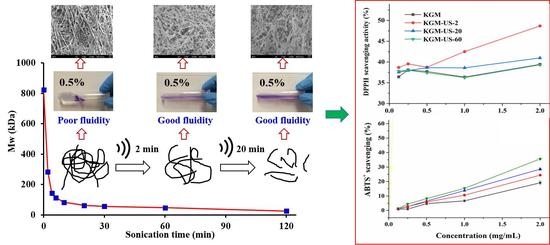
3.1. Effect of US on MW Distribution of KGM
3.2. Degradation Kinetics and Chain Scission Mechanisms
3.3. Effects of US on Rheological and Structural Characteristics of KGM
3.4. Effect of US on Chain Conformation of KGM
3.5. Effect of US on the Microstructure of KGM
3.6. US Treatment Effect on in Vitro Antioxidant Activity of KGM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- An, N.T.; Dong, N.T.; Le Dung, P.; Van Du, N. Characterization of glucomannan from some Amorphophallus species in Vietnam. Carbohyd. Polym. 2010, 80, 308–311. [Google Scholar] [CrossRef]
- Katsuraya, K.; Okuyama, K.; Hatanaka, K.; Oshima, R.; Sato, T.; Matsuzaki, K. Constitution of konjac glucomannan: Chemical analysis and 13C NMR spectroscopy. Carbohyd. Polym. 2003, 53, 183–189. [Google Scholar] [CrossRef]
- Maeda, M.; Shimahara, H.; Sugiyama, N. Detailed examination of the branched structure of konjac glucomannan. Agric. Biol. Chem. 1980, 44, 245–252. [Google Scholar] [CrossRef]
- Shimahara, H.; Suzuki, H.; Sugiyama, N.; Nisizawa, K. Isolation and characterization of oligosaccharides from an enzymic hydrolysate of konjac glucomannan. Agric. Biol. Chem. 1975, 39, 293–299. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xie, B.J.; Gan, X. Advance in the applications of konjac glucomannan and its derivatives. Carbohyd. Polym. 2005, 60, 27–31. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Konjac glucomannan, a promising polysaccharide of Amorphophallus konjac K. Koch in health care. Int. J. Biol. Macromo 2016, 92, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Herranz, B.; Borderias, A.J.; Solas, M.T.; Tovar, C.A. Influence of measurement temperature on the rheological and microstructural properties of glucomannan gels with different thermal histories. Food. Res. Int. 2012, 48, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.V.T.; Jovanovski, E.; Zurbau, A.; Mejia, S.B.; Sievenpiper, J.L.; Au-Yeung, F.; Jenkins, A.L.; Duvnjak, L.; Leiter, L.; Vuksan, V. A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am. J. Clin. Nutr. 2017, 105, 1239–1247. [Google Scholar] [CrossRef]
- Xu, X.; Li, B.; Kennedy, J.F.; Xie, B.J.; Huang, M. Characterization of konjac glucomannan-gellan gum blend films and their suitability for release of nisin incorporated therein. Carbohyd. Polym. 2007, 70, 192–197. [Google Scholar] [CrossRef]
- Zalewski, B.M.; Chmielewska, A.; Szajewska, H. The effect of glucomannan on body weight in overweight or obese children and adults: A systematic review of randomized controlled trials. Nutrition 2015, 31, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Modifications of konjac glucomannan for diverse applications. Food Chem. 2018, 256, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Surina, B.; Tegshi, M.; Taisei, K.; Hideki, N.; Takashi, Y. Sulfation and biological activities of konjac glucomannan. Carbohyd. Polym. 2013, 94, 899–903. [Google Scholar]
- Zheng, Q.R.; Wu, Y.L.; Xu, H.L.; Wang, H.J.; Tang, H.L.; Xia, X.J.; Feng, J. Immune responses to Aeromonas hydrophila infection in Schizothorax prenanti fed with oxidized konjac glucomannan and its acidolysis products. Fish. Shellfish. Immun. 2016, 49, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazzewi, F.; Elamir, A.; Tester, R.; Elzagoze, A. Effect of depolymerised konjac glucomannan on wound healing. Bioact. Carbohyd. Diet. Fibre 2015, 5, 125–128. [Google Scholar] [CrossRef]
- Chen, H.L.; Fan, Y.H.; Chen, M.E.; Chan, Y. Unhydrolyzed and hydrolyzed konjac glucomannans modulated cecal and fecal microflora in Balb/c mice. Nutrition 2005, 21, 1059–1064. [Google Scholar] [CrossRef]
- Jian, W.J.; Tu, L.Y.; Wu, L.L.; Xiong, H.J.; Pang, J.; Sun, Y.M. Physicochemical properties and cellular protection against oxidation of degraded Konjac glucomannan prepared by γ-irradiation. Food Chem. 2017, 231, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.H.; Song, A.X.; Yao, Z.P.; Wu, J.Y. Protective effects of natural and partially degraded konjac glucomannan on Bifidobacteria against antibiotic damage. Carbohyd. Polym. 2018, 181, 368–375. [Google Scholar] [CrossRef]
- Tatirat, O.; Charoenrein, S.; Kerr, W.L. Physicochemical properties of extrusion-modified konjac glucomannan. Carbohyd. Polym. 2012, 87, 1545–1551. [Google Scholar] [CrossRef]
- Mateus, M.M.; Acero, N.F.; Bordado, J.C.; dos Santos, R.G. Sonication as a foremost tool to improve cork liquefaction. Ind. Crop. Prod. 2015, 74, 9–13. [Google Scholar] [CrossRef]
- Gogate, P.R.; Prajapat, A.L. Depolymerization using sonochemical reactors: A critical review. Ultrason. Sonochem. 2015, 27, 480–494. [Google Scholar] [CrossRef]
- Bera, S.; Mondal, D.; Martin, J.T.; Singh, M. Potential effect of ultrasound on carbohydrates. Carbohyd. Res. 2015, 410, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Ogutu, F.O.; Mu, T.H.; Elahi, R.; Zhang, M.; Sun, H.N. Ultrasonic modification of selected polysaccharides-review. J. Food Process. Technol. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Pu, Y.; Zou, Q.; Hou, D.; Chen, S. Molecular weight kinetics and chain scission models for dextran polymers during ultrasonic degradation. Carbohyd. Polym. 2017, 156, 71–76. [Google Scholar]
- Li, J.; Li, B.; Geng, P.; Song, A.X.; Wu, J.Y. Ultrasonic degradation kinetics and rheological profiles of a food polysaccharide (konjac glucomannan) in water. Food Hydrocoll. 2017, 70, 14–19. [Google Scholar] [CrossRef]
- Song, A.X.; Mao, Y.H.; Siu, K.C.; Wu, J.Y. Bifidogenic effects of Cordyceps sinensis fungal exopolysaccharide and konjac glucomannan after ultrasound and acid degradation. Int. J. Biol. Macromo 2018, 111, 587–594. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Han, F.; Gao, C.; Liu, M.; Huang, F.; Zhang, B. Synthesis, optimization and characterization of acetylated corn starch with the high degree of substitution. Int. J. Biol. Macromo 2013, 59, 372–376. [Google Scholar] [CrossRef]
- Willför, S.; Pranovich, A.; Tamminen, T.; Puls, J.; Laine, C.; Suurnäkki, A.; Saake, B.; Uotila, K.; Simolin, H.; Hemming, J. Carbohydrate analysis of plant materials with uronic acid-containing polysaccharides-A comparison between different hydrolysis and subsequent chromatographic analytical techniques. Ind. Crop. Prod. 2009, 29, 571–580. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ma, L.Y.; Wang, L.; Wang, X.Y.; Nie, S.P.; Xie, M.Y.; Yin, J.Y. Monosaccharide composition analysis of arabinoxylan by high performance anion exchange chromatography with pulsed amperometric detection. Chin. J. Anal. Chem. 2017, 45, 415–422. [Google Scholar]
- Yin, J.Y.; Nie, S.P.; Guo, Q.B.; Wang, Q.; Cui, S.W.; Xie, M.Y. Effect of calcium on solution and conformational characteristics of polysaccharide from seeds of Plantago asiatica L. Carbohyd. Polym. 2015, 124, 331–336. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.M.; Chen, Y.; Yu, M.Y.; Wen, F.Y.; Zhang, H. Identification, quantification and antioxidant activity of acylated flavonol glycosides from sea buckthorn (Hippophae rhamnoides ssp. sinensis). Food Chem. 2013, 141, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Grönroos, A.; Pirkonen, P.; Ruppert, O. Ultrasonic depolymerization of aqueous carboxymethylcellulose. Ultrason. Sonochem. 2004, 11, 9–12. [Google Scholar] [CrossRef]
- Yan, J.K.; Pei, J.J.; Ma, H.L.; Wang, Z.B. Effects of ultrasound on molecular properties, structure, chain conformation and degradation kinetics of carboxylic curdlan. Carbohyd. Polym. 2015, 121, 64–70. [Google Scholar]
- Antti, G.; Pentti, P.; Hanna, K. Ultrasonic degradation of aqueous carboxymethylcellulose: Effect of viscosity, molecular mass, and concentration. Ultrason. Sonochem. 2008, 15, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Kishida, N.; Okimasu, S.; Kamata, T. Molecular weight and intrinsic viscosity of konjac gluco-mannan. Agric. Biol. Chem. 1978, 42, 1645–1650. [Google Scholar]
- Ratcliffe, I.; Williams, P.A.; Viebke, C.; Meadows, J. Physicochemical characterization of konjac glucomannan. Biomacromolecules 2005, 6, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Kök, M.S.; Abdelhameed, A.S.; Ang, S.; Morris, G.A.; Harding, S.E. A novel global hydrodynamic analysis of the molecular flexibility of the dietary fibre polysaccharide konjac glucomannan. Food Hydrocoll. 2009, 23, 1910–1917. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zivanovic, S.; Hayes, D.G.; Weiss, J. Efficient reduction of chitosan molecular weight by high-intensity ultrasound: Underlying mechanism and effect of process parameters. J. Agric. Food Chem. 2008, 56, 5112–5119. [Google Scholar] [CrossRef]
- Schmid, G. Zur Kinetik der Ultraschalldepolymerisation. Z. Phys. Chem. 1940, 186, 113–128. [Google Scholar] [CrossRef]
- Madras, G.; Kumar, S.; Chattopadhyay, S. Continuous distribution kinetics for ultrasonic degradation of polymers. Polym. Degrad. Stab. 2000, 69, 73–78. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Femenia, A.; García-Pascual, P.; Simal, S.; Rosselló, C. Effects of heat treatment and dehydration on bioactive polysaccharide acemannan and cell wall polymers from Aloe barbadensis Miller. Carbohyd. Polym. 2003, 51, 397–405. [Google Scholar] [CrossRef]
- Xing, X.; Cui, S.W.; Nie, S.P.; Phillips, G.O.; Goff, H.D.; Wang, Q. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part I. Extraction, purification, and partial structural characterization. Bioact. Carbohyd. Diet. Fibre 2014, 4, 74–83. [Google Scholar] [CrossRef]
- Burchard, W. Two-Dimensional Fourier Transform Infrared Spectroscopy Applied to Cellulose and Paper. In Light Scattering from Polysaccharides, 2nd ed.; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 199–202. [Google Scholar]
- Cui, S.W. Food Carbohydrates: Chemistry, Physical Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Picout, D.R.; Ross-Murphy, S.B. On the Mark-Houwink parameters for galactomannans. Carbohyd. Polym. 2007, 70, 145–148. [Google Scholar] [CrossRef]
- Kratky, O.; Porod, G. Röntgenuntersuchung gelöster fadenmoleküle. Recueil des Travaux Chimiques des Pays-Bas 1949, 68, 1106–1122. [Google Scholar] [CrossRef]
- Bohdanecký, M. New method for estimating the parameters of the wormlike chain model from the intrinsic viscosity of stiff-chain polymers. Macromolecules 1983, 16, 1483–1492. [Google Scholar] [CrossRef]
- Tatirat, O.; Charoenrein, S. Physicochemical properties of konjac glucomannan extracted from konjac flour by a simple centrifugation process. LWT-Food Sci. Technol. 2011, 44, 2059–2063. [Google Scholar] [CrossRef]
- Xu, W.; Wang, S.; Ye, T.; Jin, W.; Liu, J.; Lei, J.; Li, B.; Wang, C. A simple and feasible approach to purify konjac glucomannan from konjac flour-Temperature effect. Food Chem. 2014, 158, 171–176. [Google Scholar] [CrossRef]
- Guo, X.; Ye, X.Q.; Sun, Y.J.; Wu, D.; Wu, N.; Hu, Y.Q.; Chen, S.G. Ultrasound effects on the degradation kinetics, structure, and antioxidant activity of sea cucumber fucoidan. J. Agric. Food Chem. 2014, 62, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yang, Y.; Hu, X.; Zhang, Z. Effect of ultrasonic extraction conditions on antioxidative and immunomodulatory activities of a Ganoderma lucidum polysaccharide originated from fermented soybean curd residue. Food Chem. 2014, 155, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Wang, Y.Y.; Ma, H.L.; Wang, Z.B. Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from Phellinus linteus mycelia. Ultrason. Sonochem. 2016, 29, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, H.; Liu, W.; Pei, J.; Wang, Z.; Zhou, H.; Yan, J. Ultrasound enhanced production and antioxidant activity of polysaccharides from mycelial fermentation of Phellinus igniarius. Carbohyd. Polym. 2014, 113, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Y.; Ma, H.; He, R. Effect of ultrasonic degradation on in vitro antioxidant activity of polysaccharides from Porphyra yezoensis (Rhodophyta). Food Sci. Technol. Int. 2008, 14, 479–486. [Google Scholar] [CrossRef]







| Sonication Time (min) | Mw/Mn | Rh (nm) | <Rg>z (nm) | Higher-MW Region a | |
|---|---|---|---|---|---|
| α | Lp (nm) | ||||
| 0 | 1.5 | 60.0 | 104.5 | 0.79 | 10.3 |
| 2 | 1.3 | 33.6 | 55.9 | 0.51 | 7.1 |
| 4 | 1.3 | 22.7 | 39.6 | 0.62 | 7.2 |
| 6 | 1.3 | 19.7 | 33.3 | 0.65 | 7.4 |
| 10 | 1.2 | 16.5 | 28.7 | 0.69 | 7.4 |
| 20 | 1.2 | 13.8 | 25.4 | 0.73 | 7.3 |
| 30 | 1.2 | 12.9 | 24.9 | 0.77 | 7.5 |
| 60 | 1.2 | 11.1 | 26.9 | 0.95 | 9.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.-Y.; Ma, L.-Y.; Siu, K.-C.; Wu, J.-Y. Effects of Ultrasonication on the Conformational, Microstructural, and Antioxidant Properties of Konjac Glucomannan. Appl. Sci. 2019, 9, 461. https://0-doi-org.brum.beds.ac.uk/10.3390/app9030461
Yin J-Y, Ma L-Y, Siu K-C, Wu J-Y. Effects of Ultrasonication on the Conformational, Microstructural, and Antioxidant Properties of Konjac Glucomannan. Applied Sciences. 2019; 9(3):461. https://0-doi-org.brum.beds.ac.uk/10.3390/app9030461
Chicago/Turabian StyleYin, Jun-Yi, Lu-Yao Ma, Ka-Chai Siu, and Jian-Yong Wu. 2019. "Effects of Ultrasonication on the Conformational, Microstructural, and Antioxidant Properties of Konjac Glucomannan" Applied Sciences 9, no. 3: 461. https://0-doi-org.brum.beds.ac.uk/10.3390/app9030461