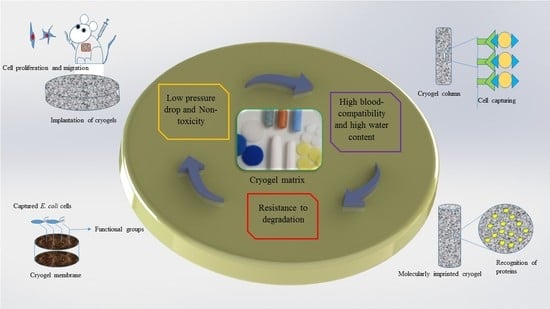
Biomedical Applications of Polymeric Cryogels
Abstract
:Featured Application
Abstract
1. Introduction
2. Preparation and Properties of Supermacroporous Cryogels
Factors Affecting Properties of Cryogels
3. Cryogels in Medical Applications
3.1. Immobilization of Biomolecules
3.2. Capturing of Target Molecules
3.3. Controlled Drug Delivery
3.4. Cryogels for Tissue Engineering
3.4.1. Bioreactors
3.4.2. Cell Separation
3.4.3. Scaffolds
3.4.4. Other Tissue Engineering Applications
4. Conclusions
Funding
Conflicts of Interest
References
- Ertürk, G.; Mattiasson, B. Cryogels-versatile tools in bioseparation. J. Chromatogr. A 2014, 1357, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, P.; Plieva, F.M.; Lozinsky, V.I.; Galaev, I.Y.; Mattiasson, B. Direct chromatographic capture of enzyme from crude homogenate using immobilized metal affinity chromatography on a continuous supermacroporous adsorbent. J. Chromatogr. A 2003, 986, 275–290. [Google Scholar] [CrossRef]
- Peppas, N.A.; Huang, Y.; Torres-Lugo, M.; Ward, J.H.; Zhang, J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu. Rev. Biomed. Eng. 2000, 2, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The potential of polymeric cryogels in bioseparation. Bioseparation 2001, 10, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Plieva, F.; Huiting, X.; Galaev, I.Y.; Bergenståhl, B.; Mattiasson, B. Macroporous elastic polyacrylamide gels prepared at subzero temperatures: Control of porous structure. J. Mater. Chem. 2006, 16, 4065–4073. [Google Scholar] [CrossRef]
- Jungbauer, A.; Hahn, R. Monoliths for fast bioseparation and bioconversion and their applications in biotechnology. J. Sep. Sci. 2004, 10–11, 767–778. [Google Scholar] [CrossRef]
- Josić, D.; Buchacher, A. Application of monoliths as supports for affinity chromatography and fast enzymatic conversion. J. Biochem. Biophys. Methods 2001, 49, 153–174. [Google Scholar] [CrossRef]
- Bencina, M.; Podgornik, A.; Strancar, A. Characterization of methacrylate monoliths for purification of DNA molecules. J. Sep. Sci. 2004, 10–11, 801–810. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef]
- Plieva, F.M.; Savina, I.N.; Deraz, S.; Andersson, J.; Galaev, I.Y.; Mattiasson, B. Characterization of supermacroporous monolithic polyacrylamide based matrices designed for chromatography of bioparticles. J. Chromatog. B 2004, 807, 129–137. [Google Scholar] [CrossRef]
- Yavuz, H.; Denizli, A. A new affinity separation medium: supermacroporous cryogels, reference module in chemistry. Mol. Sci. Chem. Eng. 2015, 4, 1–12. [Google Scholar]
- Bereli, N.; Türkmen, D.; Yavuz, H.; Galaev, I.Y.; Denizli, A. Cryogels for Affinity Chromatography in: Advanced Separations by Specialized Sorbents. In Chromatographic Science Series; Dragan, E.S., Ed.; CRC Press: Boca Raton, FL, USA, 2015; Chapter 2; pp. 39–67. [Google Scholar]
- Perçin, I.; Saĝlar, E.; Yavuz, H.; Aksöz, E.; Denizli, A. Poly(hydroxyethyl methacrylate) based affinity cryogel for plasmid DNA purification. Int. J. Biol. Macromol. 2011, 48, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Bereli, N.; Yavuz, H.; Denizli, A. Cryogels. In Encylopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Heidelberg, Germany, 2016; Volume 1, pp. 22–23. [Google Scholar]
- Andac, M.; Galaev, I.; Denizli, A. Dye attached poly(hydroxyethyl methacrylate) cryogel for albumin depletion from human serum. J. Sep. Sci. 2012, 35, 1173–1182. [Google Scholar] [CrossRef]
- Bereli, N.; Şener, G.; Yavuz, H.; Denizli, A. Oriented immobilized anti-LDL antibody carrying poly(hydroxyethyl methacrylate) cryogel for cholesterol removal from human plasma. Mater. Sci. Eng. C 2011, 31, 1078–1083. [Google Scholar] [CrossRef]
- Perçin, I.; Yavuz, H.; Aksöz, E.; Denizli, A. Mannose-specific lectin isolation from Canavalia ensiformis seeds by PHEMA-based cryogel. Biotechnol. Prog. 2012, 28, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Damshkaln, L.G.; Simenel, I.A.; Lozinsky, V.I. Study of cryostructuration of polymer systems. XV. Freeze-thaw-induced formation of cryoprecipitate matter from low-concentrated aqueous solutions of poly(vinyl alcohol). J. Appl. Polym. Sci. 1999, 74, 1978–1986. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Yavuz, H.; Denizli, A. Controlled release of mitomycin C from PHEMAH–Cu(II) cryogel membranes. Artif. Cells Nanomed. Biotechnol. 2018, 64, 1–9. [Google Scholar] [CrossRef]
- Ivanov, R.V.; Lozinsky, V.I.; Noh, S.K.; Lee, Y.R.; Han, S.S.; Lyoo, W.S. Preparation and characterization of polyacrylamide cryogels produced from a high-molecular-weight precursor. II. The influence of the molecular weight of the polymeric precursor. J. Appl. Polym. Sci. 2008, 106, 1470–1475. [Google Scholar] [CrossRef]
- Ozmen, M.M.; Dinu, M.V.; Dragan, E.S.; Okay, O. Preparation of macroporous acrylamide-based hydrogels: Cryogelation under isothermal conditions. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 1195–1202. [Google Scholar] [CrossRef]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef]
- Okay, O.; Lozinsky, V.I. Synthesis and structure–property relationships of cryogels. In Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Okay, O., Ed.; Springer International Publishing: Basel, Switzerland, 2014; Volume 263, pp. 103–157. [Google Scholar]
- Babac, C.; Yavuz, H.; Galaev, I.Y.; Pişkin, E.; Denizli, A. Binding of antibodies to concanavalin A-modified monolithic cryogel. React. Funct. Polym. 2006, 66, 1263–1271. [Google Scholar] [CrossRef]
- Alkan, H.; Bereli, N.; Baysal, Z.; Denizli, A. Selective removal of the autoantibodies from rheumatoid arthritis patient plasma using protein A carrying affinity cryogels. Biochem. Eng. J. 2010, 51, 153–159. [Google Scholar] [CrossRef]
- Kumar, A.; Rodríguez-Caballero, A.; Plieva, F.M.; Galaev, I.Y.; Nandakumar, K.S.; Kamihira, M.; Holmdahl, R.; Orfao, A.; Mattiasson, B. Affinity binding of cells to cryogel adsorbents with immobilized specific ligands: Effect of ligand coupling and matrix architecture. J. Mol. Recognit. 2005, 18, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Avcibaşi, N.; Uygun, M.; Çorman, M.E.; Akgöl, S.; Denizli, A. Application of supermacroporous monolithic hydrophobic cryogel in capturing of albumin. Appl. Biochem. Biotechnol. 2010, 162, 2232–2243. [Google Scholar] [CrossRef]
- Bereli, N.; Ertürk, G.; Denizli, A. Histidine containing macroporous affinity cryogels for immunoglobulin G purification. Sep. Sci. Technol. 2012, 47, 1813–1820. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Bereli, N.; Şenel, S. Preparation and characterization of thiophilic cryogels with 2-mercapto ethanol as the ligand for IgG purification. Colloids Surf. B 2013, 113, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Bakhshpour, M.; Derazshamshir, A.; Bereli, N.; Elkak, A.; Denizli, A. [PHEMA/PEI]-Cu(II) based immobilized metal affinity chromatography cryogels: Application on the separation of IgG from human plasma. Mater. Sci. Eng. C. 2016, 61, 824–831. [Google Scholar] [CrossRef]
- Demiryas, N.; Tüzmen, N.; Galaev, I.Y.; Pişkin, E.; Denizli, A. Poly(acrylamide-allyl glycidyl ether) cryogel as a novel stationary phase in dye-affinity chromatography. J. Appl. Polym. Sci. 2007, 105, 1808–1816. [Google Scholar] [CrossRef]
- Andac, M.; Galaev, I.Y.; Denizli, A. Molecularly imprinted poly(hydroxyethyl methacrylate) based cryogel for albumin depletion from human serum. Colloids Surf. B. 2013, 109, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Derazshamshir, A.; Baydemir, G.; Andac, M.; Say, R.; Galaev, I.Y.; Denizli, A. Molecularly imprinted PHEMA-based cryogel for depletion of hemoglobin from human blood. Macromol. Chem. Phys. 2010, 211, 657–668. [Google Scholar] [CrossRef]
- Gao, F.X.; Zhao, X.L.; He, X.W.; Li, W.Y.; Zhang, Y.K. A pH and temperature dual-responsive macroporous molecularly imprinted cryogel for enhanced recognition capability towards ovalbumin. Anal. Methods 2013, 5, 6700–6708. [Google Scholar] [CrossRef]
- Rabieizadeh, M.; Kashefimofrad, S.M.; Naeimpoor, F. Monolithic molecularly imprinted cryogel for lysozyme recognition. J. Sep. Sci. 2014, 37, 2983–2990. [Google Scholar] [CrossRef] [PubMed]
- La Spina, R.; Tripisciano, C.; Mecca, T.; Cunsolo, F.; Weber, V.; Mattiasson, B. Chemically modified poly(2-hydroxyethyl methacrylate) cryogel for the adsorption of heparin. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102B, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Bereli, N.; Ertürk, G.; Tümer, M.A.; Say, R.; Denizli, A. Oriented immobilized anti-hIgG via Fcfragment-imprinted PHEMA cryogel for IgG purification. Biomed. Chromatogr. 2013, 27, 599–607. [Google Scholar] [CrossRef]
- Baydemir, G.; Andaç, M.; Bereli, N.; Galaev, I.Y.; Say, R.; Denizli, A. Supermacroporous PHEMA based cryogel with embedded bilirubin imprinted particles. React. Funct. Polym. 2009, 69, 36–42. [Google Scholar] [CrossRef]
- Baydemir, G.; Andaç, M.; Bereli, N.; Galaev, I.Y.; Say, R.; Denizli, A. Bilirubin recognition via molecularly imprinted supermacroporous cryogels. Colloids Surf. B 2009, 68, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Baydemir, G.; Denizli, A. Heparin removal from human plasma using molecular imprinted cryogels. Artif. Cells Nanomed. Biotechnol. 2015, 43, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Özgür, E.; Bereli, N.; Türkmen, D.; Ünal, S.; Denizli, A. PHEMA cryogel for in-vitro removal of anti-dsDNA antibodies from SLE plasma. Mater. Sci. Eng. C 2011, 31, 915–920. [Google Scholar] [CrossRef]
- Demirçelik, A.H.; Andaç, M.; Andaç, C.A.; Say, R.; Denizli, A. Molecular recognition-based detoxification of aluminum in human plasma. J. Biomater. Sci. Polym. Ed. 2009, 20, 1235–1258. [Google Scholar] [CrossRef] [PubMed]
- Asliyüce, S.; Bereli, N.; Uzun, L.; Onur, M.A.; Say, R.; Denizli, A. Ion-imprinted supermacroporous cryogel, for in vitro removal of iron out of human plasma with beta thalassemia. Sep. Purif. Technol. 2010, 73, 243–249. [Google Scholar] [CrossRef]
- Alkan, H. Poly(hydroxyethyl methacrylate)-co-N-methacryloyl-(l)-histidine methyl ester based cryogel for the removal of Fe3+ from human plasma effected with Beta thalassemia. Hacettepe J. Biol. Chem. 2015, 43, 179–185. [Google Scholar]
- Yavuz, H.; Say, R.; Denizli, A. Iron removal from human plasma based on molecular recognition using imprinted beads. Mater. Sci. Eng. C 2005, 25, 521–528. [Google Scholar] [CrossRef]
- Wang, N.X.; Von Recum, H.A. Affinity-based drug delivery. Macromol. Biosci. 2011, 11, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Carino, G.P.; Mathiowitz, E. Oral insulin delivery. Adv. Drug Deliv. Rev. 1999, 35, 249–257. [Google Scholar] [CrossRef]
- Çetin, K.; Denizli, A. 5-Fluorouracil delivery from metal-ion mediated molecularly imprinted cryogel discs. Colloids Surf. B 2015, 126, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.; Langer, R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 327–341. [Google Scholar] [CrossRef]
- Öncel, P.; Çetin, K.; Topçu, A.A.; Yavuz, H.; Denizli, A. Molecularly imprinted cryogel membranes for mitomycin C delivery. J. Biomater. Sci. Polym. Ed. 2017, 28, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Caka, M.; Türkcan, C.; Aktaş Uygun, D.; Uygun, M.; Akgöl, S.; Denizli, A. Controlled release of curcumin from poly(HEMA-MAPA) membrane. Artif. Cells Nanomed. Biotechnol. 2017, 45, 426–431. [Google Scholar] [CrossRef]
- Kostova, B.; Momekova, D.; Petrov, P.; Momekov, G.; Toncheva-Moncheva, N.; Tsvetanov, C.B.; Lambov, N. Poly(ethoxytriethyleneglycol acrylate) cryogels as novel sustained drug release systems for oral application. Polymer 2011, 52, 1217–1222. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 1–19. [Google Scholar] [CrossRef]
- Kumari, J.; Kumar, A. Development of polymer based cryogel matrix for transportation and storage of mammalian cells. Sci. Rep. 2017, 7, 41551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efremenko, E.N.; Tatarinova, N.Y. The effect of long-term preservation of bacterial cells immobilized in poly(vinyl alcohol) cryogel on their viability and biosynthesis of target metabolites. Microbiology 2007, 76, 336–341. [Google Scholar] [CrossRef]
- Jain, E.; Damania, A.; Shakya, A.K.; Kumar, A.; Sarin, S.K.; Kumar, A. Fabrication of macroporous cryogels as potential hepatocyte carriers for bioartificial liver support. Colloids Surf. B 2015, 136, 761–771. [Google Scholar] [CrossRef]
- Guilherme, E.P.X.; de Oliveira, J.P.; de Carvalho, L.M.; Brandi, I.V.; Santos, S.H.S.; de Carvalho, G.G.P.; Cota, J.; Mara Aparecida de Carvalho, B. Synthesis of supermacroporous cryogel for bioreactors continuous starch hydrolysis. Electrophoresis 2017, 38, 2940–2946. [Google Scholar] [CrossRef]
- Jain, E.; Karande, A.A.; Kumar, A. Supermacroporous polymer-based cryogel bioreactor for monoclonal antibody production in continuous culture using hybridoma cells. Biotechnol. Prog. 2011, 27, 170–180. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A. Effect of plasma polymerization on physicochemical properties of biocomposite cryogels causing a differential behavior of human osteoblasts. J. Colloid Interface Sci. 2014, 431, 139–148. [Google Scholar] [CrossRef]
- Dainiak, M.B.; Plieva, F.M.; Galaev, I.Y.; Hatti-Kaul, R.; Mattiasson, B. Cell chromatography: Separation of different microbial cells using IMAC supermacroporous monolithic columns. Biotechnol. Prog. 2005, 21, 644–649. [Google Scholar] [CrossRef]
- Arvidsson, P.; Plieva, F.M.; Savina, I.N.; Lozinsky, V.I.; Fexby, S.; Bülow, L.; Yu. Galaev, I.; Mattiasson, B. Chromatography of microbial cells using continuous supermacroporous affinity and ion-exchange columns. J. Chromatogr. A 2002, 977, 27–38. [Google Scholar] [CrossRef]
- Williams, S.L.; Eccleston, M.E.; Slater, N.K.H. Affinity capture of a biotinylated retrovirus on macroporous monolithic adsorbents: Towards a rapid single-step purification process. Biotechnol. Bioeng. 2005, 89, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Noppe, W.; Plieva, F.M.; Vanhoorelbeke, K.; Deckmyn, H.; Tuncel, M.; Tuncel, A.; Galaev, I.Y.; Mattiasson, B. Macroporous monolithic gels, cryogels, with immobilized phages from phage-display library as a new platform for fast development of affinity adsorbent capable of target capture from crude feeds. J. Biotechnol. 2007, 131, 293–299. [Google Scholar] [CrossRef]
- Mishra, R.; Goel, S.K.; Gupta, K.C.; Kumar, A. Biocomposite cryogels as tissue engineered biomaterials for regeneration of critical-sized cranial bone defects. Tissue Eng. Part A 2013, 20, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Yang, Y.; Korkusuz, P.; Güzel, E.; El Haj, A.J.; Pişkin, E. Three-dimensional ingrowth of bone cells within biodegradable cryogel scaffolds in bioreactors at different regimes. Tissue Eng. Part A 2008, 14, 1743–1750. [Google Scholar] [CrossRef]
- Rezaeeyazdi, M.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable hyaluronic acid-co-gelatin cryogels for tissue-engineering applications. Materials 2018, 11, 1374. [Google Scholar] [CrossRef]
- Gürer, B.; Yılmaz, C.; Yılmaz, Ş.N.; Çabuk, S.; Bölgen, N. A novel strategy for cartilage tissue engineering: Collagenase-loaded cryogel scaffolds in a sheep model. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 313–321. [Google Scholar] [CrossRef]
- Salgado, C.L.; Grenho, L.; Fernandes, M.H.; Colaço, B.J.; Monteiro, F.J. Biodegradation, biocompatibility, and osteoconduction evaluation of collagen-nanohydroxyapatite cryogels for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2016, 104, 57–70. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Salgado, C.L.; Sahu, A.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J. Preparation and characterization of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2013, 101, 1080–1094. [Google Scholar] [CrossRef]
- Kemençe, N.; Bölgen, N. Gelatin- and hydroxyapatite-based cryogels for bone tissue engineering: Synthesis, characterization, in vitro and in vivo biocompatibility. J. Tissue Eng. Regen. Med. 2017, 11, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Eberlin, C.T.; Lu, T.; Neal, S.M.; Case, N.D.; McBride-Gagyi, S.H.; Sell, S.A. The calcification potential of cryogel scaffolds incorporated with various forms of hydroxyapatite for bone regeneration. Biomed. Mater. 2017, 12, 025005. [Google Scholar] [CrossRef] [PubMed]
- Raina, D.B.; Isaksson, H.; Teotia, A.K.; Lidgren, L.; Tägil, M.; Kumar, A. Biocomposite macroporous cryogels as potential carrier scaffolds for bone active agents augmenting bone regeneration. J. Control. Release 2016, 235, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.Y.; Inci, I.; Egri, S.; Ozturk, A.M.; Yetkin, H.; Goktas, G.; Elmas, C.; Piskin, E.; Erdogan, D. The treatment of segmental bone defects in rabbit tibiae with vascular endothelial growth factor (VEGF)-loaded gelatin/hydroxyapatite “cryogel” scaffold. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 767–774. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; Carletta, M.N.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A preliminary in vitro evaluation of the bioactive potential of cryogel scaffolds incorporated with Manuka honey for the treatment of chronic bone infections. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1918–1933. [Google Scholar] [CrossRef] [PubMed]
- Ak, F.; Oztoprak, Z.; Karakutuk, I.; Okay, O. Macroporous silk fibroin cryogels. Biomacromolecules 2013, 14, 719–727. [Google Scholar] [CrossRef]
- Mishra, R.; Raina, D.B.; Pelkonen, M.; Lidgren, L.; Tägil, M.; Kumar, A. Study of in vitro and in vivo bone formation in composite cryogels and the influence of electrical stimulation. Int. J. Biol. Sci. 2015, 11, 1325–1336. [Google Scholar] [CrossRef]
- Oelschlaeger, C.; Bossler, F.; Willenbacher, N. Synthesis, Structural and micromechanical properties of 3D hyaluronic acid-based cryogel scaffolds. Biomacromolecules 2016, 17, 580–589. [Google Scholar] [CrossRef]
- Phadke, A.; Hwang, Y.S.; Hee Kim, S.; Hyun Kim, S.; Yamaguchi, T.; Masuda, K.; Varghese, S. Effect of scaffold microarchitecture on osteogenic differentiation of human mesenchymal stem cells. Eur. Cells Mater. 2012, 25, 114–129. [Google Scholar] [CrossRef]
- Saino, E.; Fassina, L.; Van Vlierberghe, S.; Avanzini, M.A.; Dubruel, P.; Magenes, G.; Visai, L.; Benazzo, F. Effects of electromagnetic stimulation on osteogenic differentiation of human mesenchymal stromal cells seeded onto gelatin cryogel. Int. J. Immunopathol. Pharmacol. 2011, 24, 1–6. [Google Scholar] [CrossRef]
- Bölgen, N.; Yang, Y.; Korkusuz, P.; Güzel, E.; El Haj, A.J.; Pişkin, E. 3D ingrowth of bovine articular chondrocytes in biodegradable cryogel scaffolds for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2011, 10, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Bhat, S.; Nayak, V.; Kumar, A. Study of different delivery modes of chondroitin sulfate using microspheres and cryogel scaffold for application in cartilage tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 859–872. [Google Scholar] [CrossRef]
- Odabas, S.; Feichtinger, G.A.; Korkusuz, P.; Inci, I.; Bilgic, E.; Yar, A.S.; Cavusoglu, T.; Menevse, S.; Vargel, I.; Piskin, E. Auricular cartilage repair using cryogel scaffolds loaded with BMP-7-expressing primary chondrocytes. J. Tissue Eng. Regen. Med. 2013, 7, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bhat, S.; Jagdale, P.R.; Chaudhari, B.P.; Lidgren, L.; Gupta, K.C.; Kumar, A. Evaluation of three-dimensional chitosan-agarose-gelatin cryogel scaffold for the repair of subchondral cartilage defects: An in vivo study in a rabbit model. Tissue Eng. Part A 2014, 20, 3101–3111. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Chen, C.H.; Hsiao, C.Y.; Chen, J.P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Allan, I.U.; Tolhurst, B.A.; Shevchenko, R.V.; Dainiak, M.B.; Illsley, M.; Ivanov, A.; Jungvid, H.; Galaev, I.Y.; James, S.L.; Mikhalovsky, S.V.; et al. An: In vitro evaluation of fibrinogen and gelatin containing cryogels as dermal regeneration scaffolds. Biomater. Sci. 2016, 4, 1007–1014. [Google Scholar] [CrossRef]
- Dainiak, M.B.; Allan, I.U.; Savina, I.N.; Cornelio, L.; James, E.S.; James, S.L.; Mikhalovsky, S.V.; Jungvid, H.; Galaev, I.Y. Gelatin-fibrinogen cryogel dermal matrices for wound repair: Preparation, optimisation and in vitro study. Biomaterials 2010, 31, 67–76. [Google Scholar] [CrossRef]
- Takei, T.; Nakahara, H.; Tanaka, S.; Nishimata, H.; Yoshida, M.; Kawakami, K. Effect of chitosan-gluconic acid conjugate/poly(vinyl alcohol) cryogels as wound dressing on partial-thickness wounds in diabetic rats. J. Mater. Sci. Mater. Med. 2013, 24, 2479–2487. [Google Scholar] [CrossRef]
- Martínez, Y.N.; Cavello, I.; Hours, R.; Cavalitto, S.; Castro, G.R. Immobilized keratinase and enrofloxacin loaded on pectin PVA cryogel patches for antimicrobial treatment. Bioresour. Technol. 2013, 145, 280–284. [Google Scholar] [CrossRef]
- Priya, S.G.; Gupta, A.; Jain, E.; Sarkar, J.; Damania, A.; Jagdale, P.R.; Chaudhari, B.P.; Gupta, K.C.; Kumar, A. Bilayer cryogel wound dressing and skin regeneration grafts for the treatment of acute skin wounds. ACS Appl. Mater. Interfaces 2016, 8, 15145–15159. [Google Scholar] [CrossRef]
- Vrana, N.E.; Cahill, P.A.; McGuinness, G.B. Endothelialization of PVA/gelatin cryogels for vascular tissue engineering: Effect of disturbed shear stress conditions. J. Biomed. Mater. Res. Part A 2010, 94, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Leor, J. Rebuilding broken hearts. Sci. Am. 2004, 291, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Campbell, G. Campbell, G. Formulations of polyvinyl alcohol cryogel that mimic the biomechanical properties of soft tissues in the natural lumbar intervertebral disc. Spine (Phila. Pa. 1976) 2009, 34, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Temofeew, N.A.; Hixon, K.R.; McBride-Gagyi, S.H.; Sell, S.A. The fabrication of cryogel scaffolds incorporated with poloxamer 407 for potential use in the regeneration of the nucleus pulposus. J. Mater. Sci. Mater. Med. 2017, 28, 36. [Google Scholar] [CrossRef] [PubMed]
- Jurga, M.; Dainiak, M.B.; Sarnowska, A.; Jablonska, A.; Tripathi, A.; Plieva, F.M.; Savina, I.N.; Strojek, L.; Jungvid, H.; Kumar, A.; et al. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials 2011, 32, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Nayak, V.; Kumar, A. Proliferation of myoblast skeletal cells on three-dimensional supermacroporous cryogels. Int. J. Biol. Sci. 2010, 6, 371–381. [Google Scholar] [CrossRef]





| Target | Matrix | Functional Monomer/Ligand | Method | Adsorption Capacity | Reference |
|---|---|---|---|---|---|
| Human serum albumin | Poly(acrylamide-allyl glycidyl ether) [poly(AAm-AGE)] cryogel | Dye ligand Cibacron Blue | Dye-affinity- based | 74.2 mg/g | [31] |
| Human serum albumin | PHEMA cryogel | Dye ligand Cibacron Blue | Dye-affinity- based | 950 mg/g | [12] |
| Human serum albumin | Poly(glycidyl methacrylate) beads embedded PHEMA composite cryogels | Dye ligand Cibacron Blue | Dye-affinity- based | 342 mg/g | [15] |
| Human serum albumin | PHEMA-based cryogel | N-Methacryloyl-Lphenylalanine (MAPA) | Molecular imprinting | 390.2 mg/g | [32] |
| Hemoglobulin | PHEMA cryogel | N-methacryloyl-L-histidine methyl ester | Molecular imprinting | 11.4 mg/g | [33] |
| Ovalbumin | N-[3-(2-aminoethylamino) propyl] trimethoxysilane | N-isopropylacrylamide and p-vinylphenylboronic acid | Molecular imprinting | 21 mg/g | [34] |
| Lysozyme | Polyacrylamide cryogels | N,N-Methylene bisacrylamide | Molecular imprinting | 36.3 mg/g | [35] |
| Heparin | HEMA-based monolithic cryogel | L-lysine | Affinity-based | 40,500 IU/g | [36] |
| Immunoglobin G | Poly(GMA) beads embedded PHEMA composite cryogels | Iminodiacetic acid (IDA)-Cu2+ | Immobilized metal affinity chromatography based | 257 mg/g | [29] |
| Immunoglobin G | Fc fragment-imprinted PHEMA cryogel | N-Methacryloyl-(l)-cysteine methyl ester (MAC)/ Immobilization of anti-hIgG | Molecular imprinting | 86.9 mg/g | [37] |
| Immunoglobin G | PHEMA composite cryogel disks | Thiophilic ligand | Thiophilic affinity-based | 68.7 mg/g | [29] |
| Immunoglobin G | PHEMA cryogel | Poly(ethylene imine) (PEI) -Cu2+ | Immobilized metal affinity chromatography based | 72.28 mg/g | [30] |
| Bilirubin | PHEMA composite cryogel | N-methacryloyl-(L)-tyrosinemethylester | Molecular imprinting | 10.3 mg/g | [38] |
| Bilirubin | PHEMA cryogel | N-methacryloyl-(l)-tyrosinemethylester | Molecular imprinting | 3.6 mg/g | [39] |
| Heparin | PHEMA cryogel | N-[(3-dimethylamino)-propyl]methacrylamide) | Molecular imprinting | 9.36 mg/g | [40] |
| Cholesterol | PHEMA cryogel | Anti-human β-lipoprotein antibody | Affinity-based | 129 mg/g | [16] |
| Anti-dsDNA antibodies | PHEMA cryogel | Herring testes DNA | Affinity-based | 70,000 IU/g | [41] |
| IgM-antibody | PHEMA cryogel | Protein A | Affinity-based | 42.7 mg/g | [25] |
| Metal Ion | Functional Monomer/Ligand | Method | Adsorption Capacity | Reference |
|---|---|---|---|---|
| Iron(III) | N-methacryloyl-(l)-cysteine methyl ester | Ion-imprinted cryogel | 75 μg/g | [43] |
| Iron(III) | N-methacryloyl-(L)-histidine methyl ester | Affinity-based cryogel | 1.79 mg/g | [44] |
| Aluminum(III) | N-methacryloyl-L-glutamic acid | Ion-imprinted polymer | 0.76 mg/g | [42] |
| Iron(III) | N-Methacryloly-(l)-glutamic acid | Ion-imprinted polymer | 92.6 μmol/g | [45] |
| Scaffolds | Outcome | Reference |
|---|---|---|
| Bone | ||
| Collagen-nanoHA biocomposite cryogels | In vitro: Differentiation of human bone marrow stromal cells into osteoblastic phenotype. In vivo: Reduced inflammatory response by new tissue production | [72] |
| Collagen-nanoHA biocomposite cryogels | In vitro: Adherence and spreading of osteoblast cells along with high cell reproduction | [73] |
| Gelatin-HA cryogels | In vitro: Cell viability In vivo: Biocompatibility at the end of implantation within bone | [74] |
| Chitosan-gelatin composite cryogels incorporated with HA | Excellent cell compatibility and high mineralization with the usage of bone char cryogels | [75] |
| Silk fibroin, chitosan, agarose and HA with/without bioactive glass biocomposite cryogels | In vitro: No advantage of bioactive glass Reproduction of C2C12 myoblasts and mesenchymal stem cells. Potential carriers for recombinant human bone morphogenicprotein-2 (rhBMP-2) and zoledronic acid (ZA) | [76] |
| Gelatin-HA cryogels with/without local vascular endothelial growth factor (VEGF) | Successful grafting of critical-sized bone defects in white rabbits. Favorable effect in early fracture healing | [77] |
| Gelatin and silk fibroin cryogels incorporated with Manuka honey (MH) | Long-term inhibition of bacterial growth with 5% MH-silk fibroin cryogel. Promising alternatives for chronic bone infections | [78] |
| Silk fibroin cryogels | Showing good performance on compressive modulus (50 MPa) and alternatives for bone scaffolds | [79] |
| Electrically stimulated PTAC biocomposite cryogels | Supporting the movement of C2C12 myoblasts towards osteogenic lineage. In vivo and in vitro verification of osteoinductive and osteoconductive characteristics | [80] |
| 3D Hyaluronic acid-based cryogels | Recommendable for bone tissue repair confirmed with optimization experiments | [81] |
| Poly(ethylene glycol) diacrylate-co-N-acryloyl 6-aminocaproic acid cryogels | Osteogenic differentiation of human mesenchymal stem cells (hMSCs) In vitro: Cell viability and proliferation of hMSCs In vivo: Promoted ectopic bone formation | [82] |
| Gelatin cryogel discs | Confirmation of electromagnetic stimuli effect on mesenchymal stromal cells isolated from the bone marrow High cell reproduction and osteogenic differentiation Increased production of bone matrix components | [83] |
| Cartilage | ||
| Collagenase incorporated cryogel | Isolation of chondrocytes and triggered proliferation in a sheep model Synthesis of type II collagen | [71] |
| 2-hydroxyethyl methacrylate–lactate–dextran (HEMA-LLA-D)-based cryogels | Verification of biocompatibility with cartilage cells Chondrocyte attachment and infiltration 73.7 ± 3.3% cell viability after 15 days | [84] |
| Chitosan-gelatin cryogels incorporated with chondroitin sulfate loaded microparticles | Encouraged chondrocyte reproduction and differentiation Elevated matrix formation | [85] |
| Gelatin-oxidized dextran cryogels with bone morphogenetic protein-7 (BMP-7) | Improved matrix synthesis | [86] |
| Chitosan-agarose-gelatin cryogels | In vivo: Confirmed biocompatibility and production of hyaline cartilage in rabbits after implantation | [87] |
| Biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel incorporated with chitosan | Up-regulation of glycosaminoglycans (GAGs) and type II collagen (COL II) secretion | [88] |
| Skin | ||
| Fibrinogen and gelatin containing cryogels | High migration of human dermal fibroblast by elevated fibrinogen concentration | [89] |
| Gelatin-fibrinogen cryogel | Reproduction of fibroblasts and migration of them within the scaffolds | [90] |
| Chitosan-gluconic acid conjugate/poly(vinyl alcohol) cryogels | High infiltration of inflammatory cells into wound In vivo: Healing of partial-thickness wounds with incorporation of basic fibroblast growth factor | [91] |
| Keratinase and enrofloxacin loaded pectin PVA cryogel | Controllable release of antibiotics | [92] |
| Bilayer cryogels, Top layer: antiseptic layer Polyvinylpyrrolidone-iodine (PVP-I) Bottom layer: regenerative layer Gelatin cryogel | Cell infiltration and attachment Proliferation of fibroblasts and keratinocytes In vivo: Regeneration ability was proven in implanted rabbits Resembling to commercial skin regeneration product Neuskin-F | [93] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhshpour, M.; Idil, N.; Perçin, I.; Denizli, A. Biomedical Applications of Polymeric Cryogels. Appl. Sci. 2019, 9, 553. https://0-doi-org.brum.beds.ac.uk/10.3390/app9030553
Bakhshpour M, Idil N, Perçin I, Denizli A. Biomedical Applications of Polymeric Cryogels. Applied Sciences. 2019; 9(3):553. https://0-doi-org.brum.beds.ac.uk/10.3390/app9030553
Chicago/Turabian StyleBakhshpour, Monireh, Neslihan Idil, Işık Perçin, and Adil Denizli. 2019. "Biomedical Applications of Polymeric Cryogels" Applied Sciences 9, no. 3: 553. https://0-doi-org.brum.beds.ac.uk/10.3390/app9030553