Morphology-Controlled Versatile One-Pot Synthesis of Hydrophobic Gold Nanodots, Nanobars, Nanorods, and Nanowires and Their Applications in Surface-Enhanced Raman Spectroscopy
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Synthesis of Gold Nanomaterials
2.2.1. Synthesis of Gold Nanodots
2.2.2. Synthesis of Large Spherical Gold Nanoparticles Via a Seed-Growth Approach
2.2.3. Synthesis of Gold Nanobars
2.2.4. Synthesis of Long Gold Nanorods
2.2.5. Synthesis of Silk-Like Ultralong and Ultrathin Gold Nanowires
2.3. SERS Measurements
2.3.1. SERS Detection of Benzidine in Organic Solvents Using Spherical Gold Nanoparticles as a Substrate
2.3.2. SERS Detection of 3,4-Benzopyrene in Organic Solvents Using Spherical Gold Nanoparticles as a Substrate
2.3.3. SERS Detection of Malachite Green Using Long Gold Nanorods or Silk-Like Ultralong Nanowires as a Solid Substrate
3. Results and Discussion
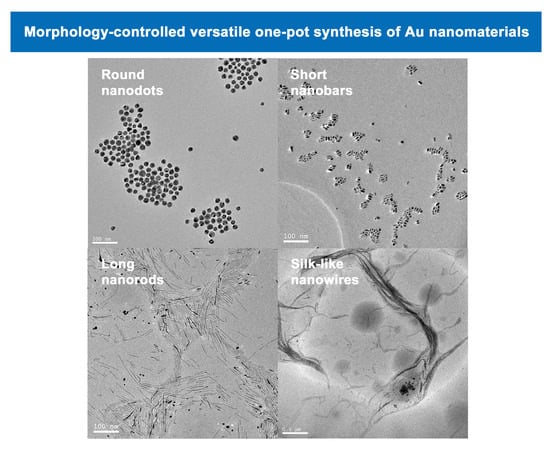
3.1. Characteristics of Au Nanomaterials by TEM and XRD
3.2. Characteristics of Large Spherical Au Nanoparticles by TEM and XRD
3.3. Detection of 3,4-Benzopyrene and Benzidine in Chloroform
3.4. Detection of Malachite Green Using Gold Nanorods or Nanowires as a Solid Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bond, C.G.; Thompson, D.T. Catalysis by Gold. Catal. Rev. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Haruta, M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A Gen. 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Porta, F.; Prati, L.; Rossi, M.; Scari, G. New Au(0) sols as precursors for heterogeneous liquid-phase oxidation catalysts. J. Catal. 2002, 211, 464–469. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Goodman, D.W. Catalytically active gold: From nanoparticles to ultrathin films. Acc. Chem. Res. 2006, 39, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Support gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Grow, M.E.; Pan, H.; Bednarek, M.; Ghann, W.E.; Zabetakis, K.; Cornish, J. Gold nanoparticle-cored poly(propyleneimine) dendrimers as a new platform for multifunctional drug delivery systems. New. J. Chem. 2011, 35, 2366–2374. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; EI-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Heller, D.A.; Winslow, M.M.; Dahlman, J.E.; Pratt, G.W.; Langer, R.; Jacks, T.; Anderson, D.G. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 2011, 12, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Theranostic nanoshells: From probe design to imaging and treatment of cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Schatz, G.C. Using theory and computation to model nanoscale properties. Proc. Natl. Acad. Sci. USA 2007, 104, 6885–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Goodman, P. Current and future uses of gold in electronics. Gold Bull. 2002, 35, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Faraday Trans. 2 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; EI-Sayed, I.H.; EI-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phy. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Park, H.; Schadt, M.J.; Lim, I.S.; Njoki, P.N.; Kim, S.H.; Jang, M.; Luo, J.; Zhong, C. Fabrication of magnetic core@shell Fe oxide@Au nanoparticles for interfacial bioactivity and bio-separation. Langmuir 2007, 23, 9050–9056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Harpster, M.H.; Park, H.J.; Johnson, P.A.; Wilson, W.C. Surface-enhanced Raman scattering detection of DNA derived from the west nile virus genome using magnetic capture of Raman-active gold nanoparticles. Anal. Chem. 2011, 83, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Tan, J.; Kan, J.; Sun, P. A fast and cost-effective detection of melamine by surface enhanced Raman spectroscopy using a novel hydrogen bonding-assisted supramolecular matrix and gold-coated magnetic nanoparticles. Appl. Sci. 2017, 7, 475. [Google Scholar] [CrossRef]
- Subramaniam, C.; Tom, R.T.; Pradeep, T. On the formation of protected gold nanoparticles from AuCl4- by the reduction using aromatic amines. J. Nanopart. Res. 2005, 7, 209–217. [Google Scholar] [CrossRef]
- Newman, J.D.S.; Blanchard, G.J. Formation of gold nanoparticles using amine reducing agents. Langmuir 2006, 22, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, H.; Osterloh, F.E. A simple large-scale synthesis of nearly monodisperse gold and silver nanoparticles with adjustable sizes and with exchangeable surfactants. Chem. Mater. 2004, 16, 2509–2511. [Google Scholar] [CrossRef]
- Sugie, A.; Somete, T.; Kanie, K.; Muramatsu, A.; Mori, A. Triethylsilane as a mild and efficient reducing agent for the preparation of alkanethiol-capped gold nanoparticles. Chem. Commun. 2008, 33, 3882–3884. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yavuz, M.S.; Tuan, H.; Korgel, B.A.; Xia, Y. Ultrathin gold nanowires can be obtained by reducing polymeric stands of oleylamine-AuCl complexes formed via aurophilic interaction. J. Am. Chem. Soc. 2008, 130, 8900–8901. [Google Scholar] [CrossRef] [PubMed]
- Takahata, R.; Yamazoe, S.; Koyasu, K.; Imura, K.; Tsukuda, T. Gold ultrathin nanorods with controlled aspect ratios and surface modifications: Formation mechanism and localized surface plasmon. J. Am. Chem. Soc. 2018, 12, 6640–6647. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Nordquist, C.D.; Jackson, T.N.; Mayer, B.R.; Mbindyo, J.; Mallouk, T.E. Electric-field assisted assembly and alignment of metallic nanowires. Appl. Phys. Lett. 2000, 77, 1399–1401. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Rivas-Murias, B.; Grzelczak, M.; Pérez-Juste, J.; Liz-Marzán, L.M.; Rivadulla, F.; Correa-Duarte, M.A. Highly transparent and conductive films of densely aligned ultrathin Au nanowire monolayers. Nano Lett. 2012, 12, 6066–6070. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Yang, Y.; You, Y.; Li, G.; Guo, J.; Yu, T.; Shen, Z.; Wu, T.; Xing, B. Simple and rapid synthesis of ultrathin gold nanowires, their self-assembly and application in surface-enhanced Raman scattering. Chem. Commun. 2009, 15, 1984–1986. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Research 2018, 7, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Handy, R.D.; Shaw, B.J. Toxic effects of nanoparticles and nanomaterials: Implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc. 2007, 9, 125–144. [Google Scholar] [CrossRef]
- Morgeneyer, M.; Aguerre-Chariol, O.; Bressot, C. STEM imaging to characterize nanoparticle emissions and help to design nanosafer paints. Chem. Eng. Res. Des. 2018, 136, 663–674. [Google Scholar] [CrossRef]
- Bressot, C.; Aubry, A.; Pagnoux, C.; Aguerre-Chariol, O.; Morgeneyer, M. Assessment of functional nanomaterials in medical applications: Can time mend public and occupational health risks related to the products’ fate? J. Toxicol. Environ. Health A 2018, 81, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, B.; Reverberi, A.P.; Varbanov, P.S. Safety opportunities for the synthesis of metal nanoparticles and short-cut approach to workplace risk evaluation. J. Clean. Prod. 2019, 209, 297–308. [Google Scholar] [CrossRef]
- Morgeneyer, M.; Ramirez, A.; Poletto, M.; Smith, S.W.; Tweedie, R.; Heng, J.; Maass, S.; Bressot, C. Particle technology as a uniform discipline? Towards a holistic approach to particles, their creation, characterisation, handling and processing! Chem. Eng. Res. Des. 2018. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neng, J.; Xiang, C.; Jia, K.; Nie, X.; Sun, P. Morphology-Controlled Versatile One-Pot Synthesis of Hydrophobic Gold Nanodots, Nanobars, Nanorods, and Nanowires and Their Applications in Surface-Enhanced Raman Spectroscopy. Appl. Sci. 2019, 9, 935. https://0-doi-org.brum.beds.ac.uk/10.3390/app9050935
Neng J, Xiang C, Jia K, Nie X, Sun P. Morphology-Controlled Versatile One-Pot Synthesis of Hydrophobic Gold Nanodots, Nanobars, Nanorods, and Nanowires and Their Applications in Surface-Enhanced Raman Spectroscopy. Applied Sciences. 2019; 9(5):935. https://0-doi-org.brum.beds.ac.uk/10.3390/app9050935
Chicago/Turabian StyleNeng, Jing, Chen Xiang, Kan Jia, Xiaohua Nie, and Peilong Sun. 2019. "Morphology-Controlled Versatile One-Pot Synthesis of Hydrophobic Gold Nanodots, Nanobars, Nanorods, and Nanowires and Their Applications in Surface-Enhanced Raman Spectroscopy" Applied Sciences 9, no. 5: 935. https://0-doi-org.brum.beds.ac.uk/10.3390/app9050935