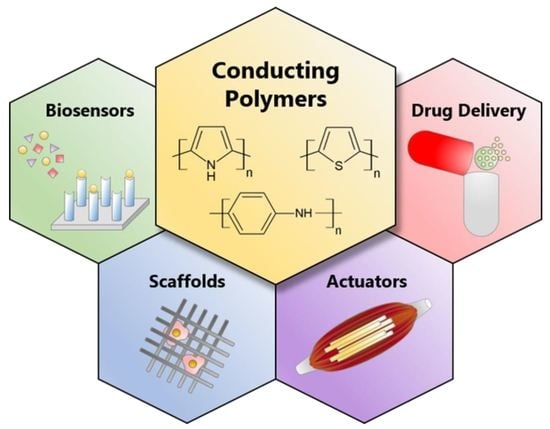
Research Progress on Conducting Polymer-Based Biomedical Applications
Abstract
:1. Introduction
2. Conducting Polymers (CPs)
3. Biomedical Applications
3.1. Biosensors
3.2. Scaffolds for Tissue Engineering
3.3. Actuators for Artificial Muscles
3.4. Drug Delivery
4. Current Stage and Challenges
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials science: A multidisciplinary endeavor. In Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Elsevier Academic Press: San Diego, CA, USA, 2004; pp. 1–9. [Google Scholar]
- Zheng, J.; Tang, M.; Hu, Y.-Y. Lithium ion pathway within Li7La3Zr2O12-polyethylene oxide composite electrolytes. Angew. Chem. Int. Ed. 2016, 55, 12538–12542. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhao, G.; Zhang, X.; Zhou, S.; Wang, P.; An, Y.; Xu, B. Graphene oxide grafted carbon fiber reinforced siliconborocarbonitride ceramics with enhanced thermal stability. Carbon 2015, 95, 157–165. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Nie, H.; Wang, C.-H. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. J. Control. Release 2007, 120, 111–121. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, J.; Pivin, J.C.; Swart, H.C. Noble metal nanoparticles embedding into polymeric materials: From fundamentals to applications. Adv. Colloid Interface Sci. 2015, 226, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef]
- Leja, K.; Lewandowicz, G. Polymer Biodegradation and Biodegradable Polymers-a Review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Li, Y. Challenges and Issues of Using Polymers as Structural Materials in MEMS: A Review. J. Microelectromech. Syst. 2018, 27, 581–598. [Google Scholar] [CrossRef]
- Fincher, C.R.; Ozaki, M.; Heeger, A.J.; MacDiarmid, A.G. Donor and acceptor states in lightly doped polyacetylene, (CH)x. Phys. Rev. B 1979, 19, 4140–4148. [Google Scholar] [CrossRef]
- Rivers, T.J.; Hudson, T.W.; Schmidt, C.E. Synthesis of a Novel, Biodegradable Electrically Conducting Polymer for Biomedical Applications. Adv. Funct. Mater. 2002, 12, 33–37. [Google Scholar] [CrossRef]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef]
- Smela, E. Conjugated polymer actuators for biomedical applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T.W. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef]
- Wang, C.H.; Dong, Y.Q.; Sengothi, K.; Tan, K.L.; Kang, E.T. In-vivo tissue response to polyaniline. Synth. Metals 1999, 102, 1313–1314. [Google Scholar] [CrossRef]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. Part A 2004, 68A, 411–422. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Kausaite, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: An in-vivo study in mice. J. Pharm. Pharm. 2007, 59, 311–315. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, B.; Sekine, J.; Luo, S.-C.; Yu, H.-H. Oligoethylene-Glycol-Functionalized Polyoxythiophenes for Cell Engineering: Syntheses, Characterizations, and Cell Compatibilities. ACS Appl. Mater. Interfaces 2012, 4, 680–686. [Google Scholar] [CrossRef]
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility, 4th ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 3–16. [Google Scholar]
- Ahmed, M.H.; Byrne, J.A.; Keyes, T.E.; Ahmed, W.; Elhissi, A.; Jackson, M.J.; Ahmed, E. Characteristics and applications of titanium oxide as a biomaterial for medical implants. In The Design and Manufacture of Medical Devices; Davim, J.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 1–57. [Google Scholar]
- Heimann, R.B. Materials science of crystalline bioceramics: A review of basic properties and applications. CMU J. 2002, 1, 23–46. [Google Scholar]
- Kohane, D.S.; Langer, R. Biocompatibility and drug delivery systems. Chem. Sci. 2010, 1, 441–446. [Google Scholar] [CrossRef]
- Slater, T.F.; Sawyer, B.; Sträuli, U. Studies on succinate-tetrazolium reductase systems: III. Points of coupling of four different tetrazolium salts. Biochim. Biophys. Acta 1963, 77, 383–393. [Google Scholar] [CrossRef]
- Haining, W.N.; Anderson, D.G.; Little, S.R.; von Berwelt-Baildon, M.S.; Cardoso, A.A.; Alves, P.; Kosmatopoulos, K.; Nadler, L.M.; Langer, R.; Kohane, D.S. pH-triggered microparticles for peptide vaccination. J. Immunol. 2004, 173, 2578. [Google Scholar] [CrossRef]
- Epstein-Barash, H.; Shichor, I.; Kwon, A.H.; Hall, S.; Lawlor, M.W.; Langer, R.; Kohane, D.S. Prolonged duration local anesthesia with minimal toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 7125–7130. [Google Scholar] [CrossRef] [Green Version]
- Yeo, Y.; Burdick, J.A.; Highley, C.B.; Marini, R.; Langer, R.; Kohane, D.S. Peritoneal application of chitosan and UV-cross-linkable chitosan. J. Biomed. Mater. Res. Part A 2006, 78A, 668–675. [Google Scholar] [CrossRef]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [Green Version]
- Kohane, D.S.; Plesnila, N.; Thomas, S.S.; Le, D.; Langer, R.; Moskowitz, M.A. Lipid–sugar particles for intracranial drug delivery: Safety and biocompatibility. Brain Res. 2002, 946, 206–213. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Bowlin, G.L.; Jhurry, D. An assessment of biopolymer- and synthetic polymer-based scaffolds for bone and vascular tissue engineering. Polym. Int. 2013, 62, 523–533. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Rao, P.S. Synthesis of polymeric nanomaterials for biomedical applications. In Nanomaterials in Tissue Engineering; Gaharwar, A.K., Sant, S., Hancock, M.J., Hacking, S.A., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 27–63. [Google Scholar]
- Lakard, B.; Ploux, L.; Anselme, K.; Lallemand, F.; Lakard, S.; Nardin, M.; Hihn, J.Y. Effect of ultrasounds on the electrochemical synthesis of polypyrrole, application to the adhesion and growth of biological cells. Bioelectrochemistry 2009, 75, 148–157. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Baharvand, H.; Kiani, S.; Al-Deyab, S.S.; Ramakrishna, S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, e17–e35. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Y.; Li, S.; Feng, T. The synthesis and characterization of a novel biodegradable and electroactive polyphosphazene for nerve regeneration. Mater. Sci. Eng. C 2010, 30, 160–166. [Google Scholar] [CrossRef]
- Huang, L.; Hu, J.; Lang, L.; Wang, X.; Zhang, P.; Jing, X.; Wang, X.; Chen, X.; Lelkes, P.I.; MacDiarmid, A.G.; et al. Synthesis and characterization of electroactive and biodegradable ABA block copolymer of polylactide and aniline pentamer. Biomaterials 2007, 28, 1741–1751. [Google Scholar] [CrossRef]
- Cui, X.; Lee, V.A.; Raphael, Y.; Wiler, J.A.; Hetke, J.F.; Anderson, D.J.; Martin, D.C. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J. Biomed. Mater. Res. 2001, 56, 261–272. [Google Scholar] [CrossRef]
- Williams, R.L.; Doherty, P.J. A preliminary assessment of poly(pyrrole) in nerve guide studies. J. Mater. Sci. Mater. Med. 1994, 5, 429–433. [Google Scholar] [CrossRef]
- Humpolicek, P.; Kasparkova, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Metals 2012, 162, 722–727. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)4. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2591–2611. [Google Scholar] [CrossRef]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Jang, J. Conducting Polymer Nanomaterials and Their Applications. In Emissive Materials Nanomaterials; Abe, A., Dusˇek, K., Kobayashi, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 189–260. [Google Scholar]
- Hong, S.Y.; Marynick, D.S. Understanding the conformational stability and electronic structures of modified polymers based on polythiophene. Macromolecules 1992, 25, 4652–4657. [Google Scholar] [CrossRef] [Green Version]
- Kundu, K.; Giri, D. Evolution of the electronic structure of cyclic polythiophene upon bipolaron doping. J. Chem. Phys. 1996, 105, 11075–11080. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, Y.; Yang, X.; Chen, J.; Fu, H.; Cheng, T.; Wang, Y. Conducting polymers in environmental analysis. TrAC Trends Anal. Chem. 2012, 39, 163–179. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Grapenson, S.; Jakob, A. Conductive polypyrrole nanofibers via electrospinning: Electrical and morphological properties. Polymer 2006, 47, 1597–1603. [Google Scholar] [CrossRef]
- Aoki, T.; Tanino, M.; Sanui, K.; Ogata, N.; Kumakura, K. Secretory function of adrenal chromaffin cells cultured on polypyrrole films. Biomaterials 1996, 17, 1971–1974. [Google Scholar] [CrossRef]
- Lee, J.-W.; Serna, F.; Nickels, J.; Schmidt, C.E. Carboxylic Acid-Functionalized Conductive Polypyrrole as a Bioactive Platform for Cell Adhesion. Biomacromolecules 2006, 7, 1692–1695. [Google Scholar] [CrossRef] [Green Version]
- Castano, H.; O’Rear, E.A.; McFetridge, P.S.; Sikavitsas, V.I. Polypyrrole Thin Films Formed by Admicellar Polymerization Support the Osteogenic Differentiation of Mesenchymal Stem Cells. Macromol. Biosci. 2004, 4, 785–794. [Google Scholar] [CrossRef]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-based glucose biosensor: A review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Kim, Y.; Cook, S.; Tuladhar, S.M.; Choulis, S.A.; Nelson, J.; Durrant, J.R.; Bradley, D.D.C.; Giles, M.; McCulloch, I.; Ha, C.-S.; et al. A strong regioregularity effect in self-organizing conjugated polymer films and high-efficiency polythiophene:fullerene solar cells. Nat. Mater. 2006, 5, 197–203. [Google Scholar] [CrossRef]
- Oh, J.-W.; Choi, J.; Luong, B.; Kim, N. Effects of polythiophene as a photosensitizer on dynamic and steady-state photorefractive performance in polymeric composites. Macromol. Res. 2010, 18, 8–13. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Peramo, A.; Urbanchek, M.G.; Spanninga, S.A.; Povlich, L.K.; Cederna, P.; Martin, D.C. In Situ Polymerization of a Conductive Polymer in Acellular Muscle Tissue Constructs. Tissue Eng. Part A 2008, 14, 423–432. [Google Scholar] [CrossRef]
- Andrade, C.; Oliveira, M.D.; Faulin, T.; Hering, V.; Abdalla, D.S.P. Biosensors for detection of low-density lipoprotein and its modified forms. In Biosensors for Health, Environment and Biosecurity; Serra, P.A., Ed.; Intech: Rijeka, Croatia, 2011; pp. 215–240. [Google Scholar]
- Borisov, S.M.; Wolfbeis, O.S. Optical Biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Ramanathan, K.; Danielsson, B. Principles and applications of thermal biosensors. Biosens. Bioelectron. 2001, 16, 417–423. [Google Scholar] [CrossRef]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Arshak, K.; Velusamy, V.; Korostynska, O.; Oliwa-Stasiak, K.; Adley, C. Conducting Polymers and Their Applications to Biosensors: Emphasizing on Foodborne Pathogen Detection. IEEE Sens. J. 2009, 9, 1942–1951. [Google Scholar] [CrossRef]
- Newman, J.D.; Turner, A.P.F. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef]
- Wang, J. Glucose Biosensors: 40 Years of Advances and Challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Fogh-Andersen, N.; D’Orazio, P. Proposal for standardizing direct-reading biosensors for blood glucose. Clin. Chem. 1998, 44, 655–659. [Google Scholar]
- Adeloju, S.B.; Moline, A.N. Fabrication of ultra-thin polypyrrole–glucose oxidase film from supporting electrolyte-free monomer solution for potentiometric biosensing of glucose. Biosens. Bioelectron. 2001, 16, 133–139. [Google Scholar] [CrossRef]
- Das, P.; Das, M.; Chinnadayyala, S.R.; Singha, I.M.; Goswami, P. Recent advances on developing 3rd generation enzyme electrode for biosensor applications. Biosens. Bioelectron. 2016, 79, 386–397. [Google Scholar] [CrossRef]
- Kumar, A.; Rajesh; Chaubey, A.; Grover, S.K.; Malhotra, B.D. Immobilization of cholesterol oxidase and potassium ferricyanide on dodecylbenzene sulfonate ion-doped polypyrrole film. J. Appl. Polym. Sci. 2001, 82, 3486–3491. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Ling-Chung, S.K. Conducting polymer gas sensors part II: Response of polypyrrole to methanol vapour. Sens. Actuators 1989, 19, 141–150. [Google Scholar] [CrossRef]
- Dobay, R.; Harsányi, G.; Visy, C. Conducting polymer based electrochemical sensors on thick film substrate. Electroanalysis 1999, 11, 804–808. [Google Scholar] [CrossRef]
- Krutovertsev, S.A.; Sorokin, S.I.; Zorin, A.V.; Letuchy, Y.A.; Antonova, O.Y. Polymer film-based sensors for ammonia detection. Sens. Actuators B 1992, 7, 492–494. [Google Scholar] [CrossRef]
- Lepsenyi, I.; Reichardt, A.; Inzelt, G.; Harsanyi, G. Highly sensitive and selective polymer based gas sensors. In Proceedings of the 12th European Microelectronics and Packaging Conference—IMAPS Europe, Harrogate, UK, 7–9 June 1999; pp. 301–305. [Google Scholar]
- Matindoust, S.; Farzi, A.; Nejad, M.B.; Abadi, M.H.S.; Zou, Z.; Zheng, L.-R. Ammonia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater. Electron. 2017, 28, 7760–7768. [Google Scholar] [CrossRef]
- Nohria, R.; Khillan, R.K.; Su, Y.; Dikshit, R.; Lvov, Y.; Varahramyan, K. Humidity sensor based on ultrathin polyaniline film deposited using layer-by-layer nano-assembly. Sens. Actuators B 2006, 114, 218–222. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Ding, W.; Ishida, T. Humidity control in a closed system utilizing conducting polymers. RSC Adv. 2018, 8, 12540–12546. [Google Scholar] [CrossRef] [Green Version]
- Tully, E.; Higson, S.P.; O’Kennedy, R. The development of a ‘labeless’ immunosensor for the detection of Listeria monocytogenes cell surface protein, Internalin B. Biosens. Bioelectron. 2008, 23, 906–912. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Chaubey, A.; Singh, S. Prospects of conducting polymers in biosensors. Anal. Chim. Acta 2006, 578, 59–74. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef]
- Jayasree, R.; Chandrasekar, R.; Cindrella, L. Synthesis and characterization of polypyrrole-platinum composite for use as electrode material. Polym. Compos. 2012, 33, 1652–1657. [Google Scholar] [CrossRef]
- Molino, P.J.; Higgins, M.J.; Innis, P.C.; Kapsa, R.M.I.; Wallace, G.G. Fibronectin and Bovine Serum Albumin Adsorption and Conformational Dynamics on Inherently Conducting Polymers: A QCM-D Study. Langmuir 2012, 28, 8433–8445. [Google Scholar] [CrossRef]
- Sadekar, A.G.; Mohite, D.; Mulik, S.; Chandrasekaran, N.; Sotiriou-Leventis, C.; Leventis, N. Robust PEDOT films by covalent bonding to substrates using in tandem sol-gel, surface initiated free-radical and redox polymerization. J. Mater. Chem. 2012, 22, 100–108. [Google Scholar] [CrossRef]
- Karim, F.; Fakhruddin, A.N.M. Recent advances in the development of biosensor for phenol: A review. Rev. Environ. Sci. Biotechnol. 2012, 11, 261–274. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Z.; Shao, X.; Zhou, C. Protein adsorption and cytocompatibility of poly(L-lactic acid) surfaces modified with biomacromolecules. J. Appl. Polym. Sci. 2012, 125, E501–E510. [Google Scholar] [CrossRef]
- Chaubey, A.; Gerard, M.; Singhal, R.; Singh, V.S.; Malhotra, B.D. Immobilization of lactate dehydrogenase on electrochemically prepared polypyrrole–polyvinylsulphonate composite films for application to lactate biosensors. Electrochim. Acta 2001, 46, 723–729. [Google Scholar] [CrossRef]
- Ramanathan, K.; Pandey, S.S.; Kumar, R.; Gulati, A.; Murthy, A.S.N.; Malhotra, B.D. Covalent immobilization of glucose oxidase to poly(O-amino benzoic acid) for application to glucose biosensor. J. Appl. Polym. Sci. 2000, 78, 662–667. [Google Scholar] [CrossRef]
- Pang, X.; Imin, P.; Zhitomirsky, I.; Adronov, A. Amperometric Detection of Glucose Using a Conjugated Polyelectrolyte Complex with Single-Walled Carbon Nanotubes. Macromolecules 2010, 43, 10376–10381. [Google Scholar] [CrossRef]
- He, S.; Buelt, A.A.; Hanley, J.M.; Morgan, B.P.; Tennyson, A.G.; Smith, R.C. Sterically Encumbered Bipyridyl-Derivatized Conjugated Polymers and Metallopolymers Incorporating Phenylenevinylene, Phenyleneethynylene, and Fluorenylene Segments. Macromolecules 2012, 45, 6344–6352. [Google Scholar] [CrossRef]
- Mousty, C.; Galland, B.; Cosnier, S. Electrogeneration of a hydrophilic cross-linked polypyrrole film for enzyme electrode fabrication. Application to the amperometric detection of glucose. Electroanalysis 2001, 13, 186–190. [Google Scholar] [CrossRef]
- Fabiano, S.; Tran-Minh, C.; Piro, B.; Anh Dang, L.; Chau Pham, M.; Vittori, O. Poly 3,4-ethylenedioxythiophene as an entrapment support for amperometric enzyme sensor. Mater. Sci. Eng. C 2002, 21, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef]
- Gao, M.; Dai, L.; Wallace, G.G. Biosensors Based on Aligned Carbon Nanotubes Coated with Inherently Conducting Polymers. Electroanalysis 2003, 15, 1089–1094. [Google Scholar] [CrossRef]
- Gao, M.; Huang, S.; Dai, L.; Wallace, G.; Gao, R.; Wang, Z. Aligned coaxial nanowires of carbon nanotubes sheathed with conducting polymers. Angew. Chem. Int. Ed. 2000, 39, 3664–3667. [Google Scholar] [CrossRef]
- Pedrotty, D.M.; Koh, J.; Davis, B.H.; Taylor, D.A.; Wolf, P.; Niklason, L.E. Engineering skeletal myoblasts: Roles of three-dimensional culture and electrical stimulation. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1620–H1626. [Google Scholar] [CrossRef]
- Kawahara, Y.; Yamaoka, K.; Iwata, M.; Fujimura, M.; Kajiume, T.; Magaki, T.; Takeda, M.; Ide, T.; Kataoka, K.; Asashima, M.; et al. Novel Electrical Stimulation Sets the Cultured Myoblast Contractile Function to ‘On’. Pathobiology 2006, 73, 288–294. [Google Scholar] [CrossRef]
- Barbier-Chassefiere, V.; Garcia-Filipe, S.; Yue, X.L.; Kerros, M.E.; Petit, E.; Kern, P.; Saffar, J.L.; Papy-Garcia, D.; Caruelle, J.P.; Barritault, D. Matrix therapy in regenerative medicine, a new approach to chronic wound healing. J. Biomed. Mater. Res. A 2009, 90, 641–647. [Google Scholar] [CrossRef]
- Bach, A.D.; Beier, J.P.; Stern-Staeter, J.; Horch, R.E. Skeletal muscle tissue engineering. J. Cell. Mol. Med. 2004, 8, 413–422. [Google Scholar] [CrossRef]
- Novikov, L.N.; Novikova, L.N.; Mosahebi, A.; Wiberg, M.; Terenghi, G.; Kellerth, J.-O. A novel biodegradable implant for neuronal rescue and regeneration after spinal cord injury. Biomaterials 2002, 23, 3369–3376. [Google Scholar] [CrossRef]
- Wei, Q.; Becherer, T.; Angioletti-Uberti, S.; Dzubiella, J.; Wischke, C.; Neffe, A.T.; Lendlein, A.; Ballauff, M.; Haag, R. Protein interactions with polymer coatings and biomaterials. Angew. Chem. Int. Ed. 2014, 53, 8004–8031. [Google Scholar] [CrossRef]
- Wei, Q.; Haag, R. Universal polymer coatings and their representative biomedical applications. Mater. Horiz. 2015, 2, 567–577. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L.; Yu, G. Multifunctional Nanostructured Conductive Polymer Gels: Synthesis, Properties, and Applications. Acc. Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, J.; Zhang, D.; Zhang, P.; Ou, J.; Liu, B.; Yang, S. Investigation on cell biocompatible behaviors of polyaniline film fabricated via electroless surface polymerization. Appl. Surf. Sci. 2010, 256, 3427–3431. [Google Scholar] [CrossRef]
- Zhang, Z.; Roy, R.; Dugré, F.J.; Tessier, D.; Dao, L.H. In vitro biocompatibility study of electrically conductive polypyrrole-coated polyester fabrics. J. Biomed. Mater. Res. 2001, 57, 63–71. [Google Scholar] [CrossRef]
- Spearman, B.S.; Hodge, A.J.; Porter, J.L.; Hardy, J.G.; Davis, Z.D.; Xu, T.; Zhang, X.; Schmidt, C.E.; Hamilton, M.C.; Lipke, E.A. Conductive interpenetrating networks of polypyrrole and polycaprolactone encourage electrophysiological development of cardiac cells. Acta Biomater. 2015, 28, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Broda, C.R.; Lee, J.Y.; Sirivisoot, S.; Schmidt, C.E.; Harrison, B.S. A chemically polymerized electrically conducting composite of polypyrrole nanoparticles and polyurethane for tissue engineering. J. Biomed. Mater. Res. Part A 2011, 98A, 509–516. [Google Scholar] [CrossRef]
- Guo, B.; Glavas, L.; Albertsson, A.-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Lynn, D.M.; Farhadi, J.; Martin, I.; Shastri, V.; Langer, R. Erodible Conducting Polymers for Potential Biomedical Applications. Angew. Chem. Int. Ed. 2002, 41, 141–144. [Google Scholar] [CrossRef]
- Guimard, N.K.E.; Sessler, J.L.; Schmidt, C.E. Toward a Biocompatible and Biodegradable Copolymer Incorporating Electroactive Oligothiophene Units. Macromolecules 2009, 42, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomater. 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [Green Version]
- Arioz, I.; Erol, O.; Bakan, G.; Dikecoglu, F.B.; Topal, A.E.; Urel, M.; Dana, A.; Tekinay, A.B.; Guler, M.O. Biocompatible Electroactive Tetra(aniline)-Conjugated Peptide Nanofibers for Neural Differentiation. Acs Appl. Mater. Interfaces 2018, 10, 308–317. [Google Scholar] [CrossRef]
- Jiang, T.; Carbone, E.J.; Lo, K.W.H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, K.; Sun, B.; Fang, J.; Zhang, K.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X. The aligned core–sheath nanofibers with electrical conductivity for neural tissue engineering. J. Mater. Chem. B 2014, 2, 7945–7954. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Ebrahimi, M.; Bae, H.; Nguyen, H.K.; Salehi, S.; Kim, S.B.; Kumatani, A.; Matsue, T.; Shi, X.; Nakajima, K.; et al. Gelatin–Polyaniline Composite Nanofibers Enhanced Excitation–Contraction Coupling System Maturation in Myotubes. ACS Appl. Mater. Interfaces 2017, 9, 42444–42458. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Hu, T.; Guo, B.; Ma, P.X. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. 2017, 59, 68–81. [Google Scholar] [CrossRef]
- Lau, H.K.; Kiick, K.L. Opportunities for Multicomponent Hybrid Hydrogels in Biomedical Applications. Biomacromolecules 2015, 16, 28–42. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Wang, B.; Yan, S.; Zhang, K.; Ding, J.; Yin, J. Self-Healing Supramolecular Self-Assembled Hydrogels Based on Poly(l-glutamic acid). Biomacromolecules 2015, 16, 3508–3518. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-Functionalized Chitosan/Pluronic Hydrogels for Tissue Adhesives and Hemostatic Materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef]
- Guo, B.-L.; Gao, Q.-Y. Preparation and properties of a pH/temperature-responsive carboxymethyl chitosan/poly(N-isopropylacrylamide)semi-IPN hydrogel for oral delivery of drugs. Carbohydr. Res. 2007, 342, 2416–2422. [Google Scholar] [CrossRef]
- Mawad, D.; Artzy-Schnirman, A.; Tonkin, J.; Ramos, J.; Inal, S.; Mahat, M.M.; Darwish, N.; Zwi-Dantsis, L.; Malliaras, G.G.; Gooding, J.J.; et al. Electroconductive Hydrogel Based on Functional Poly(Ethylenedioxy Thiophene). Chem. Mater. 2016, 28, 6080–6088. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Wu, J.; Sun, H.; Lin, J.; Fan, S.; Hu, D. Polyaniline/polyacrylamide conducting composite hydrogel with a porous structure. Carbohydr. Polym. 2008, 74, 215–219. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Q.; Wu, J.; Li, Q. A multifunctional hydrogel with high-conductivity, pH-responsive, and release properties from polyacrylate/polyptrrole. J. Appl. Polym. Sci. 2010, 116, 1376–1383. [Google Scholar] [CrossRef]
- Mawad, D.; Lauto, A.; Wallace, G.G. Conductive polymer hydrogels. In Polymeric Hydrogels as Smart Biomaterials; Kalia, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 19–44. [Google Scholar]
- Das, S.; Chatterjee, D.P.; Ghosh, R.; Nandi, A.K. Water soluble polythiophenes: Preparation and applications. RSC Adv. 2015, 5, 20160–20177. [Google Scholar] [CrossRef]
- Mawad, D.; Stewart, E.; Officer, D.L.; Romeo, T.; Wagner, P.; Wagner, K.; Wallace, G.G. A Single Component Conducting Polymer Hydrogel as a Scaffold for Tissue Engineering. Adv. Funct. Mater. 2012, 22, 2692–2699. [Google Scholar] [CrossRef]
- Bendrea, A.-D.; Cianga, L.; Cianga, I. Review paper: Progress in the Field of Conducting Polymers for Tissue Engineering Applications. J. Biomater. Appl. 2011, 26, 3–84. [Google Scholar] [CrossRef]
- Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotechnol. 2013, 24, 847–854. [Google Scholar] [CrossRef]
- Kim, B.-S.; Park, I.-K.; Hoshiba, T.; Jiang, H.-L.; Choi, Y.-J.; Akaike, T.; Cho, C.-S. Design of artificial extracellular matrices for tissue engineering. Prog. Polym. Sci. 2011, 36, 238–268. [Google Scholar] [CrossRef]
- Arias-Pardilla, J.; Otero, T.F.; Martínez, J.G.; Ismail, Y.A. Biomimetic sensing-actuators based on conducting polymers. In Fundaments and Applications of Conducting Polymers; de Jesus Motheo, A., Ed.; Intech: Rijeka, Croatia, 2012. [Google Scholar]
- Fukushima, T.; Asaka, K.; Kosaka, A.; Aida, T. Fully Plastic Actuator through Layer-by-Layer Casting with Ionic-Liquid-Based Bucky Gel. Angew. Chem. Int. Ed. 2005, 44, 2410–2413. [Google Scholar] [CrossRef]
- Kaneto, K. Conducting Polymers. In Soft Actuators: Materials, Modeling, Applications, and Future Perspectives; Asaka, K., Okuzaki, H., Eds.; Springer: Tokyo, Japan, 2014; pp. 95–109. [Google Scholar]
- García-Córdova, F.; Valero, L.; Ismail, Y.A.; Otero, T.F. Biomimetic polypyrrole based all three-in-one triple layer sensing actuators exchanging cations. J. Mater. Chem. 2011, 21, 17265–17272. [Google Scholar] [CrossRef]
- Madden, J.D.; Cush, R.A.; Kanigan, T.S.; Hunter, I.W. Fast contracting polypyrrole actuators. Synth. Metals 2000, 113, 185–192. [Google Scholar] [CrossRef]
- Mirfakhrai, T.; Madden, J.D.W.; Baughman, R.H. Polymer artificial muscles. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- Madden, J.D.W.; Schmid, B.; Hechinger, M.; Lafontaine, S.R.; Madden, P.G.A.; Hover, F.S.; Kimball, R.; Hunter, I.W. Application of polypyrrole actuators: Feasibility of variable camber foils. IEEE J. Ocean. Eng. 2004, 29, 738–749. [Google Scholar] [CrossRef]
- Madden, J.D.; Madden, P.G.; Hunter, I.W. Conducting polymer actuators as engineering materials. In Proceedings of the SPIE’s 9th Annual International Symposium on Smart Structures and Materials, San Diego, CA, USA, 8–12 March 2015; SPIE: Bellingham, WA, USA; p. 15. [Google Scholar]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef]
- Thompson, B.C.; Moulton, S.E.; Ding, J.; Richardson, R.; Cameron, A.; O’Leary, S.; Wallace, G.G.; Clark, G.M. Optimising the incorporation and release of a neurotrophic factor using conducting polypyrrole. J. Control. Release 2006, 116, 285–294. [Google Scholar] [CrossRef]
- Armelin, E.; Gomes, A.L.; Perez-Madrigal, M.M.; Puiggali, J.; Franco, L.; del Valle, L.J.; Rodriguez-Galan, A.; Campos, J.S.d.C.; Ferrer-Anglada, N.; Aleman, C. Biodegradable free-standing nanomembranes of conducting polymer:polyester blends as bioactive platforms for tissue engineering. J. Mater. Chem. 2012, 22, 585–594. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Yan-Peng, J.; Fu-Zhai, C. Surface modification of polyester biomaterials for tissue engineering. Biomed. Mater. 2007, 2, R24. [Google Scholar]
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.-C. Electroactive hydrophilic polylactide surface by covalent modification with tetraaniline. Macromolecules 2012, 45, 652–659. [Google Scholar] [CrossRef]
- Ates, M. A review study of (bio)sensor systems based on conducting polymers. Mater. Sci. Eng. C 2013, 33, 1853–1859. [Google Scholar] [CrossRef]
- Sharma, M.; Waterhouse, G.I.N.; Loader, S.W.C.; Garg, S.; Svirskis, D. High surface area polypyrrole scaffolds for tunable drug delivery. Int. J. Pharm. 2013, 443, 163–168. [Google Scholar] [CrossRef]
- Abidian, M.R.; Kim, D.H.; Martin, D.C. Conducting-polymer nanotubes for controlled drug release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef]











| Polymer | Chemical Structure | Applications | References |
|---|---|---|---|
| Polypyrrole (PPy) |  | Fuel cells, corrosion protection, computer displays, microsurgical tools, biosensors | [33,46,47,48,49] |
| Polyaniline (PANI) |  | Biosensors, neural probes, drug delivery, tissue engineering | [38,50] |
| Polythiophene (PT) |  | Biosensors, solar cells, tissue engineering, photosensitizers, supercapacitors | [41,51,52,53] |
| Poly(3,4-ethyelenedioxythiophene) (PEDOT) |  | Neural electrodes, nerve grafts, heart muscle patches | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Jung, J.; Chang, M. Research Progress on Conducting Polymer-Based Biomedical Applications. Appl. Sci. 2019, 9, 1070. https://0-doi-org.brum.beds.ac.uk/10.3390/app9061070
Park Y, Jung J, Chang M. Research Progress on Conducting Polymer-Based Biomedical Applications. Applied Sciences. 2019; 9(6):1070. https://0-doi-org.brum.beds.ac.uk/10.3390/app9061070
Chicago/Turabian StylePark, Yohan, Jaehan Jung, and Mincheol Chang. 2019. "Research Progress on Conducting Polymer-Based Biomedical Applications" Applied Sciences 9, no. 6: 1070. https://0-doi-org.brum.beds.ac.uk/10.3390/app9061070