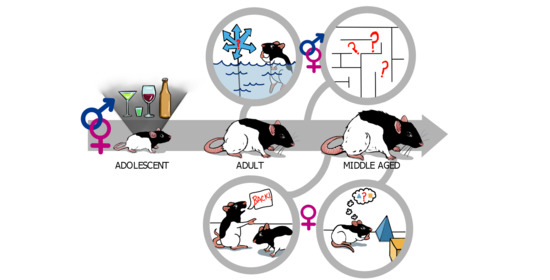
Adolescent Alcohol Exposure Produces Protracted Cognitive-Behavioral Impairments in Adult Male and Female Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Adolescent Intermittent Ethanol (AIE) Exposure Paradigm
2.3. Radial Arm Water Maze
2.4. Hebb-Williams Maze
2.5. Tube Dominance Test
2.6. Novel Object Recognition Test
2.7. Statistical Analyses
3. Results
3.1. AIE Impairs Spatial Navigation and Alters Search Strategies in the RAWM
3.2. AIE Impairs Working Memory in Hebb-Williams Maze
3.3. AIE Increases Submissive Behaviors in Tube Dominance Test
3.4. AIE Impairs NOR in Females
4. Discussion
4.1. AIE Impairs Cognitive Functioning and Flexibility
4.2. AIE Increases Submissive Phenotypes, Indicating Potential Emotional Dysregulation and Stress Susceptibility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crews, F.T.; Robinson, D.L.; Chandler, L.J.; Ehlers, C.L.; Mulholland, P.J.; Pandey, S.C.; Rodd, Z.A.; Spear, L.P.; Swartzwelder, H.S.; Vetreno, R.P. Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings. Alcohol. Clin. Exp. Res. 2019, 43, 1806–1822. [Google Scholar] [CrossRef] [Green Version]
- Fergusson, D.M.; Lynskey, M.T. Alcohol Misuse and Adolescent Sexual Behaviors and Risk Taking. Pediatrics 1996, 98, 91–96. [Google Scholar]
- Chassin, L.; Pitts, S.C.; Prost, J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes. J. Consult. Clin. Psychol. 2002, 70, 67–78. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Schweinsburg, A.D.; Pulido, C.; Tapert, S.F. Adolescent Binge Drinking Linked to Abnormal Spatial Working Memory Brain Activation: Differential Gender Effects. Alcohol. Clin. Exp. Res. 2011, 35, 1831–1841. [Google Scholar] [CrossRef]
- Vetreno, R.P.; Crews, F.T. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Coleman, L.G.; He, J.; Lee, J.; Styner, M.; Crews, F.T. Adolescent Binge Drinking Alters Adult Brain Neurotransmitter Gene Expression, Behavior, Brain Regional Volumes, and Neurochemistry in Mice. Alcohol. Clin. Exp. Res. 2011, 35, 671–688. [Google Scholar] [CrossRef] [Green Version]
- Gass, J.T.; Glen, W.B.; McGonigal, J.T.; Trantham-Davidson, H.; Lopez, M.F.; Randall, P.K.; Yaxley, R.; Floresco, S.B.; Chandler, L.J. Adolescent Alcohol Exposure Reduces Behavioral Flexibility, Promotes Disinhibition, and Increases Resistance to Extinction of Ethanol Self-Administration in Adulthood. Neuropsychopharmacology 2014, 39, 2570–2583. [Google Scholar] [CrossRef]
- Johnston, L.D.; Miech, R.A.; O’Malley, P.; Bachman, J.G.; Schulenberg, J.E.; Patrick, M.E. Demographic subgroup trends among adolescents in the use of various licit and illicit drugs, 1975. Monit. Future Occas. Pap. 2020, 94, 1–821. [Google Scholar]
- Vetreno, R.P.; Crews, F.T. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and toll-like receptors in the adult prefrontal cortex. Neuroscience 2012, 226, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Vetreno, R.P.; Bohnsack, J.P.; Kusumo, H.; Liu, W.; Pandey, S.C.; Crews, F.T. Neuroimmune and epigenetic involvement in adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons: Restoration with voluntary exercise. Addict. Biol. 2020, 25, e12731. [Google Scholar] [CrossRef] [Green Version]
- Swartzwelder, H.S.; Acheson, S.K.; Miller, K.M.; Sexton, H.G.; Liu, W.; Crews, F.T.; Risher, M.-L. Adolescent Intermittent Alcohol Exposure: Deficits in Object Recognition Memory and Forebrain Cholinergic Markers. PLoS ONE 2015, 10, e0140042. [Google Scholar] [CrossRef]
- Varlinskaya, E.I.; Spear, L.P. Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiol. Behav. 2015, 148, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Sircar, R.; Sircar, D. Adolescent Rats Exposed to Repeated Ethanol Treatment Show Lingering Behavioral Impairments. Alcohol. Clin. Exp. Res. 2005, 29, 1402–1410. [Google Scholar] [CrossRef]
- Albert, P.R. Why is depression more prevalent in women? J. Psychiatry Neurosci. JPN 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Larrieu, T.; Sandi, C. Stress-Induced Depression: Is Social Rank a Predictive Risk Factor? BioEssays 2018, 40, 1800012. [Google Scholar] [CrossRef]
- Wetherall, K.; Robb, K.A.; O’Connor, R.C. Social rank theory of depression: A systematic review of self-perceptions of social rank and their relationship with depressive symptoms and suicide risk. J. Affect. Disord. 2019, 246, 300–319. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, P.; Allan, S. The role of defeat and entrapment (arrested flight) in depression: An exploration of an evolutionary view. Psychol. Med. 1998, 28, 585–598. [Google Scholar] [CrossRef]
- Price, J.S.; Sloman, L. Depression as yielding behavior: An animal model based on Schjelderup-Ebbe’s pecking order. Ethol. Sociobiol. 1987, 8, 85–98. [Google Scholar] [CrossRef]
- Komori, T.; Makinodan, M.; Kishimoto, T. Social status and modern-type depression: A review. Brain Behav. 2019, 9, e01464. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.M.; Peñasco, S.; Hernández, M.-D.; Gil, A.; Borcel, E.; Moya, M.; Giné, E.; López-Moreno, J.A.; Guerri, C.; López-Gallardo, M.; et al. Long-Term Effects of Intermittent Adolescent Alcohol Exposure in Male and Female Rats. Front. Behav. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Vetreno, R.P.; Crews, F.T. Adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons and neuroimmune activation are prevented by exercise and indomethacin. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Shukitt-Hale, B.; McEwen, J.J.; Szprengiel, A.; Joseph, J.A. Effect of age on the radial arm water maze—A test of spatial learning and memory. Neurobiol. Aging 2004, 25, 223–229. [Google Scholar] [CrossRef]
- Diamond, D.M.; Park, C.R.; Heman, K.L.; Rose, G.M. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 1999, 9, 542–552. [Google Scholar] [CrossRef]
- Arendash, G.W.; King, D.L.; Gordon, M.N.; Morgan, D.; Hatcher, J.M.; Hope, C.E.; Diamond, D.M. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001, 891, 42–53. [Google Scholar] [CrossRef]
- Rabinovitch, M.S.; Rosvold, H.E. A closed-field intelligence test for rats. Can. J. Psychol. Can. Psychol. 1951, 5, 122–128. [Google Scholar] [CrossRef]
- Shore, D.I.; Stanford, L.; MacInnes, W.J.; Brown, R.E.; Klein, R.M. Of mice and men: Virtual Hebb—Williams mazes permit comparison of spatial learning across species. Cogn. Affect. Behav. Neurosci. 2001, 1, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindzey, G.; Winston, H.; Manosevitz, M. Social Dominance in Inbred Mouse Strains. Nature 1961, 191, 474–476. [Google Scholar] [CrossRef]
- Coleman, L.G.; Liu, W.; Oguz, I.; Styner, M.; Crews, F.T. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol. Biochem. Behav. 2014, 116, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Risher, M.-L.; Fleming, R.L.; Boutros, N.; Semenova, S.; Wilson, W.A.; Levin, E.D.; Markou, A.; Swartzwelder, H.S.; Acheson, S.K. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: Radial-arm maze performance and operant food reinforced responding. PLoS ONE 2013, 8, e62940. [Google Scholar] [CrossRef] [PubMed]
- Varlinskaya, E.I.; Kim, E.U.; Spear, L.P. Chronic intermittent ethanol exposure during adolescence: Effects on stress-induced social alterations and social drinking in adulthood. Brain Res. 2017, 1654, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Dannenhoffer, C.A.; Kim, E.U.; Saalfield, J.; Werner, D.F.; Varlinskaya, E.I.; Spear, L.P. Oxytocin and vasopressin modulation of social anxiety following adolescent intermittent ethanol exposure. Psychopharmacology (Berl.) 2018, 235, 3065–3077. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.M.; Bryant, K.G.; Osborne, J.I.; Chandler, L.J. Age and Sex Interact to Mediate the Effects of Intermittent, High-Dose Ethanol Exposure on Behavioral Flexibility. Front. Pharmacol. 2017, 8, 450. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.M.; Risher, M.-L.; Acheson, S.K.; Darlow, M.; Sexton, H.G.; Schramm-Sapyta, N.; Swartzwelder, H.S. Behavioral Inefficiency on a Risky Decision-Making Task in Adulthood after Adolescent Intermittent Ethanol Exposure in Rats. Sci. Rep. 2017, 7, 4680. [Google Scholar] [CrossRef]
- Sey, N.Y.A.; Gómez-A, A.; Madayag, A.C.; Boettiger, C.A.; Robinson, D.L. Adolescent intermittent ethanol impairs behavioral flexibility in a rat foraging task in adulthood. Behav. Brain Res. 2019, 373, 112085. [Google Scholar] [CrossRef]
- Impairment of Hebb-Williams maze performance following prolonged alcohol consumption in rats. Pharmacol. Biochem. Behav. 1976, 5, 85–86. [CrossRef]
- Oliveira, A.C.; Pereira, M.C.; da Silva Santana, L.N.; Fernandes, R.M.; Teixeira, F.B.; Oliveira, G.B.; Fernandes, L.M.; Fontes-Júnior, E.A.; Prediger, R.D.; Crespo-López, M.E.; et al. Chronic ethanol exposure during adolescence through early adulthood in female rats induces emotional and memory deficits associated with morphological and molecular alterations in hippocampus. J. Psychopharmacol. Oxf. Engl. 2015, 29, 712–724. [Google Scholar] [CrossRef]
- Wang, F.; Kessels, H.W.; Hu, H. The mouse that roared: Neural mechanisms of social hierarchy. Trends Neurosci. 2014, 37, 674–682. [Google Scholar] [CrossRef]
- Colas-Zelin, D.; Light, K.R.; Kolata, S.; Wass, C.; Denman-Brice, A.; Rios, C.; Szalk, K.; Matzel, L.D. The imposition of, but not the propensity for, social subordination impairs exploratory behaviors and general cognitive abilities. Behav. Brain Res. 2012, 232, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Criado, J.R.; Wills, D.N.; Liu, W.; Crews, F.T. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: Correlation with behavioral pathology. Neuroscience 2011, 199, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Füllgrabe, M.W.; Vengeliene, V.; Spanagel, R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol. Biochem. Behav. 2007, 86, 320–326. [Google Scholar] [CrossRef]
- Siegmund, S.; Vengeliene, V.; Singer, M.V.; Spanagel, R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol. Clin. Exp. Res. 2005, 29, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, J.; Zhu, H.; Zhang, Q.; Lin, Z.; Hu, H. Bidirectional Control of Social Hierarchy by Synaptic Efficacy in Medial Prefrontal Cortex. Science 2011, 334, 693–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadwater, M.A.; Lee, S.-H.; Yu, Y.; Zhu, H.; Crews, F.T.; Robinson, D.L.; Shih, Y.-Y.I. Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addict. Biol. 2018, 23, 810–823. [Google Scholar] [CrossRef]
- Medina, K.L.; McQueeny, T.; Nagel, B.J.; Hanson, K.L.; Schweinsburg, A.D.; Tapert, S.F. Prefrontal Cortex Volumes in Adolescents With Alcohol Use Disorders: Unique Gender Effects. Alcohol. Clin. Exp. Res. 2008, 32, 386–394. [Google Scholar] [CrossRef]
- Müller, C.P.; Kalinichenko, L.S.; Tiesel, J.; Witt, M.; Stöckl, T.; Sprenger, E.; Fuchser, J.; Beckmann, J.; Praetner, M.; Huber, S.E.; et al. Paradoxical antidepressant effects of alcohol are related to acid sphingomyelinase and its control of sphingolipid homeostasis. Acta Neuropathol. (Berl.) 2017, 133, 463–483. [Google Scholar] [CrossRef] [Green Version]
- Filiano, A.J.; Martens, L.H.; Young, A.H.; Warmus, B.A.; Zhou, P.; Diaz-Ramirez, G.; Jiao, J.; Zhang, Z.; Huang, E.J.; Gao, F.-B.; et al. Dissociation of Frontotemporal Dementia–Related Deficits and Neuroinflammation in Progranulin Haploinsufficient Mice. J. Neurosci. 2013, 33, 5352–5361. [Google Scholar] [CrossRef] [Green Version]
- Bunnell, B.N.; Bemporad, J.R.; Flesher, C.K. Septal forebrain lesions and social dominance behavior in the hooded rat. Psychon. Sci. 1966, 6, 207–208. [Google Scholar] [CrossRef] [Green Version]
- Bandler, R.J. Facilitation of Aggressive Behaviour in Rat by Direct Cholinergic Stimulation of the Hypothalamus. Nature 1969, 224, 1035–1036. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macht, V.; Elchert, N.; Crews, F. Adolescent Alcohol Exposure Produces Protracted Cognitive-Behavioral Impairments in Adult Male and Female Rats. Brain Sci. 2020, 10, 785. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10110785
Macht V, Elchert N, Crews F. Adolescent Alcohol Exposure Produces Protracted Cognitive-Behavioral Impairments in Adult Male and Female Rats. Brain Sciences. 2020; 10(11):785. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10110785
Chicago/Turabian StyleMacht, Victoria, Natalie Elchert, and Fulton Crews. 2020. "Adolescent Alcohol Exposure Produces Protracted Cognitive-Behavioral Impairments in Adult Male and Female Rats" Brain Sciences 10, no. 11: 785. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10110785