Adolescent Awkwardness: Alterations in Temporal Control Characteristics of Posture with Maturation and the Relation to Movement Exploration
Abstract
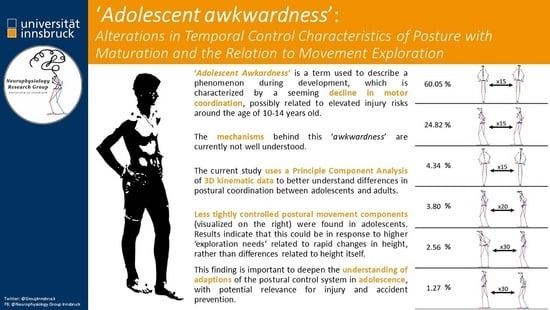
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement Procedure
2.3. Instrumentation
2.4. Data Processing
2.5. Statistics
3. Results
3.1. PCA Results
3.2. Main Results—Motor Control Differences between Adolescents vs. Adults
3.2.1. Coordinative Structure—rVARk
3.2.2. Temporal Control Characteristics—Nk
3.3. Secondary Results—Differences between Adolescents, Smaller and Taller Adults
3.3.1. Relative Variance—rVARk
3.3.2. Number of Zero-Crossings—Nk
4. Discussion
4.1. Main Results—Motor Control Differences between Adolescents vs. Adults
4.2. Secondary Results—Differences between Adolescents, Smaller and Taller Adults
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quatman-Yates, C.C.; Quatman, C.E.; Meszaros, A.J.; Paterno, M.V.; Hewett, T.E. A systematic review of sensorimotor function during adolescence: A developmental stage of increased motor awkwardness? Br. J. Sports Med. 2012, 46, 649–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viel, S.; Vaugoyeau, M.; Assaiante, C. Adolescence: A Transient Period of Proprioceptive Neglect in Sensory Integration of Postural Control. Motor Control. 2009, 13, 25–42. [Google Scholar] [CrossRef] [PubMed]
- John, C.; Rahlf, A.L.; Hamacher, D.; Zech, A. Influence of biological maturity on static and dynamic postural control among male youth soccer players. Gait Posture 2019, 68, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Barela, J.A.; Jeka, J.J.; Clark, J.E. Postural control in children. Exp. Brain Res. 2003, 150, 434–442. [Google Scholar] [CrossRef] [PubMed]
- McCollum, G.; Leen, T.K. Form and Exploration of Mechanical Stability Limits in Erect Stance. J. Mot. Behav. 1989, 21, 225–244. [Google Scholar] [CrossRef]
- Woollacott, M.H.; Shumway-Cook, A. Changes in Posture Control across the Life Span—A Systems Approach. Phys. Ther. 1990, 70, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Conforto, S.; Lopez, L.; Renzi, P.; D’Alessio, T. The development of postural strategies in children: A factorial design study. J. Neuroeng. Rehabil. 2005, 2, 29. [Google Scholar] [CrossRef] [Green Version]
- Kirshenbaum, N.; Riach, C.; Starkes, J. Non-linear development of postural control and strategy use in young children: A longitudinal study. Exp. Brain Res. 2001, 140, 420–431. [Google Scholar] [CrossRef]
- Rinaldi, N.M.; Polastri, P.F.; Barela, J.A. Age-related changes in postural control sensory reweighting. Neurosci. Lett. 2009, 467, 225–229. [Google Scholar] [CrossRef]
- Micheli, L.J. Overuse injuries in children’s sports: The growth factor. Orthop. Clin. North Am. 1983, 14, 337–360. [Google Scholar]
- Pickett, K.; Konczak, J. Measuring kinaesthetic sensitivity in typically developing children. Dev. Med. Child Neurol. 2009, 51, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.; Menon, V.; Reiss, A.L. Maturation of Brain Function Associated with Response Inhibition. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 1231–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallau, S.; Vaugoyeau, M.; Assaiante, C. Postural Strategies and Sensory Integration: No Turning Point between Childhood and Adolescence. PLoS ONE 2010, 5, e13078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bult, H.J.; Barendrecht, M.; Tak, I.J.R. Injury Risk and Injury Burden Are Related to Age Group and Peak Height Velocity Among Talented Male Youth Soccer Players. Orthop. J. Sports Med. 2018, 6. [Google Scholar] [CrossRef]
- van der Sluis, A.; Elferink-Gemser, M.T.; Coelho-e-Silva, M.J.; Nijboer, J.A.; Brink, M.S.; Visscher, C. Sport Injuries Aligned to Peak Height Velocity in Talented Pubertal Soccer Players. Int. J. Sports Med. 2014, 35, 351–355. [Google Scholar] [CrossRef]
- Emmerik, R. van, Wegen E van. On the Functional Aspects of Variability in Postural Control. Exerc. Sport Sci. Rev. 2002, 30, 177–183. [Google Scholar] [CrossRef]
- Riccio, G.E.; Stoffregen, T.A. Affordances as constraints on the control of stance. Hum. Mov. Sci. 1988, 7, 265–300. [Google Scholar] [CrossRef]
- Stoffregen, T.A.; Yang, C.-M.; Bardy, B.G. Affordance Judgments and Nonlocomotor Body Movement. Ecol. Psychol. 2005, 17, 75–104. [Google Scholar] [CrossRef]
- Mark, L.S.; Balliett, J.A.; Craver, K.D.; Douglas, S.D.; Fox, T. What an Actor Must Do in Order to Perceive the Affordance for Sitting. Ecol. Psychol. 1990, 2, 325–366. [Google Scholar] [CrossRef]
- van Andel, S.; Cole, M.H.; Pepping, G.-J. A systematic review on perceptual-motor calibration to changes in action capabilities. Hum. Mov. Sci. 2017, 51, 59–71. [Google Scholar] [CrossRef]
- Franchak, J.M.; Adolph, K.E. Gut estimates: Pregnant women adapt to changing possibilities for squeezing through doorways. Atten. Percept. Psychophys. 2014, 76, 460–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogamba, M.I.; Loverro, K.L.; Laudicina, N.M.; Gill, S.V.; Lewis, C.L. Changes in Gait with Anteriorly Added Mass: A Pregnancy Simulation Study. J. Appl. Biomech. 2016, 32, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Todorov, E.; Jordan, M.I. Optimal feedback control as a theory of motor coordination. Nat. Neurosci. 2002, 5, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.H. Optimal feedback control and the neural basis of volitional motor control. Nat. Rev. Neurosci. 2004, 5, 532–545. [Google Scholar] [CrossRef]
- Honegger, F.; Tielkens, R.J.M.; Allum, J.H.J. Movement strategies and sensory reweighting in tandem stance: Differences between trained tightrope walkers and untrained subjects. Neuroscience 2013, 254, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.; Trippel, M.; Discher, M.; Dietz, V. Influence of Subjects’ Height on the Stabilization of Posture. Acta Oto-Laryngol. 1992, 112, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.C.; Luna NM, S.; Mochizuki, L.; Barbieri, F.; Santos, S.; Greve, J.M.D.A. The influence of anthropometric factors on postural balance: The relationship between body composition and posturographic measurements in young adults. Clinics 2012, 67, 1433–1441. [Google Scholar] [CrossRef]
- Fabunmi, A.A.; Gbiri, C.A. Relationship between balance performance in the elderly and some anthropometric variables. Afr. J. Med. Med. Sci. 2008, 37, 321–326. [Google Scholar]
- Kejonen, P.; Kauranen, K.; Vanharanta, H. The relationship between anthropometric factors and body-balancing movements in postural balance. Arch. Phys. Med. Rehabil. 2003, 84, 17–22. [Google Scholar] [CrossRef]
- Palluel, E.; Nougier, V.; Olivier, I. Postural control and attentional demand during adolescence. Brain Res. 2010, 1358, 151–159. [Google Scholar] [CrossRef]
- Ghislieri, M.; Gastaldi, L.; Pastorelli, S.; Tadano, S.; Agostini, V. Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors 2019, 19, 4075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federolf, P.A. A novel approach to study human posture control: “Principal movements” obtained from a principal component analysis of kinematic marker data. J. Biomech. 2016, 49, 364–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daffertshofer, A.; Lamoth, C.J.; Meijer, O.G.; Beek, P.J. PCA in studying coordination and variability: A tutorial. Clin. Biomech. 2004, 19, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Federolf, P.; Roos, L.; Nigg, B.M. Analysis of the multi-segmental postural movement strategies utilized in bipedal, tandem and one-leg stance as quantified by a principal component decomposition of marker coordinates. J. Biomech. 2013, 46, 2626–2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, A.; Meulenbroek, R.; Haid, T.; Federolf, P. Postural reconfiguration and cycle-to-cycle variability in patients with work-related musculoskeletal disorders compared to healthy controls and in relation to pain emerging during a repetitive movement task. Clin. Biomech. 2018, 54, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Promsri, A.; Haid, T.; Federolf, P. How does lower limb dominance influence postural control movements during single leg stance? Hum. Mov. Sci. 2018, 58, 165–174. [Google Scholar] [CrossRef]
- Promsri, A.; Longo, A.; Haid, T.; Doix AC, M.; Federolf, P. Leg Dominance as a Risk Factor for Lower-Limb Injuries in Downhill Skiers—A Pilot Study into Possible Mechanisms. Int. J. Environ. Res. Public Health 2019, 16, 3399. [Google Scholar] [CrossRef] [Green Version]
- Haid, T.H.; Doix, A.C.M.; Nigg, B.M.; Federolf, P.A. Age Effects in Postural Control Analyzed via a Principal Component Analysis of Kinematic Data and Interpreted in Relation to Predictions of the Optimal Feedback Control Theory. Front. Aging Neurosci 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Wachholz, F.; Tiribello, F.; Promsri, A.; Federolf, P. Should the Minimal Intervention Principle Be Considered When Investigating Dual-Tasking Effects on Postural Control? Brain Sci. 2020, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Haid, T.H.; Zago, M.; Promsri, A.; Doix AC, M.; Federolf, P.A. PManalyzer: A Software Facilitating the Study of Sensorimotor Control of Whole-Body Movements. Front. Neuroinform. 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Gløersen, Ø.; Federolf, P. Predicting Missing Marker Trajectories in Human Motion Data Using Marker Intercorrelations. PLoS ONE 2016, 11, e0152616. [Google Scholar] [CrossRef]
- Federolf, P.A. A Novel Approach to Solve the “Missing Marker Problem” in Marker-Based Motion Analysis That Exploits the Segment Coordination Patterns in Multi-Limb Motion Data. PLoS ONE 2013, 8, e78689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gløersen, Ø.; Myklebust, H.; Hallén, J.; Federolf, P. Technique analysis in elite athletes using principal component analysis. J. Sports Sci. 2017, 1–9. [Google Scholar] [CrossRef]
- de Leva, P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. J. Biomech. 1996, 29, 1223–1230. [Google Scholar] [CrossRef]
- Troje, N.F. Decomposing biological motion: A framework for analysis and synthesis of human gait patterns. J. Vis. 2002, 2, 371–387. [Google Scholar] [CrossRef]
- Bockemühl, T.; Troje, N.F.; Dürr, V. Inter-joint coupling and joint angle synergies of human catching movements. Hum. Mov. Sci. 2010, 29, 73–93. [Google Scholar] [CrossRef] [Green Version]
- Verrel, J.; Lövdén, M.; Schellenbach, M.; Schaefer, S.; Lindenberger, U. Interacting effects of cognitive load and adult age on the regularity of whole-body motion during treadmill walking. Psychol. Aging 2009, 24, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Wachholz, F.; Kockum, T.; Haid, T.; Federolf, P. Changed Temporal Structure of Neuromuscular Control, Rather Than Changed Intersegment Coordination, Explains Altered Stabilographic Regularity after a Moderate Perturbation of the Postural Control System. Entropy 2019, 21, 614. [Google Scholar] [CrossRef] [Green Version]
- Haid, T.; Federolf, P. Human Postural Control: Assessment of Two Alternative Interpretations of Center of Pressure Sample Entropy through a Principal Component Factorization of Whole-Body Kinematics. Entropy 2018, 20, 30. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, R. Parametric measures of effect size. In The Handbook of Research Synthesis; Russell Sage Foundation: New York, NY, USA, 1994; pp. 231–244. [Google Scholar]
- Hepple, R.T. Sarcopenia-A Critical Perspective. Sci. Aging Knowl. Environ. 2003, 2003, pe31. [Google Scholar] [CrossRef]
- Promsri, A.; Haid, T.; Werner, I.; Federolf, P. Leg Dominance Effects on Postural Control When Performing Challenging Balance Exercises. Brain Sci. 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Andel, S.; McGuckian, T.B.; Chalkley, D.; Cole, M.H.; Pepping, G.J. Principles of the Guidance of Exploration for Orientation and Specification of Action. Front. Behav. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Pepping, G.-J.; Li, F.-X. The Role of Haptic Exploration of Ground Surface Information in Perception of Overhead Reachability. J. Mot. Behav. 2008, 40, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.J. The Ecological Approach to Visual Perception: Classic Edition; Psychology Press: London, UK, 2014. [Google Scholar]
- Withagen, R.; De Poel, H.J.; Araújo, D.; Pepping, G.J. Affordances can invite behavior: Reconsidering the relationship between affordances and agency. New Ideas Psychol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Cisek, P.; Kalaska, J.F. Neural Mechanisms for Interacting with a World Full of Action Choices. Annu. Rev. Neurosci. 2010, 33, 269–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Participants | Adolescents n = 12 | Adults ≤ 180 cm n = 9 | Adults ≥ 190 cm n = 5 | p-Value ANOVA |
|---|---|---|---|---|
| Age [years] | 13.2 ± 0.9 | 25.4 ± 3.1 | 27.4 ± 2.2 | <0.001 |
| Height [cm] | 168.1 ± 8.8 | 177.9 ± 3.0 | 192.0 ± 2.5 | <0.001 |
| Weight [kg] | 59.8 ± 10.4 | 74.2 ± 4.1 | 87.8 ± 8.0 | <0.001 |
| rVARk | Adolescents Mean | Adults Mean | p-Value | r | Cumulative Adolescents | Cumulative Adults |
|---|---|---|---|---|---|---|
| rVAR1 | 51.2 ± 22.6 | 62.2 ± 14.5 | 0.274 | 0.222 | 51.2 | 62.2 |
| rVAR2 | 31.1 ± 19.9 | 28.3 ± 14.9 | 0.820 | 0.050 | 82.3 | 90.5 |
| rVAR3 | 4.9 ± 3.9 | 2.8 ± 2.3 | 0.131 | 0.303 | 87.2 | 93.3 |
| rVAR4 | 5.2 ± 6.0 | 2.2 ± 1.6 | 0.036 | 0.414 | 92.4 | 95.5 |
| rVAR5 | 2.8 ± 1.8 | 1.4 ± 1.5 | 0.011 | 0.494 | 95.2 | 96.9 |
| rVAR6 | 1.4 ± 1.5 | 0.8 ± 0.6 | 0.322 | 0.202 | 96.6 | 97.7 |
| rVAR7 | 0.7 ± 0.4 | 0.6 ± 0.5 | 0.274 | 0.222 | 97.3 | 98.3 |
| rVAR8 | 0.7 ± 1.0 | 0.3 ± 0.2 | 0.145 | 0.293 | 98.0 | 98.6 |
| rVAR9 | 0.3 ± 0.2 | 0.2 ± 0.3 | 0.041 | 0.403 | 98.3 | 98.8 |
| rVARk | Adolescents Mean | Adults Mean | p-Value | r | Cumulative Adolescents | Cumulative Adults |
|---|---|---|---|---|---|---|
| rVAR1 | 61.0 ± 19.3 | 61.9 ± 18.4 | 0.940 | 0.020 | 61.0 | 61.9 |
| rVAR2 | 21.5 ± 19.4 | 23.5 ± 15.0 | 0.252 | 0.232 | 82.5 | 85.4 |
| rVAR3 | 5.0 ± 3.7 | 4.0 ± 5.3 | 0.231 | 0.242 | 87.5 | 89.4 |
| rVAR4 | 4.4 ± 4.8 | 3.4 ± 4.7 | 0.462 | 0.151 | 91.9 | 92.8 |
| rVAR5 | 3.4 ± 2.5 | 2.5 ± 2.3 | 0.193 | 0.262 | 95.3 | 95.3 |
| rVAR6 | 1.7 ± 1.6 | 1.5 ± 1.7 | 0.705 | 0.081 | 97.0 | 96.8 |
| rVAR7 | 0.7 ± 0.4 | 1.0 ± 1.0 | 0.494 | 0.141 | 97.7 | 97.8 |
| rVAR8 | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.595 | 0.111 | 98.2 | 98.3 |
| rVAR9 | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.432 | 0.161 | 98.5 | 98.5 |
| Nk | Adolescents Mean | Adults Mean | p-Value Group | ηp2 |
|---|---|---|---|---|
| N1 | 105.9 ± 24.3 | 89.0 ± 27.0 | 0.119 | 0.098 |
| N2 | 116.0 ± 19.8 | 134.4 ± 19.7 | 0.004 * | 0.298 |
| N3 | 131.7 ± 18.8 | 139.0 ± 18.0 | 0.200 | 0.068 |
| N4 | 129.7 ± 17.4 | 143.0 ± 17.2 | 0.020 | 0.206 |
| N5 | 117.8 ± 10.8 | 134.2 ± 16.5 | 0.001 * | 0.350 |
| N6 | 143.9 ± 13.1 | 159.2 ± 13.6 | 0.001 * | 0.379 |
| N7 | 142.3 ± 14.1 | 154.7 ± 17.3 | 0.026 | 0.191 |
| N8 | 152.5 ± 9.6 | 154.6 ± 15.1 | 0.355 | 0.036 |
| N9 | 164.3 ± 10.7 | 169.1 ± 11.1 | 0.122 | 0.097 |
| rVARk | Adolescents Mean | Adults ≤ 180 Mean | Adults ≥ 190 Mean | p-Value | ηp2 |
|---|---|---|---|---|---|
| rVAR1 | 51.2 ± 22.6 | 58.8 ± 14.1 | 68.2 ± 14.7 | 0.345 | 0.445 |
| rVAR2 | 31.1 ± 19.9 | 34.1 ± 13.6 | 17.9 ± 9.7 | 0.125 | 0.094 |
| rVAR3 | 4.9 ± 3.9 | 1.6 ± 1.3 | 5.0 ± 2.2 | 0.011 | 0.303 |
| rVAR4 | 5.2 ± 6.0 | 1.7 ± 0.9 | 3.0 ± 2.4 | 0.054 | 0.167 |
| rVAR5 | 2.8 ± 1.8 | 0.8 ± 0.1 | 2.4 ± 2.3 | 0.021 | 0.248 |
| rVAR6 | 1.4 ± 1.5 | 0.8 ± 0.1 | 0.8 ± 0.5 | 0.569 | 0.038 |
| rVAR7 | 0.7 ± 0.4 | 0.4 ± 0.1 | 0.9 ± 0.8 | 0.124 | 0.095 |
| rVAR8 | 0.7 ± 1.0 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.254 | 0.032 |
| rVAR9 | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.038 | 0.197 |
| Nk | Adolescents Mean | Adults ≤ 180 Mean | Adults ≥ 190 Mean | p-Value | ηp2 | Adolescents Adults ≤ 180 | Adolescents Adults ≥ 190 | Adults ≤ 180 Adults ≥ 190 |
|---|---|---|---|---|---|---|---|---|
| N1 | 105.9 ± 24.3 | 94.2 ± 29.2 | 83.7 ± 22.2 | 0.227 | 0.121 | 0.873 | 0.306 | 1.000 |
| N2 | 116.0 ± 19.8 | 140.2 ± 17.0 | 128.5 ± 22.7 | 0.007 * | 0.348 | 0.006 * | 0.448 | 0.580 |
| N3 | 131.7 ± 18.8 | 142.2 ± 19.2 | 135.9 ± 15.8 | 0.351 | 0.087 | 0.458 | 1.000 | 1.000 |
| N4 | 129.7 ± 17.4 | 146.5 ± 14.6 | 139.5 ± 21.2 | 0.012 | 0.322 | 0.011 | 1.000 | 0.179 |
| N5 | 117.8 ± 10.8 | 140.8 ± 10.1 | 127.7 ± 22.4 | 0.002 * | 0.429 | 0.001 * | 0.385 | 0.265 |
| N6 | 143.9 ± 13.1 | 163.4 ± 13.5 | 155.0 ± 12.6 | 0.001 * | 0.475 | 0.001 * | 0.352 | 0.154 |
| N7 | 142.3 ± 14.1 | 159.9 ± 17.1 | 149.4 ± 16.4 | 0.001 * | 0.440 | 0.002 * | 1.000 | 0.012 |
| N8 | 152.5 ± 9.6 | 162.5 ± 10.6 | 146.6 ± 17.2 | 0.024 | 0.277 | 0.114 | 0.878 | 0.033 |
| N9 | 164.3 ± 10.7 | 173.3 ± 11.2 | 164.9 ± 9.3 | 0.086 | 0.192 | 0.109 | 1.000 | 0.339 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachholz, F.; Tiribello, F.; Mohr, M.; van Andel, S.; Federolf, P. Adolescent Awkwardness: Alterations in Temporal Control Characteristics of Posture with Maturation and the Relation to Movement Exploration. Brain Sci. 2020, 10, 216. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10040216
Wachholz F, Tiribello F, Mohr M, van Andel S, Federolf P. Adolescent Awkwardness: Alterations in Temporal Control Characteristics of Posture with Maturation and the Relation to Movement Exploration. Brain Sciences. 2020; 10(4):216. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10040216
Chicago/Turabian StyleWachholz, Felix, Federico Tiribello, Maurice Mohr, Steven van Andel, and Peter Federolf. 2020. "Adolescent Awkwardness: Alterations in Temporal Control Characteristics of Posture with Maturation and the Relation to Movement Exploration" Brain Sciences 10, no. 4: 216. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10040216