Sesamol Ameliorates Renal Injury-Mediated Atherosclerosis via Inhibition of Oxidative Stress/IKKα/p53
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Antibodies, and Cells
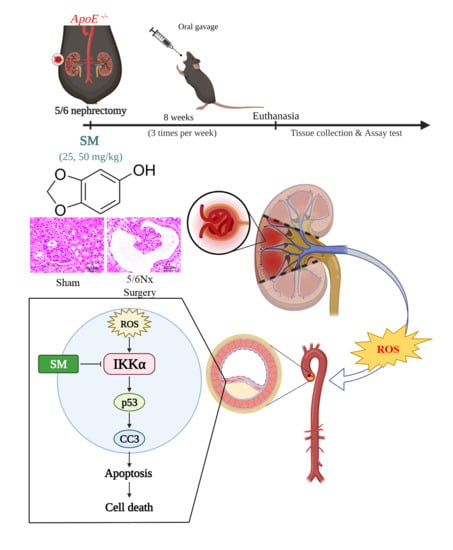
2.2. Animal Model and Experimental Design
2.3. Lipid Peroxidation Assay, Oil Red O Staining, and Histology Staining of Aortas
2.4. Measurement of Cell Viability
2.5. Measurement of Intracellular ROS Levels in Cells
2.6. Apoptosis Assay
2.7. Protein Extraction and Western Blotting
2.8. Quantitative Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. Effects of SM on ROS Levels and Atherosclerosis in 5/6 Nx ApoE–/– KO Mice
3.2. In Vitro Activity of SM on HAECs Injured Via H2O2 Treatment
3.3. Effects of SM on the Apoptosis of HAECs Exposed to H2O2
3.4. Protective Effects of SM against H2O2-Induced Endothelial Cell Apoptosis Is Mediated via the IKKα Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wanner, C.; Amann, K.; Shoji, T. The heart and vascular system in dialysis. Lancet 2016, 388, 276–284. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Wang, Y.; Nabi, X. Protective effect of Ziziphora clinopodioides flavonoids against H2O2-induced oxidative stress in HUVEC cells. Biomed. Pharm. 2019, 117, 109156. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H. The effects of exogenous H2O2 on cell death, reactive oxygen species and glutathione levels in calf pulmonary artery and human umbilical vein endothelial cells. Int. J. Mol. Med. 2013, 31, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Sorriento, D.; Santulli, G.; Del Giudice, C.; Anastasio, A.; Trimarco, B.; Iaccarino, G. Endothelial cells are able to synthesize and release catecholamines both in vitro and in vivo. Hypertension 2012, 60, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. Sci. 2016, 2016, 9152732. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Huang, Y. Chinese Herbal Medicine on Cardiovascular Diseases and the Mechanisms of Action. Front. Pharm. 2016, 7, 469. [Google Scholar] [CrossRef] [Green Version]
- Mashour, N.H.; Lin, G.I.; Frishman, W.H. Herbal medicine for the treatment of cardiovascular disease: Clinical considerations. Arch. Intern Med. 1998, 158, 2225–2234. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chen, F.Y.; Lee, A.S.; Ting, K.H.; Chang, C.M.; Hsu, J.F.; Lee, W.S.; Sheu, J.R.; Chen, C.H.; Shen, M.Y. Sesamol reduces the atherogenicity of electronegative L5 LDL in vivo and in vitro. J. Nat. Prod. 2015, 78, 225–233. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, B.; Wang, Y.; Zou, C.; Qiao, Q.; Diao, Z.; Mi, Y.; Zhu, D.; Liu, X. Sesamol Induces Human Hepatocellular Carcinoma Cells Apoptosis by Impairing Mitochondrial Function and Suppressing Autophagy. Sci. Rep. 2017, 7, 45728. [Google Scholar] [CrossRef]
- Ruankham, W.; Suwanjang, W.; Wongchitrat, P.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Sesamin and sesamol attenuate H2O2 -induced oxidative stress on human neuronal cells via the SIRT1-SIRT3-FOXO3a signaling pathway. Nutr. Neurosci. 2019, 24, 90–101. [Google Scholar] [CrossRef]
- Henrich, T.J.; Hatano, H.; Bacon, O.; Hogan, L.E.; Rutishauser, R.; Hill, A.; Kearney, M.F.; Anderson, E.M.; Buchbinder, S.P.; Cohen, S.E.; et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med. 2017, 14, e1002417. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kaur, I.P. Development and evaluation of sesamol as an antiaging agent. Int. J. Derm. 2006, 45, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Khullar, N.; Kakkar, V.; Kaur, I.P. Hepatoprotective effects of sesamol loaded solid lipid nanoparticles in carbon tetrachloride induced sub-chronic hepatotoxicity in rats. Environ. Toxicol. 2016, 31, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.Z.; Wan, C.H.; Hsu, H.F.; Lin, Y.M.; Liu, M.Y. The prophylactic protective effect of sesamol against ferric-nitrilotriacetate-induced acute renal injury in mice. Food Chem. Toxicol. 2008, 46, 2736–2741. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhou, P.; Sun, Y.; Meng, X.; Dai, Z.; Sun, G.; Sun, X. Protective Effects and Target Network Analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion Injury: A Comprehensive Overview of Experimental Studies. Cells 2018, 7, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.W.; Byun, M.S.; Yi, D.; Lee, J.H.; Jeon, S.Y.; Jung, G.; Lee, H.N.; Sohn, B.K.; Lee, J.Y.; Kim, Y.K.; et al. Coffee intake and decreased amyloid pathology in human brain. Transl. Psychiatry 2019, 9, 270. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Saatman, K.E.; Chen, L. Therapeutic potential of natural compounds from Chinese medicine in acute and subacute phases of ischemic stroke. Neural Regen. Res. 2020, 15, 416–424. [Google Scholar]
- Wiese, C.B.; Zhong, J.; Xu, Z.Q.; Zhang, Y.; Ramirez Solano, M.A.; Zhu, W.; Linton, M.F.; Sheng, Q.; Kon, V.; Vickers, K.C. Dual inhibition of endothelial miR-92a-3p and miR-489-3p reduces renal injury-associated atherosclerosis. Atherosclerosis 2019, 282, 121–131. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubina, P.; Lahera, V.; Luno, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. Suppl. 2008, 74, S4–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geetha, T.; Rohit, B.; Pal, K.I. Sesamol: An efficient antioxidant with potential therapeutic benefits. Med. Chem. 2009, 5, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Hacker, H.; Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, 2006, re13. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [Green Version]
- Kamata, H.; Manabe, T.; Oka, S.; Kamata, K.; Hirata, H. Hydrogen peroxide activates IkappaB kinases through phosphorylation of serine residues in the activation loops. FEBS Lett. 2002, 519, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.C.; Ju, T.K.; Hung, M.C.; Chen, C.C. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol. Cell 2007, 26, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef]
- Shen, M.Y.; Chen, F.Y.; Hsu, J.F.; Fu, R.H.; Chang, C.M.; Chang, C.T.; Liu, C.H.; Wu, J.R.; Lee, A.S.; Chan, H.C.; et al. Plasma L5 levels are elevated in ischemic stroke patients and enhance platelet aggregation. Blood 2016, 127, 1336–1345. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.H.; Naito, M.; Tsujihara, N.; Osawa, T. Sesamolin inhibits lipid peroxidation in rat liver and kidney. J. Nutr. 1998, 128, 1018–1022. [Google Scholar] [CrossRef] [Green Version]
- Manzini, S.; Busnelli, M.; Parolini, C.; Minoli, L.; Ossoli, A.; Brambilla, E.; Simonelli, S.; Lekka, E.; Persidis, A.; Scanziani, E.; et al. Topiramate protects apoE-deficient mice from kidney damage without affecting plasma lipids. Pharm. Res. 2019, 141, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, M.; Manzini, S.; Bonacina, F.; Soldati, S.; Barbieri, S.S.; Amadio, P.; Sandrini, L.; Arnaboldi, F.; Donetti, E.; Laaksonen, R.; et al. Fenretinide treatment accelerates atherosclerosis development in apoE-deficient mice in spite of beneficial metabolic effects. Br. J. Pharm. 2020, 177, 328–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, M.C. Inhibitory effects and actions of pentacyclic triterpenes upon glycation. BioMedicine 2015, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, Y.; Zhang, Y.; Liu, F.; Zhu, L.; Pan, S.; Qiu, C.; Guo, Y.; Yang, T.; Wang, J. Matrine-Type Alkaloids Inhibit Advanced Glycation End Products Induced Reactive Oxygen Species-Mediated Apoptosis of Aortic Endothelial Cells In Vivo and In Vitro by Targeting MKK3 and p38MAPK Signaling. J. Am. Heart Assoc. 2017, 6, e007441. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Gene Expression qPCR Primers | ||
|---|---|---|
| GAPDH | 5′-AGAAGGCTGGGGCTCATTTG-3′ | 5′-AGGGGCCATCCACAGTCTTC-3′ |
| p53 | 5′-TACAAGAAGTCACAGCACAT-3′ | 5′-GATAGGTCGGCGGTTCAT-3′ |
| IKKα | 5′-GCCAGGGAGACTTGATGG-3′ | 5′-GAGGTCTGTGCTTTAGCTGCTT-3′ |
| Caspase-3 | 5′-GCGATGGAGAATGTGCATAAATTC-3′ | 5′-GGGAAACCAACAGTACTCAGTCCT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-S.; Tsai, P.-H.; Tseng, K.-F.; Chen, F.-Y.; Yang, W.-C.; Shen, M.-Y. Sesamol Ameliorates Renal Injury-Mediated Atherosclerosis via Inhibition of Oxidative Stress/IKKα/p53. Antioxidants 2021, 10, 1519. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10101519
Wang J-S, Tsai P-H, Tseng K-F, Chen F-Y, Yang W-C, Shen M-Y. Sesamol Ameliorates Renal Injury-Mediated Atherosclerosis via Inhibition of Oxidative Stress/IKKα/p53. Antioxidants. 2021; 10(10):1519. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10101519
Chicago/Turabian StyleWang, Jie-Sian, Ping-Hsuan Tsai, Kuo-Feng Tseng, Fang-Yu Chen, Wen-Chin Yang, and Ming-Yi Shen. 2021. "Sesamol Ameliorates Renal Injury-Mediated Atherosclerosis via Inhibition of Oxidative Stress/IKKα/p53" Antioxidants 10, no. 10: 1519. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10101519