Valorization of Agro-Industrial Wastes by Ultrasound-Assisted Extraction as a Source of Proteins, Antioxidants and Cutin: A Cascade Approach
Abstract
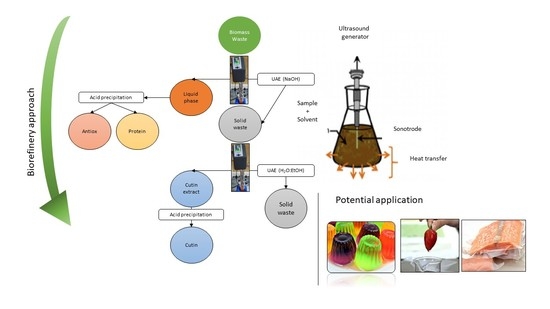
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Ultrasound-Assisted Extraction (UAE)
2.3. Characterization of the Obtained Fractions
2.3.1. Extraction Yield
2.3.2. Total Proteins Content by the Bradford Method
2.3.3. Total Phenolic Content (TPC)
2.3.4. Antioxidant Activity
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.7. Gas Chromatography–Mass Spectrometry (GC-MS)
2.3.8. Thermal Properties
2.4. Statistical Analysis
3. Results
3.1. Proteins Extraction
3.2. Antioxidants Extraction
3.2.1. Extraction Yield of Antioxidants
3.2.2. Total Phenolic Content (TPC)
3.2.3. Antioxidant Activity
3.3. Cutin Extraction
3.3.1. Extraction Yield of Cutin
3.3.2. FTIR Analysis
3.3.3. GC-MS Analysis
3.3.4. Thermal Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO World Food and Agriculture—Statistical Yearbook 2021; FAO: Rome, Italy, 2021.
- Basri, M.S.M.; Shah, N.N.A.K.; Sulaiman, A.; Tawakkal, I.S.M.A.; Nor, M.Z.M.; Ariffin, S.H.; Ghani, N.H.A.; Salleh, F.S.M. Progress in the valorization of fruit and vegetable wastes: Active packaging, biocomposites, by-products, and innovative technologies used for bioactive compound extraction. Polymers 2021, 13, 3503. [Google Scholar] [CrossRef] [PubMed]
- Del Rio Osorio, L.L.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Value-added products from fruit and vegetable wastes: A review. Clean-Soil Air Water 2021, 49, 2000376. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel approaches in the valorization of agricultural wastes and their applications. J. Agric. Food Chem. 2021, 2022, 6787–6804. [Google Scholar] [CrossRef]
- Mikula, K.; Gersz, A.; Witek-Krowiak, A.; Skrzypczak, D.; Izydorczyk, G.; Chojnacka, K. Agrochemicals in view of circular economy. In Smart Agrochemicals for Sustainable Agriculture; Academic Press: Cambridge, MA, USA, 2022; pp. 57–80. [Google Scholar] [CrossRef]
- Mohanty, A.; Mankoti, M.; Rout, P.R.; Meena, S.S.; Dewan, S.; Kalia, B.; Varjani, S.; Wong, J.W.C.; Banu, J.R. Sustainable utilization of food waste for bioenergy production: A step towards circular bioeconomy. Int. J. Food Microbiol. 2022, 365, 109538. [Google Scholar] [CrossRef]
- Massias, A.; Boisard, S.; Baccaunaud, M.; Leal Calderon, F.; Subra-Paternault, P. Recovery of phenolics from apple peels using CO2 + ethanol extraction: Kinetics and antioxidant activity of extracts. J. Supercrit. Fluids 2015, 98, 172–182. [Google Scholar] [CrossRef]
- Chaudhari, S.A.; Singhal, R.S. Cutin from Watermelon Peels: A novel inducer for cutinase production and its physicochemical characterization. Int. J. Biol. Macromol. 2015, 79, 398–404. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ballesteros, M.; Negro, M.J. Biorefineries for the valorization of food processing waste. In The Interaction of Food Industry and Environment; Academic Press: Cambridge, MA, USA, 2020; pp. 155–190. [Google Scholar] [CrossRef]
- Cifarelli, A.; Cigognini, I.M.; Bolzoni, L.; Montanari, A. Physical–chemical characteristics of cutin separated from tomato waste for the preparation of bio-lacquers. Adv. Sci. Eng. 2019, 11, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Benítez, J.J.; Castillo, P.M.; del Río, J.C.; León-Camacho, M.; Domínguez, E.; Heredia, A.; Guzmán-Puyol, S.; Athanassiou, A.; Heredia-Guerrero, J.A. Valorization of tomato processing by-products: Fatty acid extraction and production of bio-based materials. Materials 2018, 11, 2211. [Google Scholar] [CrossRef]
- Misran, A.; HaniffJaafar, A. Protein. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132784. [Google Scholar]
- Illera, A.E.; Beltrán, S.; Sanz, M.T. Structural changes of a protein extract from apple with polyphenoloxidase activity obtained by cationic reversed micellar extraction induced by high-pressure carbon dioxide and thermosonication. Sci. Rep. 2019, 9, 13749. [Google Scholar] [CrossRef]
- Mechmeche, M.; Kachouri, F.; Chouabi, M.; Ksontini, H.; Setti, K.; Hamdi, M. Optimization of extraction parameters of protein isolate from tomato seed using response surface methodology. Food Anal. Methods 2017, 10, 809–819. [Google Scholar] [CrossRef]
- Qin, D.; Wang, Y.; Wu, Y.; Kong, X.; Liu, L.; Li, Z.; Xi, J. Optimization of protein extraction from watermelon seeds by liquid-phase pulsed discharge based on energy input for scale-up application. LWT 2021, 152, 112355. [Google Scholar] [CrossRef]
- Gadalkar, S.M.; Rathod, V.K. Extraction of watermelon seed proteins with enhanced functional properties using ultrasound. Prep. Biochem. Biotechnol. 2020, 50, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Behere, M.; Patil, S.S.; Rathod, V.K. Rapid extraction of watermelon seed proteins using microwave and its functional properties. Prep. Biochem. Biotechnol. 2021, 51, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of pulsed electric fields to improve product yield and waste valorization in industrial tomato processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Kehili, M.; Schmidt, L.M.; Reynolds, W.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Biorefinery cascade processing for creating added value on tomato industrial by-products from tunisia. Biotechnol. Biofuels 2016, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- da Silva, L.C.; Viganó, J.; de Souza Mesquita, L.M.; Dias, A.L.B.; de Souza, M.C.; Sanches, V.L.; Chaves, J.O.; Pizani, R.S.; Contieri, L.S.; Rostagno, M.A. Recent advances and trends in extraction techniques to recover polyphenols compounds from apple by-products. Food Chem. X 2021, 12, 100133. [Google Scholar] [CrossRef]
- Neglo, D.; Tettey, C.O.; Essuman, E.K.; Kortei, N.K.; Boakye, A.A.; Hunkpe, G.; Amarh, F.; Kwashie, P.; Devi, W.S. Comparative antioxidant and antimicrobial activities of the peels, rind, pulp and seeds of watermelon (Citrullus Lanatus) fruit. Sci. Afr. 2021, 11, e00582. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aadil, R.M. An update on functional, nutraceutical and industrial applications of watermelon by-products: A comprehensive review. Trends Food Sci. Technol. 2021, 114, 275–291. [Google Scholar] [CrossRef]
- Chada, P.S.N.; Santos, P.H.; Rodrigues, L.G.G.; Goulart, G.A.S.; Azevedo dos Santos, J.D.; Maraschin, M.; Lanza, M. Non-conventional techniques for the extraction of antioxidant compounds and lycopene from industrial tomato pomace (Solanum lycopersicum L.) using spouted bed drying as a pre-treatment. Food Chem. X 2022, 13, 100237. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Saad, A.M.; El-Saadony, M.T.; El-Tahan, A.M.; Sayed, S.; Moustafa, M.A.M.; Taha, A.E.; Taha, T.F.; Ramadan, M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against spodoptera littoralis. Saudi J. Biol. Sci. 2021, 28, 5674–5683. [Google Scholar] [CrossRef] [PubMed]
- Casazza, A.A.; Pettinato, M.; Perego, P. Polyphenols from Apple Skins: A study on microwave-assisted extraction optimization and exhausted solid characterization. Sep. Purif. Technol. 2020, 240, 116640. [Google Scholar] [CrossRef]
- Gulsunoglu, Z.; Purves, R.; Karbancioglu-Guler, F.; Kilic-Akyilmaz, M. Enhancement of phenolic antioxidants in industrial apple waste by fermentation with Aspergillus Spp. Biocatal. Agric. Biotechnol. 2020, 25, 101562. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Heredia, A.; Domínguez, E.; Cingolani, R.; Bayer, I.S.; Athanassiou, A.; Benítez, J.J. Cutin from agro-waste as a raw material for the production of bioplastics. J. Exp. Bot. 2017, 68, 5401–5410. [Google Scholar] [CrossRef] [Green Version]
- Reynoud, N.; Petit, J.; Bres, C.; Lahaye, M.; Rothan, C.; Marion, D.; Bakan, B. The complex architecture of plant cuticles and its relation to multiple biological functions. Front. Plant Sci. 2021, 12, 782773. [Google Scholar] [CrossRef]
- Kallio, H.; Nieminen, R.; Tuomasjukka, S.; Hakala, M. Cutin composition of five finnish berries. J. Agric. Food Chem. 2006, 54, 457–462. [Google Scholar] [CrossRef]
- Manrich, A.; Moreira, F.K.V.; Otoni, C.G.; Lorevice, M.V.; Martins, M.A.; Mattoso, L.H.C. Hydrophobic edible films made up of tomato cutin and pectin. Carbohydr. Polym. 2017, 164, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cifarelli, A.; Cigognini, I.; Bolzoni, L.; Montanari, A. Cutin isolated from tomato processing by-products: Extraction methods and characterization. In Proceedings of the CYPRUS 2016 4th International Conference on Sustainable Solid Waste Management, Limassol, Cyprus, 23–25 June 2016; pp. 1–20. [Google Scholar]
- Arrieta-Baez, D.; de Jesús Perea Flores, M.; Méndez-Méndez, J.V.; Mendoza León, H.F.; Gómez-Patiño, M.B. Structural studies of the cutin from two apple varieties: Golden Delicious and Red Delicious (Malus Domestica). Molecules 2020, 25, 5955. [Google Scholar] [CrossRef] [PubMed]
- Hood, C.; Laredo, T.; Marangoni, A.G.; Pensini, E. Water-repellent films from corn protein and tomato cutin. J. Appl. Polym. Sci. 2021, 138, 50831. [Google Scholar] [CrossRef]
- Madzuki, I.N.; Tan, J.M.; Mohamad Shalan, N.A.A.; Mohd Isa, N.S. Tomato peel-cutin based film mitigates the deterioration of Calamansi (Citrofortunella Microcarpa). IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012013. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Sankaranarayanan, A.R.; Nivedhitha, L. Platform Chemical biorefinery and agroindustrial waste management. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 379–391. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S. Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: A critical review. Bioresour. Technol. 2022, 343, 126126. [Google Scholar] [CrossRef]
- Casa, M.; Miccio, M.; De Feo, G.; Paulillo, A.; Chirone, R.; Paulillo, D.; Lettieri, P.; Chirone, R. A brief overview on valorization of industrial tomato by-products using the biorefinery cascade approach. Detritus 2021, 15, 31. [Google Scholar] [CrossRef]
- Patsalou, M.; Chrysargyris, A.; Tzortzakis, N.; Koutinas, M. A biorefinery for conversion of citrus peel waste into essential oils, pectin, fertilizer and succinic acid via different fermentation strategies. Waste Manag. 2020, 113, 469–477. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Castro, E.; Cardona, C.A. A model biorefinery for avocado (Persea Americana Mill.) processing. Bioresour. Technol. 2017, 243, 17–29. [Google Scholar] [CrossRef]
- Karanicola, P.; Patsalou, M.; Stergiou, P.Y.; Kavallieratou, A.; Evripidou, N.; Christou, P.; Panagiotou, G.; Damianou, C.; Papamichael, E.M.; Koutinas, M. Ultrasound-assisted dilute acid hydrolysis for production of essential oils, pectin and bacterial cellulose via a citrus processing waste biorefinery. Bioresour. Technol. 2021, 342, 126010. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Ostojić, J.; Miletić, V.; Djaković, S.; Bosiljkov, T.; Zorić, Z.; Ježek, D.; Rimac Brnčić, S.; Brnčić, M. Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel waste. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Zorpas, A.A.; Dorado, M.P. Biorefinery concept for the industrial valorization of tomato processing by-products. In Tomato Processing by-Products; Academic Press: Cambridge, MA, USA, 2022; pp. 371–420. [Google Scholar] [CrossRef]
- Civan, M.; Kumcuoglu, S. Green Ultrasound-assisted extraction of carotenoid and capsaicinoid from the pulp of hot pepper paste based on the bio-refinery concept. LWT 2019, 113, 108320. [Google Scholar] [CrossRef]
- Pandey, A.; Belwal, T.; Sekar, K.C.; Bhatt, I.D.; Rawal, R.S. Optimization of ultrasonic-assisted extraction (UAE) of phenolics and antioxidant compounds from rhizomes of rheum moorcroftianum using response surface methodology (RSM). Ind. Crops Prod. 2018, 119, 218–225. [Google Scholar] [CrossRef]
- Luo, X.; Cui, J.; Zhang, H.; Duan, Y.; Zhang, D.; Cai, M.; Chen, G. Ultrasound assisted extraction of polyphenolic compounds from red sorghum (Sorghum bicolor L.) bran and their biological activities and polyphenolic compositions. Ind. Crops Prod. 2018, 112, 296–304. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Priya, B.; Al-Dhabi, N.A.; Ponmurugan, K.; Moorthy, I.G.; Sivarajasekar, N. Ultrasound assisted citric acid mediated pectin extraction from industrial waste of musa balbisiana. Ultrason. Sonochem. 2017, 35, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Mikani, M. Lycopene Green Ultrasound-assisted extraction using edible oil accompany with response surface methodology (rsm) optimization performance: Application in tomato processing wastes. Microchem. J. 2019, 146, 1033–1042. [Google Scholar] [CrossRef]
- Buvaneshwaran, M.; Radhakrishnan, M.; Natarajan, V. Influence of ultrasound-assisted extraction techniques on the valorization of agro-based industrial organic waste—A review. J. Food Process Eng. 2022, e14012. [Google Scholar] [CrossRef]
- Dorosh, O.; Moreira, M.M.; Rodrigues, F.; Peixoto, A.F.; Freire, C.; Morais, S.; Delerue-Matos, C. Vine-canes valorisation: Ultrasound-assisted extraction from lab to pilot scale. Molecules 2020, 25, 1739. [Google Scholar] [CrossRef]
- Baker, P.W.; Preskett, D.; Krienke, D.; Runager, K.S.; Hastrup, A.C.S.; Charlton, A. Pre-processing waste tomatoes into separated streams with the intention of recovering protein: Towards an integrated fruit and vegetable biorefinery approach to waste minimization. Waste Biomass Valorization 2022, 1, 3463–3473. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of microwave-assisted extraction of cocoa bean shell waste and evaluation of its antioxidant, physicochemical and functional properties. LWT 2020, 127, 109361. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Szabo, K.; Diaconeasa, Z.; Cătoi, A.F.; Vodnar, D.C. Screening of ten tomato varieties processing waste for bioactive components and their related antioxidant and antimicrobial activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Piyakina, G.A.; Yunusov, T.S. General characteristics of the proteins of tomato seed flour and tomato skin flour. Chem. Nat. Compd. 1995, 31, 495–499. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M. Physicochemical composition of tomato seed oil for an edible use: The effect of cultivar. Int. Food Res. J. 2016, 23, 583–591. [Google Scholar]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, Z.; Li, G.; Zhao, X.; Wu, H.; Ren, Y. Tomato peel powder as fat replacement in low-fat sausages: Formulations with mechanically crushed powder exhibit higher stability than those with airflow ultra-micro crushed powder. Eur. J. Lipid Sci. Technol. 2016, 118, 175–184. [Google Scholar] [CrossRef]
- García Herrera, P.; Sánchez-Mata, M.C.; Cámara, M. Nutritional characterization of tomato fiber as a useful ingredient for food industry. Innov. Food Sci. Emerg. Technol. 2010, 11, 707–711. [Google Scholar] [CrossRef]
- Abu-Hiamed, H.A.A. Chemical composition, flavonoids and β-sitosterol contents of pulp and rind of watermelon (Citrullus Lanatus) fruit. Pakistan J. Nutr. 2017, 16, 502–507. [Google Scholar] [CrossRef]
- Dibanda Romelle, F.; Rani, A.P.; Sai Manohar, R. Chemical composition of some selected fruit peels a phenolic-rich herbal tea from stathmostelma sp. (statroltea) inhibits in vitro pancreatic lipase activity view project chemical composition of some selected fruit peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Hasanin, M.S.; Hashem, A.H. Eco-friendly, economic fungal universal medium from watermelon peel waste. J. Microbiol. Methods 2020, 168, 105802. [Google Scholar] [CrossRef] [PubMed]
- Tropea, A.; Ferracane, A.; Albergamo, A.; Potortì, A.G.; Lo Turco, V.; Di Bella, G. Single cell protein production through multi food-waste substrate fermentation. Fermentation 2022, 8, 91. [Google Scholar] [CrossRef]
- Shivamathi, C.S.S.; Moorthy, I.G.; Kumar, R.S.V.; Soosai, M.R.; Maran, J.P.; Kumar, R.S.V.; Varalakshmi, P. Optimization of ultrasound assisted extraction of pectin from custard apple peel: Potential and new source. Carbohydr. Polym. 2019, 225, 115240. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of gold milenium and papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef]
- Chakraborty, S.; Uppaluri, R.; Das, C. Optimization of ultrasound-assisted extraction (UAE) process for the recovery of bioactive compounds from bitter gourd using response surface methodology (RSM). Food Bioprod. Process. 2020, 120, 114–122. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, C.; Feng, Y.; Zhang, J.; He, Y.; Duan, Y.; Zhang, H.; Ma, H. Effects of ultrasound-assisted extraction on the structural, functional and antioxidant properties of Dolichos lablab L. protein. Process Biochem. 2021, 101, 274–284. [Google Scholar] [CrossRef]
- Ngo, N.T.T.; Shahidi, F. Functional properties of protein isolates from camelina (camelina sativa (l.) crantz) and flixweed (Sophia, Descurainis sophia L.) seed meals. Food Prod. Process. Nutr. 2021, 3, 31. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Solanki, H.A.; Gupta, U. Quantification of ash and selected primary metabolites from non-edible parts of several fruits. Int. J. Pharm. Pharm. Sci. 2015, 7, 288. [Google Scholar]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef] [PubMed]
- PA Silva, Y.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.L.; Ferreira, T.A.P.C. Characterization of tomato processing by-product for use as a potential functional food ingredient: Nutritional composition, antioxidant activity and bioactive compounds. Int. J. Food Sci. Nutr. 2019, 70, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Prandi, B.; Faccini, A.; Lambertini, F.; Bencivenni, M.; Jorba, M.; Van Droogenbroek, B.; Bruggeman, G.; Schöber, J.; Petrusan, J.; Elst, K.; et al. Food wastes from agrifood industry as possible sources of proteins: A detailed molecular view on the composition of the nitrogen fraction, amino acid profile and racemisation degree of 39 food waste streams. Food Chem. 2019, 286, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Mallek-Ayadi, S.; Bahloul, N.; Kechaou, N. Characterization, phenolic compounds and functional properties of Cucumis melo L. peels. Food Chem. 2017, 221, 1691–1697. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.Y. Development of cold plasma pretreatment for improving phenolics extractability from tomato pomace. Innov. Food Sci. Emerg. Technol. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Ma, Q.; Bi, J.; Yi, J.; Wu, X.; Li, X.; Zhao, Y. Stability of phenolic compounds and drying characteristics of apple peel as affected by three drying treatments. Food Sci. Hum. Wellness 2021, 10, 174–182. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Fuentes, E.; Carle, R.; Astudillo, L.; Guzmán, L.; Gutiérrez, M.; Carrasco, G.; Palomo, I. Antioxidant and antiplatelet activities in extracts from green and fully ripe tomato fruits (Solanum lycopersicum) and pomace from industrial tomato processing. Evid.-Based Complement. Altern. Med. 2013, 2013, 867578. [Google Scholar] [CrossRef]
- Valdez-Morales, M.; Espinosa-Alonso, L.G.; Espinoza-Torres, L.C.; Delgado-Vargas, F.; Medina-Godoy, S. phenolic content and antioxidant and antimutagenic activities in tomato peel, seeds, and byproducts. J. Agric. Food Chem. 2014, 62, 5281–5289. [Google Scholar] [CrossRef]
- Pettinato, M.; Casazza, A.A.; Ferrari, P.F.; Perego, P. Optimization and modeling of solid-liquid multivariable extractor (solve): A new solution for tomato waste valorization. Chem. Eng. Res. Des. 2022, 182, 465–477. [Google Scholar] [CrossRef]
- Gharbi, S.; Renda, G.; La Barbera, L.; Amri, M.; Messina, C.M.; Santulli, A. Tunisian tomato by-products, as a potential source of natural bioactive compounds. Nat. Prod. Res. 2017, 31, 626–631. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.H.F.; Visentainer, J.V. Antioxidant activity, phenolics and uplc–esi(–)–ms of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Kim, S.J.; Matsushita, Y.; Fukushima, K.; Aoki, D.; Yagami, S.; Yuk, H.G.; Lee, S.C. Antioxidant activity of a hydrothermal extract from watermelons. LWT-Food Sci. Technol. 2014, 59, 361–368. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Al-Juhaimi, F.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. J. Food Meas. Charact. 2020, 14, 1784–1793. [Google Scholar] [CrossRef]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Okatan, V.; Güçlü, S.F.; Çolak, A.M. Determination of some chemical characteristics and total antioxidant capacity in apple varieties grown in Posof/Ardahan region. Int. J. Agric. Environ. Food Sci. 2018, 2, 131–134. [Google Scholar] [CrossRef]
- Gecer, M.K.; Ozkan, G.; Sagbas, H.I.; Ilhan, G.; Gundogdu, M.; Ercisli, S. Some important horticultural properties of summer apple genotypes from coruh valley in Turkey. Int. J. Fruit Sci. 2020, 20, S1406–S1416. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Martín-González, M.F.S.; Hirst, P.; Ballard, T.S. Optimizing microwave-assisted extraction of phenolic antioxidants from red delicious and jonathan apple pomace. J. Food Process Eng. 2015, 38, 571–582. [Google Scholar] [CrossRef]
- Blidi, S.; Bikaki, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. A comparative evaluation of bio-solvents for the efficient extraction of polyphenolic phytochemicals: Apple waste peels as a case study. Waste Biomass Valorization 2015, 6, 1125–1133. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Managbanag Salas, F.; Algodon Salas, R.; Notarte Pole, V.; Amarila Quevedo, M. Shelf-life and free radical scavenging activity of tomato (Lycopersicon Esculentum Mill.) fruits coated with safe phytochemicals. J. Food Nutr. Sci. 2015, 3, 94. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and carotenoid pigments in the peel and flesh of commercial apple fruit varieties. Food Res. Int. 2014, 65, 272–281. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y. Lo Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr. Polym. 2015, 122, 60–68. [Google Scholar] [CrossRef]
- de Souza, C.G.; Rodrigues, T.H.S.; e Silva, L.M.A.; Ribeiro, P.R.V.; de Brito, E.S. Sequential extraction of flavonoids and pectin from yellow passion fruit rind using pressurized solvent or ultrasound. J. Sci. Food Agric. 2018, 98, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.F.; Irwin, P.; Fett, W.F.; O’Connor, J.V.; Parris, N. Preparation, isolation, and characterization of cutin monomers and oligomers from tomato peels. J. Agric. Food Chem. 1999, 47, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, S.; Molina, I. Isolation and compositional analysis of plant cuticle lipid polyester monomers. J. Vis. Exp. 2015, 2015, 53386. [Google Scholar] [CrossRef] [PubMed]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical characteristics of biomass ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Philippe, G.; Geneix, N.; Petit, J.; Guillon, F.; Sandt, C.; Rothan, C.; Lahaye, M.; Marion, D.; Bakan, B. Assembly of tomato fruit cuticles: A cross-talk between the cutin polyester and cell wall polysaccharides. New Phytol. 2020, 226, 809–822. [Google Scholar] [CrossRef]
- Leide, J.; Nierop, K.G.J.; Deininger, A.C.; Staiger, S.; Riederer, M.; De Leeuw, J.W. Leaf cuticle analyses: Implications for the existence of cutan/non-ester cutin and its biosynthetic origin. Ann. Bot. 2020, 126, 141–162. [Google Scholar] [CrossRef]
- Hernández Velasco, B.L.; Arrieta-Baez, D.; Cortez Sotelo, P.I.; Méndez-Méndez, J.V.; Berdeja Martínez, B.M.; Gómez-Patiño, M.B. Comparative studies of cutins from lime (Citrus aurantifolia) and grapefruit (Citrus paradisi) after TFA hydrolysis. Phytochemistry 2017, 144, 78–86. [Google Scholar] [CrossRef]
- Razeq, F.M.; Kosma, D.K.; França, D.; Rowland, O.; Molina, I. Extracellular lipids of camelina sativa: Characterization of cutin and suberin reveals typical polyester monomers and unusual dicarboxylic fatty acids. Phytochemistry 2021, 184, 112665. [Google Scholar] [CrossRef]
- Eke, R.; Ejiofor, E.; Oyedemi, S.; Onoja, S.; Omeh, N. Evaluation of nutritional composition of Citrullus Lanatus Linn. (watermelon) seed and biochemical assessment of the seed oil in rats. J. Food Biochem. 2021, 45, e13763. [Google Scholar] [CrossRef]
- Petchsomrit, A.; McDermott, M.I.; Chanroj, S.; Choksawangkarn, W. Watermelon seeds and peels: Fatty acid composition and cosmeceutical potential. OCL 2020, 27, 54. [Google Scholar] [CrossRef]
- Bada, J.C.; León-Camacho, M.; Copovi, P.; Alonso, L. Characterization of apple seed oil with denomination of origin from Asturias, Spain. Grasas Aceites 2014, 65, e027. [Google Scholar] [CrossRef]
- Akšić, M.F.; Lazarević, K.; Šegan, S.; Natić, M.; Tosti, T.; Ćirić, I.; Meland, M. Assessing the fatty acid, carotenoid, and tocopherol compositions of seeds from apple cultivars (Malus Domestica Borkh.) grown in Norway. Foods 2021, 10, 1956. [Google Scholar] [CrossRef]
- Velcheva, M.P.; Espelie, K.E.; Ivanov, C.P. Aliphatic composition of cutin from inner seed coat of apple. Phytochemistry 1981, 20, 2225–2227. [Google Scholar] [CrossRef]
- Leide, J.; Xavier de Souza, A.; Papp, I.; Riederer, M. Specific Characteristics of the apple fruit cuticle: Investigation of early and late season cultivars ‘Prima’ and ‘Florina’ (Malus Domestica Borkh.). Sci. Hortic. 2018, 229, 137–147. [Google Scholar] [CrossRef]
- Guzmán, P.; Fernández, V.; Graça, J.; Cabral, V.; Kayali, N.; Khayet, M.; Gil, L. Chemical and structural analysis of Eucalyptus Globulus and E. Camaldulensis leaf cuticles: A Lipidized Cell Wall Region. Front. Plant Sci. 2014, 5, 481. [Google Scholar] [CrossRef]
- He, M.; Qin, C.X.; Wang, X.; Ding, N.Z. Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Benítez, J.J.; García-Segura, R.; Heredia, A. Plant biopolyester cutin: A tough way to its chemical synthesis. Biochim. Biophys. Acta-Gen. Subj. 2004, 1674, 1–3. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Cutin from plants. Biopolym. Online 2005, 1–12. [Google Scholar] [CrossRef]
- Tedeschi, G.; Ceseracciu, L.; Dastmalchi, K.; Itin, B.; Stark, R.E.; Heredia, A.; Athanassiou, A.; Heredia-Guerrero, A. Sustainable fabrication of plant cuticle-like packaging films from tomato pomace agro-waste, beeswax, and alginate. ACS Sustain. Chem. Eng. 2018, 6, 14955–14966. [Google Scholar] [CrossRef]
- Aloui, H.; Ghazouani, Z.; Khwaldia, K. Bioactive coatings enriched with cuticle components from tomato wastes for cherry tomatoes preservation. Waste Biomass Valorization 2021, 12, 6155–6163. [Google Scholar] [CrossRef]




| Waste | YP (wt.%) | Protein Content (mg BSA/g Extract) | YAx (wt.%) | YC (wt.%) | Non-Hydrolysable Cutin * (wt.%) |
|---|---|---|---|---|---|
| TP | 9 ± 1 a | 590 ± 3 a | 36 ± 5 a | 14 ± 2 a | 42 ± 4 ab |
| WP | 7 ± 2 ab | 857 ± 1 b | 25 ± 6 b | 7 ± 1 b | 55 ± 9 a |
| AP | 5 ± 1 b | 625 ± 2 c | 32 ± 3 ab | 20 ± 1 c | 40 ± 6 b |
| Waste | TPC (mg GAE/100 g dm) | ABTS (µmol TE/100 g dm) | FRAP (µmol TE/100 g dm) | DPPH (µmol TE/100 g dm) |
|---|---|---|---|---|
| TP | 103.5 ± 0.9 a | 279 ± 3 a | 264 ± 2 a | 159 ± 1 a |
| WP | 107.2 ± 0.2 b | 356 ± 1 b | 507 ± 4 b | 158 ± 1 a |
| AP | 61.4 ± 0.1 c | 1559 ± 20 c | 1767 ± 5 c | 902 ± 16 b |
| Run | EtOH (wt.%) | Energy (kWs) | Amplitude (%) | Ratio (mL/g) | pH | Acid | Yield (wt.%) |
|---|---|---|---|---|---|---|---|
| 1 | 40 | 20 | 100 | 80 | 5.5 | Hydrochloric | 1.2 |
| 2 | 40 | 60 | 100 | 80 | 2.5 | Citric | 14.3 |
| 3 | 40 | 20 | 60 | 40 | 2.5 | Hydrochloric | 0.0 |
| 4 | 100 | 60 | 60 | 80 | 5.5 | Hydrochloric | 0.7 |
| 5 | 100 | 20 | 100 | 40 | 2.5 | Hydrochloric | 2.5 |
| 6 | 40 | 20 | 60 | 80 | 5.5 | Citric | 2.4 |
| 7 | 40 | 60 | 60 | 40 | 2.5 | Citric | 10.3 |
| 8 | 100 | 20 | 60 | 40 | 5.5 | Citric | 7.4 |
| 9 | 100 | 60 | 60 | 80 | 2.5 | Hydrochloric | 4.6 |
| 10 | 40 | 60 | 100 | 40 | 5.5 | Hydrochloric | 0.0 |
| 11 | 100 | 20 | 100 | 80 | 2.5 | Citric | 4.9 |
| 12 | 100 | 20 | 100 | 40 | 5.5 | Citric | 5.3 |
| Compound | TP | WP | AP | |||
|---|---|---|---|---|---|---|
| Quality | wt.% | Quality | wt.% | Quality | wt.% | |
| Tetradecanoic acid | 96 | 0.40 ± 0.10 | 98 | 0.55 ± 0.02 | - | - |
| Hexadecanoic acid | 96 | 13.02 ± 2.05 | 99 | 43.00 ± 1.02 | 99 | 8.45 ± 0.21 |
| (Z)-hexadec-9-enoic acid | 90 | 0.20 ± 0.05 | - | - | - | - |
| (E)-hexadec-9-enoic acid | 46 | 0.23 ± 0.04 | 86 | 1.00 ± 0.61 | - | - |
| Heptadecanoic acid | 98 | 0.13 ± 0.03 | 96 | 0.58 ± 0.01 | - | - |
| (9Z,12Z)-octadeca-9,12-dienoic acid | 96 | 9.01 ± 2.02 | 99 | 11.10 ± 0.41 | 96 | 20.02 ± 1.23 |
| (Z)-octadec-9-enoic acid | 96 | 5.02 ± 1.00 | - | - | - | - |
| (E)-octadec-9-enoic acid | - | - | - | - | 99 | 12.17 ± 0.49 |
| Octadecanoic acid | 99 | 3.00 ± 1.10 | 99 | 12.02 ± 1.02 | 99 | 4.52 ± 0.31 |
| Icosanoic acid | 96 | 1.21 ± 0.74 | - | - | 97 | 2.22 ± 0.14 |
| Docosanoic acid | - | - | 96 | 1.39 ± 0.18 | ||
| 10,16-dihydroxyhexadecanoic acid | 46 | 59.00 ± 7.02 | - | - | 60 | 6.64 ± 0.33 |
| 9,10-Dihydroxyoctadecanedioic acid | - | - | 43 | 2.51 ± 0.60 | 43 | 3.18 ± 0.11 |
| 9,10,18-trihydroxyoctadecanoic acid | 87 | 0.61 ± 0.04 | - | - | 90 | 10.53 ± 0.61 |
| (3S)-3-methyl-2-oxopentanoic acid | 47 | 0.32 ± 0.13 | - | - | - | - |
| (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid | - | - | 99 | 16.00 ± 0.52 | 53 | 4.02 ± 2.00 |
| (2E,4E)-octadeca-2,4-dienoic acid | - | - | 42 | 1.31 ± 0.24 | ||
| Pentan-2-ol | - | - | 41 | 0.55 ± 0.03 | - | - |
| (E,7R,11R)-3,7,11,15-tetramethylhexadec-2-en-1-ol | - | - | 97 | 0.80 ± 0.12 | - | - |
| [(Z)-octadec-9-enyl] formate | - | - | - | - | 78 | 1.00 ± 0.12 |
| 8-[3-(8-hydroxyoctyl)oxiran-2-yl]octanoic acid | - | - | - | - | 53 | 1.72 ± 0.35 |
| Compound. | TP | WP | AP | |||
|---|---|---|---|---|---|---|
| This work | [11] | This work | [117] | This work | [37] | |
| Hexadecanoic acid | 13.02 ± 2.05 | 1–2 | 43.00 ± 1.02 | 28.42 ± 3.30 | 8.45 ± 0.21 | 1–2 |
| (9Z,12Z)-octadeca-9,12-dienoic acid | 9.01 ± 2.02 | 2–4 | 11.10 ± 0.41 | 15.86 ± 0.77 | 20.02 ± 1.23 | 1 |
| (E)-octadec-9-enoic acid | - | - | - | - | 12.17 ± 0.49 | - |
| (Z)-octadec-9-enoic acid | 5.02 ± 1.00 | - | - | - | - | - |
| (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid | - | - | 16.00 ± 0.52 | - | 4.02 ± 2.00 | - |
| Octadecanoic acid | 3.00 ± 1.10 | 0.8 | 12.02 ± 1.02 | - | 4.52 ± 0.31 | - |
| 9,10-Dihydroxyoctadecanedioic acid | - | - | 2.51 ± 0.60 | - | 3.18 ± 0.11 | - |
| 10,16-dihydroxyhexadecanoic acid | 59.00 ± 7.02 | 62–82 | - | - | 6.64 ± 0.33 | 15–19 |
| 9,10,18-trihydroxyoctadecanoic acid | 0.61 ± 0.04 | - | - | - | 10.53 ± 0.61 | 18–21 |
| Waste | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | Solid residue (wt.%) | Tg (°C) |
|---|---|---|---|---|---|
| TP | 301 ± 3 a | 372 ± 2 a | 445 ± 1 a | 19 ± 1 a | - |
| WP | 199 ± 2 b | 306 ± 2 b | 447 ± 1 a | 18 ± 1 a | 36 ± 4 a |
| AP | 203 ± 2 b | 302 ± 2 b | 471 ± 3 b | 18 ± 1 a | 24 ± 4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mellinas, C.; Solaberrieta, I.; Pelegrín, C.J.; Jiménez, A.; Garrigós, M.C. Valorization of Agro-Industrial Wastes by Ultrasound-Assisted Extraction as a Source of Proteins, Antioxidants and Cutin: A Cascade Approach. Antioxidants 2022, 11, 1739. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11091739
Mellinas C, Solaberrieta I, Pelegrín CJ, Jiménez A, Garrigós MC. Valorization of Agro-Industrial Wastes by Ultrasound-Assisted Extraction as a Source of Proteins, Antioxidants and Cutin: A Cascade Approach. Antioxidants. 2022; 11(9):1739. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11091739
Chicago/Turabian StyleMellinas, Cristina, Ignacio Solaberrieta, Carlos Javier Pelegrín, Alfonso Jiménez, and María Carmen Garrigós. 2022. "Valorization of Agro-Industrial Wastes by Ultrasound-Assisted Extraction as a Source of Proteins, Antioxidants and Cutin: A Cascade Approach" Antioxidants 11, no. 9: 1739. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11091739