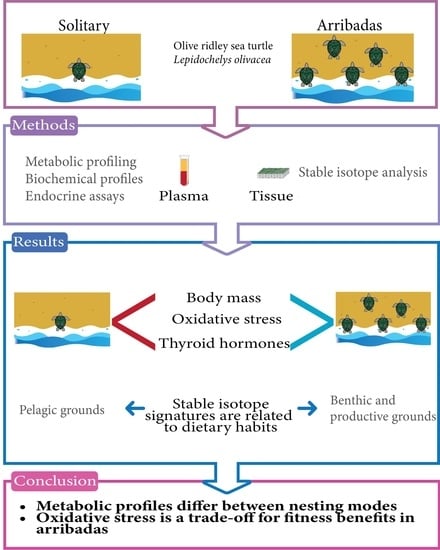
Oxidative Stress Is a Potential Cost of Synchronous Nesting in Olive Ridley Sea Turtles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Biochemical Assays
2.3. Metabolite Profiling
2.4. Stable Isotopes
2.5. Statistical Analyses
3. Results
3.1. Biochemical Profiles
3.2. Metabolite Profiling
3.3. Stable Isotopes
3.4. Oxidative Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Metcalfe, N.B.; Monaghan, P. Does Reproduction Cause Oxidative Stress? An Open Question. Trends Ecol. Evol. 2013, 28, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alvarez, C.; Bertrand, S.; Devevey, G.; Prost, J.; Faivre, B.; Sorci, G. Increased Susceptibility to Oxidative Stress as a Proximate Cost of Reproduction. Ecol. Lett. 2004, 7, 363–368. [Google Scholar] [CrossRef]
- Dowling, D.K.; Simmons, L.W. Reactive Oxygen Species as Universal Constraints in Life-History Evolution. Proc. R. Soc. B Biol. Sci. 2009, 276, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative Stress as a Mediator of Life History Trade-Offs: Mechanisms, Measurements and Interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Garratt, M.; Vasilaki, A.; Stockley, P.; McArdle, F.; Jackson, M.; Hurst, J.L. Is Oxidative Stress a Physiological Cost of Reproduction? An Experimental Test in House Mice. Proc. R. Soc. B Biol. Sci. 2011, 278, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Ołdakowski, Ł.; Piotrowska, Z.; Chrza̧ścik, K.M.; Sadowska, E.T.; Koteja, P.; Taylor, J.R.E. Is Reproduction Costly? No Increase of Oxidative Damage in Breeding Bank Voles. J. Exp. Biol. 2012, 215, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A Comparative and Evolutionary Approach to Oxidative Stress in Fish: A Review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Costantini, D. Meta-Analysis Reveals That Reproductive Strategies Are Associated with Sexual Differences in Oxidative Balance across Vertebrates. Curr. Zool. 2018, 64, 1–11. [Google Scholar] [CrossRef]
- Godoy, R.S.; Lanés, L.E.K.; Castro, B.D.; Weber, V.; Wingen, N.; Pires, M.M.; Oliveira, G.T.; Maltchik, L. Oxidative Stress Resistance in a Short-Lived Neotropical Annual Killifish. Biogerontology 2020, 21, 217–229. [Google Scholar] [CrossRef]
- Georgiev, A.V.; Thompson, M.E.; Mandalaywala, T.M.; Maestripieri, D. Oxidative Stress as an Indicator of the Costs of Reproduction among Free-Ranging Rhesus Macaques. J. Exp. Biol. 2015, 218, 1981–1985. [Google Scholar] [CrossRef] [Green Version]
- Sharick, J.T.; Vazquez-Medina, J.P.; Ortiz, R.M.; Crocker, D.E. Oxidative Stress Is a Potential Cost of Breeding in Male and Female Northern Elephant Seals. Funct. Ecol. 2015, 29, 367–376. [Google Scholar] [CrossRef]
- Fletcher, Q.E.; Selman, C.; Boutin, S.; McAdam, A.G.; Woods, S.B.; Seo, A.Y.; Leeuwenburgh, C.; Speakman, J.R.; Humphries, M.M. Oxidative Damage Increases with Reproductive Energy Expenditure and Is Reduced by Food-Supplementation. Evolution 2013, 67, 1527–1536. [Google Scholar] [CrossRef]
- Dornfeld, T.C.; Robinson, N.J.; Tomillo, P.S.; Paladino, F.V. Ecology of Solitary Nesting Olive Ridley Sea Turtles at Playa Grande, Costa Rica. Mar. Biol. 2015, 162, 123–139. [Google Scholar] [CrossRef]
- Bernardo, J.; Plotkin, P.T. An Evolutionary Perspective on the Arribada Phenomenon and Reproductive Behavioral Polymorphism of Olive Ridley Sea Turtles (Lepidochelys Olivacea). In Biology and Conservation of Ridley Sea Turtles; Plotkin, P.T., Ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2007; pp. 59–87. [Google Scholar]
- Ocana, M.; Harfush-Melendez, M.; Heppell, S. Mass Nesting of Olive Ridley Sea Turtles Lepidochelys Olivacea at La Escobilla, Mexico: Linking Nest Density and Rates of Destruction. Endanger. Species Res. 2012, 16, 45–54. [Google Scholar] [CrossRef]
- Hirth, H.F. Some Aspects of the Nesting Behavior and Reproductive Biology of Sea Turtles. Integr. Comp. Biol. 1980, 20, 507–523. [Google Scholar] [CrossRef]
- Plotkin, P.T.; Byles, R.A.; Rostal, D.C.; Owens, D.W. Independent versus Socially Facilitated Oceanic Migrations of the Olive Ridley, Lepidochelys Olivacea. Mar. Biol. 1995, 122, 137–143. [Google Scholar] [CrossRef]
- Plotkin, P.T.; Owens, D.W.; Byles, R.A.; Patterson, R. Departure of Male Olive Ridley Turtles (Lepidochelys olivacea) from a Nearshore Breeding Ground. Herpetologica 1996, 52, 1–7. [Google Scholar]
- Jensen, M.P.; Abreu-Grobois, F.A.; Frydenberg, J.; Loeschcke, V. Microsatellites Provide Insight into Contrasting Mating Patterns in Arribada vs. Non-Arribada Olive Ridley Sea Turtle Rookeries. Mol. Ecol. 2006, 15, 2567–2575. [Google Scholar] [CrossRef]
- Eckrich, C.E.; Owen, D.W. Solitary versus Arribada Nesting in the Olive Ridley Sea Turtles (Lepidochelys olivacea): A Test of the Predator-Satiation Hypothesis. Herpetologica 1995, 51, 349–354. [Google Scholar]
- Honarvar, S.; O’Connor, M.P.; Spotila, J.R. Density-Dependent Effects on Hatching Success of the Olive Ridley Turtle, Lepidochelys Olivacea. Oecologia 2008, 157, 221–230. [Google Scholar] [CrossRef]
- Bézy, V.S.; Valverde, R.A.; Plante, C.J. Olive Ridley Sea Turtle Hatching Success as a Function of Microbial Abundance and the Microenvironment of In Situ Nest Sand at Ostional, Costa Rica. J. Mar. Biol. 2014, 2014, 351921. [Google Scholar] [CrossRef]
- Williamson, S.A.; Evans, R.G.; Robinson, N.J.; Reina, R.D. Synchronised Nesting Aggregations Are Associated with Enhanced Capacity for Extended Embryonic Arrest in Olive Ridley Sea Turtles. Sci. Rep. 2019, 9, 9783. [Google Scholar] [CrossRef] [Green Version]
- Dutton, P.H. Methods for Collection and Preservation of Samples for Sea Turtle Genetic Studies. In Proceedings of the Proceedings of the International Symposium, Miami, FL, USA, 12–14 September 1995; pp. 17–24. [Google Scholar]
- Espinoza-Romo, B.A.; Sainz-Hernández, J.C.; Ley-Quiñónez, C.P.; Hart, C.E.; Leal-Moreno, R.; Aguirre, A.A.; Zavala-Norzagaray, A.A. Blood Biochemistry of Olive Ridley (Lepidochelys olivacea) Sea Turtles Foraging in Northern Sinaloa, Mexico. PLoS ONE 2018, 13, e0199825. [Google Scholar] [CrossRef]
- Mettee, N. Sample Collection Techniques. Available online: http://www.seaturtleguardian.org/sample-collection-techniques (accessed on 6 September 2022).
- Allen, C.D.; Robbins, M.N.; Eguchi, T.; Owens, D.W.; Meylan, A.B.; Meylan, P.A.; Kellar, N.M.; Schwenter, J.A.; Nollens, H.H.; LeRoux, R.A.; et al. First Assessment of the Sex Ratio for an East Pacific Green Sea Turtle Foraging Aggregation: Validation and Application of a Testosterone ELISA. PLoS ONE 2015, 10, e0138861. [Google Scholar] [CrossRef]
- Arango, B.G.; Harfush-Meléndez, M.; Marmolejo-Valencia, J.A.; Merchant-Larios, H.; Crocker, D.E. Blood Oxygen Stores of Olive Ridley Sea Turtles, Lepidochelys Olivacea Are Highly Variable among Individuals during Arribada Nesting. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2020, 19, 185–194. [Google Scholar] [CrossRef]
- Vazquez-Medina, J.P.; Zenteno-Savin, T.; Tift, M.S.; Forman, H.J.; Crocker, D.E.; Ortiz, R.M. Apnea Stimulates the Adaptive Response to Oxidative Stress in Elephant Seal Pups. J. Exp. Biol. 2011, 214, 4193–4200. [Google Scholar] [CrossRef]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality Control for Plant Metabolomics: Reporting MSI-Compliant Studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using Metaboanalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinforma. 2016, 2016, 14.10.1–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, A.; Cubedo-Ruiz, E.A.; Winkler, R. Improved Representation of Biological Information by Using Correlation as Distance Function for Heatmap Cluster Analysis. Am. J. Plant Sci. 2017, 08, 502–516. [Google Scholar] [CrossRef]
- Frolkis, A.; Knox, C.; Lim, E.; Jewison, T.; Law, V.; Hau, D.D.; Liu, P.; Gautam, B.; Ly, S.; Guo, A.C.; et al. SMPDB: The Small Molecule Pathway Database. Nucleic Acids Res. 2009, 38, 480–487. [Google Scholar] [CrossRef]
- Plotkin, P.T.; Rostal, D.C.; Byles, R.A.; Owens, D.W. Reproductive and Developmental Synchrony in Female Lepidochelys Olivacea. J. Herpetol. 1997, 31, 17–22. [Google Scholar] [CrossRef]
- Owens, D.W. The Comparative Reproductive Physiology of Sea Turtles. Integr. Comp. Biol. 1980, 20, 549–563. [Google Scholar] [CrossRef]
- Rostal, D.C.; Grumbles, J.S.; Byles, R.A.; Marquez-M, R.; Owens, D.W. Nesting Physiology of Kemp’s Ridley Sea Turtles, Lepidochelys Kempi, at Rancho Nuevo, Tamaulipas, Mexico, with Observations on Population Estimates. Chelonian Conserv. Biol. 1997, 2, 538–547. [Google Scholar]
- Osawa, T.; Shimamura, T.; Saito, K.; Hasegawa, Y.; Ishii, N.; Nishida, M.; Ando, R.; Kondo, A.; Anwar, M.; Tsuchida, R.; et al. Phosphoethanolamine Accumulation Protects Cancer Cells under Glutamine Starvation through Downregulation of PCYT2. Cell Rep. 2019, 29, 89–103.e7. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Zhang, A. Metabolic Profiling and Biomarkers Analysis of XinQiXu Syndrome; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128031186. [Google Scholar]
- Felig, P. The Glucose-Alanine Cycle. Metabolism 1973, 22, 179–207. [Google Scholar] [CrossRef]
- Palmieri, L.; Pardo, B.; Lasorsa, F.M.; Del Arco, A.; Kobayashi, K.; Iijima, M.; Runswick, M.J.; Walker, J.E.; Saheki, T.; Satrústegui, J.; et al. Citrin and Aralar1 Are Ca2+-Stimulated Aspartate/Glutamate Transporters in Mitochondria. EMBO J. 2001, 20, 5060–5069. [Google Scholar] [CrossRef]
- Tripathy, B. An Assessment of Solitary and Arribada Nesting of Olive Ridley Sea Turtles (Lepidochelys olivacea) at the Rushikulya Rookery of Orissa, India. Asiat. Herpetol. Res. 2008, 11, 136–142. [Google Scholar]
- Hamann, M.; Limpus, C.J.; Whittier, J.M. Patterns of Lipid Storage and Mobilisation in the Female Green Sea Turtle (Chelonia mydas). J. Comp. Physiol. B 2002, 172, 485–493. [Google Scholar] [CrossRef]
- Goldberg, D.W.; Leitao, S.A.T.; Godfrey, M.H.; Lopez, G.G.; Santos, A.J.B.; Neves, F.A.; de Souza, E.P.G.; Moura, A.S.; Bastos, J.d.C.; Bastos, V.L.F.d.C. Ghrelin and Leptin Modulate the Feeding Behaviour of the Hawksbill Turtle Eretmochelys imbricata during Nesting Season. Conserv. Physiol. 2013, 1, 1–13. [Google Scholar] [CrossRef]
- Plot, V.; Jenkins, T.; Robin, J.-P.; Fossette, S.; Georges, J.-Y. Leatherback Turtles Are Capital Breeders: Morphometric and Physiological Evidence from Longitudinal Monitoring. Physiol. Biochem. Zool. 2013, 86, 385–397. [Google Scholar] [CrossRef]
- Owens, D.W.; Morris, Y.A. The Comparative Endocrinology of Sea Turtles. Copeia 1985, 3, 723–735. [Google Scholar] [CrossRef]
- Peavey, L.E.; Popp, B.N.; Pitman, R.L.; Gaines, S.D.; Arthur, K.E.; Kelez, S.; Seminoff, J.A. Opportunism on the High Seas: Foraging Ecology of Olive Ridley Turtles in the Eastern Pacific Ocean. Front. Mar. Sci. 2017, 4, 348. [Google Scholar] [CrossRef]
- Fleming, A.H.; Kellar, N.M.; Allen, C.D.; Kurle, C.M. The Utility of Combining Stable Isotope and Hormone Analyses for Marine Megafauna Research. Front. Mar. Sci. 2018, 5, 338. [Google Scholar] [CrossRef]
- Ensminger, D.C.; Salvador-pascual, A.; Arango, B.G.; Allen, K.N.; Vazquez-Medina, J.P. Fasting Ameliorates Oxidative Stress: A Review of Physiological Strategies across Life History Events in Wild Vertebrates. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2021, 256. [Google Scholar] [CrossRef]
- Stier, A.; Dupoué, A.; Picard, D.; Angelier, F.; Brischoux, F.; Lourdais, O. Oxidative Stress in a Capital Breeder (Vipera aspis) Facing Pregnancy and Water Constraints. J. Exp. Biol. 2017, 220, 1792–1796. [Google Scholar] [CrossRef]
- Cohen, A.; Klasing, K.; Ricklefs, R. Measuring Circulating Antioxidants in Wild Birds. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2007, 147, 110–121. [Google Scholar] [CrossRef]
- Deutsch, C.J.; Haley, M.P.; Le Boeuf, B.J. Reproductive Effort of Male Northern Elephant Seals: Estimates from Mass Loss. Can. J. Zool. 1990, 68, 2580–2593. [Google Scholar] [CrossRef]
- Deutsch, C.J.; Crocker, D.E.; Costa, D.P.; Le Boeuf, B.J. Sex- and Age-Related Variation in Reproductive Effort of Northern Elephant Seals. In Elephant seals: Population Ecology, Behavior, and Physiologypopulation Ecology, Behavior, and Physiology; Le Boeuf, B.J., Laws, R.M., Eds.; University of California Press: Berkeley, CA, USA, 1994; pp. 169–210. ISBN 9780520328150. [Google Scholar]
- Le Boeuf, B.J. Male-Male Competition and Reproductive Success in Elephant Seals. Am. Zool. 1974, 14, 163–176. [Google Scholar] [CrossRef]
- Haley, M.P.; Deutsch, C.J.; Le Boeuf, B.J. Size, Dominance and Copulatory Success in Male Northern Elephant Seals, Mirounga Angustirostris. Anim. Behav. 1994, 48, 1249–1260. [Google Scholar] [CrossRef]
- Crocker, D.E.; Houser, D.S.; Webb, P.M. Impact of Body Reserves on Energy Expenditure, Water Flux, and Mating Success in Breeding Male Northern Elephant Seals. Physiol. Biochem. Zool. 2012, 85, 11–20. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arango, B.G.; Ensminger, D.C.; Moreno-Santillán, D.D.; Harfush-Meléndez, M.; López-Reyes, E.M.; Marmolejo-Valencia, J.A.; Merchant-Larios, H.; Crocker, D.E.; Vázquez-Medina, J.P. Oxidative Stress Is a Potential Cost of Synchronous Nesting in Olive Ridley Sea Turtles. Antioxidants 2022, 11, 1772. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11091772
Arango BG, Ensminger DC, Moreno-Santillán DD, Harfush-Meléndez M, López-Reyes EM, Marmolejo-Valencia JA, Merchant-Larios H, Crocker DE, Vázquez-Medina JP. Oxidative Stress Is a Potential Cost of Synchronous Nesting in Olive Ridley Sea Turtles. Antioxidants. 2022; 11(9):1772. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11091772
Chicago/Turabian StyleArango, B. Gabriela, David C. Ensminger, Diana Daniela Moreno-Santillán, Martha Harfush-Meléndez, Elpidio Marcelino López-Reyes, José Alejandro Marmolejo-Valencia, Horacio Merchant-Larios, Daniel E. Crocker, and José Pablo Vázquez-Medina. 2022. "Oxidative Stress Is a Potential Cost of Synchronous Nesting in Olive Ridley Sea Turtles" Antioxidants 11, no. 9: 1772. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11091772