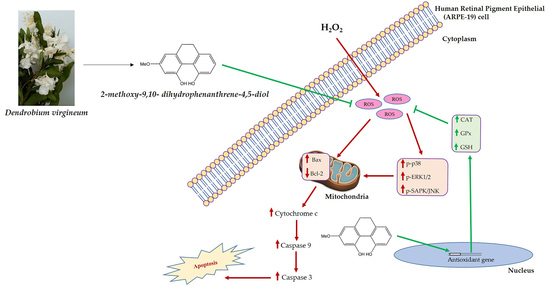
Isolation and Identification of Dihydrophenanthrene Derivatives from Dendrobium virgineum with Protective Effects against Hydrogen-Peroxide-Induced Oxidative Stress of Human Retinal Pigment Epithelium ARPE-19 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Culture of ARPE-19 Cells
2.5. Cell Viability Assay
2.6. Cytotoxicity Assay of Compounds 1–9
2.7. Determining the Optimal H2O2 Concentration for Cytotoxicity Induction
2.8. Assessing the Effect of Compound 3 on ARPE-19 Cells Exposed to H2O2
2.9. Evaluation of Reactive Oxygen Species (ROS) Production
2.10. Western Blot Analysis
2.11. Caspase-9 and -3 Activities
2.12. SOD, GPx, CAT, and GSH Determination
2.13. Statistical Analysis
3. Results
3.1. Structural Characterization
3.2. Evaluation of the Effects of Compounds (1–9) on Viability of ARPE-19 Cells
3.3. Evaluation of the Effect of H2O2 on Viability and ROS Production of ARPE-19 Cells
3.4. Evaluation of the Effect of Compounds (1–9) on Cell Viability of Oxidative-Stress-Induced ARPE-19 Cells
3.5. Evaluation of the Effect of Compound 3 on Cell Viability and ROS Production in Oxidative-Stress-Induced ARPE-19 Cells
3.6. Evaluation of the Effect of Compound 3 on MAPKs Protein Expression in Oxidative-Stress-Induced ARPE-19 Cells
3.7. Evaluation of the Effect of Compound 3 on Apoptosis Protein Expression in Oxidative-Stress-Induced ARPE-19 Cells
3.8. Effect of Compound 3 on Caspase-9 and Caspase-3 Activities in ARPE-19 Cells under Oxidative Stress
3.9. Evaluation of the Effect of Compound 3 on SOD, CAT, and GPx Activities as well as GSH Levels in ARPE-19 Cells under Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, C.W.; Yanagi, Y.; Lee, W.K.; Ogura, Y.; Yeo, I.; Wong, T.Y.; Cheung, C.M.G. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog. Retin. Eye Res. 2016, 53, 107–139. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Tiarnán, D.L.K.; Guymer, R.H.; Chakravarthy, U.; Steffen Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.W. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.L.; Cukras, C.A.; Chew, E.Y. Age-related macular degeneration: Epidemiology and clinical aspects. Adv. Exp. Med. Biol. 2021, 1256, 1–31. [Google Scholar]
- Singh, N.; Srinivasan, S.; Muralidharan, V.; Roy, R.; Jayprakash, V.; Raman, R. Prevention of age-related macular degeneration. Asia Pac. J. Ophthalmol. 2017, 6, 520–526. [Google Scholar] [CrossRef]
- Taylor, D.J.; Hobby, A.E.; Binns, A.M.; Crabb, D.P. How does age-related macular degeneration affect real-world visual ability and quality of life? A systematic review. BMJ Open 2016, 6, e011504. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef] [Green Version]
- García-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and intermediate age-related macular degeneration: Update and clinical review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Stahl, A. The diagnosis and treatment of age-related macular degeneration. Dtsch. Arztebl. Int. 2020, 117, 513–520. [Google Scholar] [CrossRef]
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the epidemiology of age-related macular degeneration. Asia Pac. J. Ophthalmol. 2017, 6, 493–497. [Google Scholar]
- Ding, X.; Patel, M.; Chan, C.C. Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009, 28, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, P.; Yang, Y.C.; Paraoan, L. Directional protein secretion by the retinal pigment epithelium: Roles in retinal health and the development of age-related macular degeneration. J. Cell. Mol. Med. 2013, 17, 833–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-related macular degeneration: Role of oxidative stress and blood vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal pigment epithelium and age-related macular degeneration: A review of major disease mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Bao, X.L.; Cong, Y.Y.; Fan, B.; Li, G.Y. Autophagy in age-related macular degeneration: A regulatory mechanism of oxidative stress. Oxidative Med. Cell. Longev. 2020, 2020, 2896036. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, C.; Michel, F.; Colvez, A.; Lacroux, A.; Delage, M.; Vernet, M.H. Associations of cardiovascular disease and its risk factors with age-related macular degeneration: The POLA study. Ophthalmic Epidemiol. 2001, 8, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Terluk, M.R.; Ebeling, M.C.; Fisher, C.R.; Kapphahn, R.J.; Yuan, C.; Kartha, R.V.; Montezuma, S.R.; Ferrington, D.A. N-acetyl-L-cysteine protects human retinal pigment epithelial cells from oxidative damage: Implications for age-related macular degeneration. Oxidative Med. Cell. Longev. 2019, 2019, 5174957. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019, 24, 101201. [Google Scholar] [CrossRef]
- Rohowetz, L.J.; Kraus, J.G.; Koulen, P. Reactive oxygen species-mediated damage of retinal neurons: Drug development targets for therapies of chronic neurodegeneration of the retina. Int. J. Mol. Sci. 2018, 19, 3362. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.Q.; Huang, Y.; Feng, S.Y.; Zheng, Z.C.; Liu, X.J.; Liu, M.M. Label-free detection of hydrogen peroxide-induced oxidative stress in human retinal pigment epithelium cells via laser tweezers Raman spectroscopy. Biomed. Opt. Express 2019, 10, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Domènech, E.B.; Marfany, G. The relevance of oxidative stress in the pathogenesis and therapy of retinal dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grune, T.; Jung, T.; Merker, K.; Davies, K.J. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int. J. Biochem. Cell Biol. 2004, 36, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, V.; Rodriguez, G.E.; Savoldelli, M.; Gioia, R.; Forlino, A.; Mazzini, G.; Pennati, M.; Zaffaroni, N.; Scovassi, A.I.; Torriglia, A. Characterization of stress response in human retinal epithelial cells. J. Cell. Mol. Med. 2013, 17, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Hecquet, C.; Lefevre, G.; Valtink, M.; Engelmann, K.; Mascarelli, F. Activation and role of map kinase-dependent pathways in retinal pigment epithelial cells: Erk and Rpe cell proliferation. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3091–3098. [Google Scholar]
- Vaseva, A.V.; Moll, U.M. The mitochondrial P53 pathway. Biochim. Biophys. Acta 2009, 1787, 414–420. [Google Scholar] [CrossRef] [Green Version]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [Green Version]
- Plestina-Borjan, I.; Katusic, D.; Medvidovic-Grubisic, M.; Supe-Domic, D.; Bucan, K.; Tandara, L.; Rogosic, V. Association of age-related macular degeneration with erythrocyte antioxidant enzymes activity and serum total antioxidant status. Oxidative Med. Cell. Longev. 2015, 2015, 804054. [Google Scholar] [CrossRef]
- Liles, M.R.; Newsome, D.A.; Oliver, P.D. Antioxidant enzymes in the aging human retinal pigment epithelium. Arch. Ophthalmol. 1991, 109, 1285–1288. [Google Scholar] [CrossRef]
- Luo, X.; Gu, S.; Zhang, Y.; Zhang, J. Kinsenoside ameliorates oxidative stress-induced Rpe cell apoptosis and inhibits angiogenesis via Erk/P38/Nf-Κb/VEGF signaling. Front. Pharmacol. 2018, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nashine, S.; Kanodia, R.; Nesburn, A.B.; Soman, G.; Kuppermann, B.D.; Kenney, M.C. Nutraceutical effects of Emblica officinalis in age-related macular degeneration. Aging 2019, 11, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Han, J.; Zhu, L.; Chen, Z.; Li, J.; Gu, Y.; Wang, F.; Wang, T.; Yue, Y.; Shang, J. Protective activities of Dendrobium huoshanense C. Z. Tang Et S. J. Cheng polysaccharide against high-cholesterol diet-induced atherosclerosis in zebrafish. Oxidative Med. Cell. Longev. 2020, 2020, 8365056. [Google Scholar] [CrossRef] [PubMed]
- Yeow, L.C.; Chew, B.L.; Sreeramanan, S. Elevation of secondary metabolites production through light-emitting diodes (LEDs) illumination in protocorm-like bodies (PLBs) of Dendrobium hybrid orchid rich in phytochemicals with therapeutic effects. Biotechnol. Rep. 2020, 27, e00497. [Google Scholar] [CrossRef]
- Hossain, M.M. Therapeutic orchids: Traditional uses and recent advances-an overview. Fitoterapia 2011, 82, 102–140. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Koskela, A.; Kaarniranta, K.; Blasiak, J. Dietary polyphenols in age-related macular degeneration: Protection against oxidative stress and beyond. Oxidative Med. Cell. Longev. 2019, 2019, 9682318. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and zeaxanthin and their roles in age-related macular degeneration—Neurodegenerative disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Wen, J.; Cheng, H.Y.; Feng, Y.F.; Huang, H.; Liao, Y.J.; Liu, Y.C.; Yang, S.Y.; Xia, Z.Y. Sulforaphane mitigates retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Oxidative Med. Cell. Longev. 2019, 2019, 9682318. [Google Scholar]
- Cheng, H.Y.; Liu, Y.C.; Huang, H.; Yang, S.Y.; Liao, Y.J.; Wen, J.; Xia, Z.Y. Inhibitory effects of sulforaphane on retinal neovascularization via retinal pigment epithelial cells: Evidences from in vitro and in vivo studies. Oxidative Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar]
- Li, Y.; Li, W.; Lv, L.; Chen, Q.; Li, M.; Liu, H.; Cheng, Y.; Zhou, L.; Li, C.; Yao, Y. Zeaxanthin attenuates retinal microglia activation and inflammatory response in vitro and in vivo. Nutrients 2022, 14, 827. [Google Scholar]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.M.; Atanasov, A.G.; Trombetta, D. Flavonoids and age-related macular degeneration: An updated review of prospective cohort studies. Molecules 2018, 23, 2058. [Google Scholar]
- Gorusupudi, A.; Nelson, K.; Bernstein, P.S. The age-related eye disease 2 study: Micronutrients in the treatment of macular degeneration. Adv. Nutr. 2017, 8, 40–53. [Google Scholar] [CrossRef] [Green Version]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L.; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar]
- Wei, X.; Chen, D.; Yi, Y.; Qi, H.; Gao, X.; Fang, H.; Gu, Q.; Wang, L.; Gu, L. Syringic acid extracted from Herba Dendrobii prevents diabetic cataract pathogenesis by inhibiting aldose reductase activity. Evid. Based Complement. Altern. Med. 2012, 2012, 426537. [Google Scholar] [CrossRef] [Green Version]
- Vaddhanaphuti, C. The Thai state and ethnic minorities: From assimilation to selective integration. In Ethnic Conflicts in Southeast Asia; Institute of Southeast Asian Studies: Singapore, 2005. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Xie, H.; Yoshikawa, M. Antiallergic phenanthrenes and stilbenes from the tubers of Gymnadenia conopsea. Planta Med. 2004, 70, 847–855. [Google Scholar] [CrossRef]
- Chen, Y.; Junju, X.; Hong, Y.; Chen, Q.; Yanli, Z.; Liqin, W.; Ying, L.; Jihua, W. Cytotoxic phenolics from Bulbophyllum odoratissimum. Food Chem. 2008, 107, 169–173. [Google Scholar] [CrossRef]
- Sritularak, B.; Anuwat, M.; Likhitwitayawuid, K. A new phenanthrenequinone from Dendrobium draconis. J. Asian Nat. Prod. Res. 2011, 13, 251–255. [Google Scholar] [CrossRef]
- Bertelli, D.; Papotti, G.; Bortolotti, L.; Marcazzan, G.L.; Plessi, M. ¹H-NMR Simultaneous identification of health-relevant compounds in propolis extracts. Phytochem. Anal. 2012, 23, 260–266. [Google Scholar] [CrossRef]
- Majumder, P.L.; Pal, S. Cumulatin and tristin, two bibenzyl derivatives from the orchids Dendrobium cumulatum and Bulbophyllum triste. Phytochemistry 1993, 32, 1561–1565. [Google Scholar] [CrossRef]
- Hu, J.M.; Chen, J.J.; Yu, H.; Zhao, Y.X.; Zhou, J. Five new compounds from Dendrobium longicornu. Planta Med. 2008, 74, 535–539. [Google Scholar] [CrossRef]
- Li, S.C.; Shichao, H.; Shijie, Z.; Xingmei, D.; Haoyu, Y.; Jie, S.; Aihua, P.; Lijuan, C. Elution–extrusion counter-current chromatography separation of five bioactive compounds from Dendrobium chrysototxum Lindl. J. Chromatogr. A 2011, 1218, 3124–3128. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, F.; Yang, L.J.; Chun, Z.; Bao, J.K.; Zhang, G.L. Anti-inflammatory phenanthrene derivatives from stems of Dendrobium denneanum. Phytochemistry 2013, 95, 242–251. [Google Scholar] [CrossRef]
- Na Ranong, S.; Likhitwitayawuid, K.; Mekboonsonglarp, W.; Sritularak, B. New dihydrophenanthrenes from Dendrobium infundibulum. Nat. Prod. Res. 2019, 33, 420–426. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, X.; Zhang, M.; Zhao, C.; Mei, C.; Li, P. Mapk pathway inhibitors attenuated hydrogen peroxide induced damage in neural cells. Biomed. Res. Int. 2019, 2019, 5962014. [Google Scholar] [CrossRef]
- Teiji, W.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar]
- Yue, J.; López, J.M. Understanding Mapk signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [Green Version]
- Amina, B.; Touil, A.; Bensouici, C.; Bendif, H.; Rhouati, S. Phenanthrene and dihydrophenanthrene derivatives from Dioscorea communis with anticholinesterase, and antioxidant activities. Nat. Prod. Res. 2019, 33, 3278–3282. [Google Scholar]
- Muangnoi, C.; Phumsuay, R.; Jongjitphisut, N.; Waikasikorn, P.; Sangsawat, M.; Rashatasakhon, P.; Paraoan, L.; Rojsitthisak, P. Protective effects of a lutein ester prodrug, lutein diglutaric acid, against H2O2-induced oxidative stress in human retinal pigment epithelial cells. Int. J. Mol. Sci. 2021, 22, 4722. [Google Scholar] [CrossRef]
- Muangnoi, C.; Sharif, U.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Paraoan, L. Protective effects of curcumin ester prodrug, curcumin diethyl disuccinate against H2O2-induced oxidative stress in human retinal pigment epithelial cells: Potential therapeutic avenues for age-related macular degeneration. Int. J. Mol. Sci. 2019, 20, 3367. [Google Scholar] [CrossRef] [Green Version]
- Warinhomhoun, S.; Muangnoi, C.; Buranasudja, V.; Mekboonsonglarp, W.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Antioxidant activities and protective effects of dendropachol, a new bisbibenzyl compound from Dendrobium pachyglossum, on hydrogen peroxide-induced oxidative stress in HaCat keratinocytes. Antioxidants 2021, 10, 252. [Google Scholar] [CrossRef]
- Badalamenti, N.; Russi, S.; Bruno, M.; Maresca, V.; Vaglica, A.; Ilardi, V.; Zanfardino, A.; Di Napoli, M.; Varcamonti, M.; Cianciullo, P.; et al. Dihydrophenanthrenes from a sicilian accession of Himantoglossum robertianum (Loisel.) P. delforge showed antioxidant, antimicrobial, and antiproliferative activities. Plants 2021, 10, 2776. [Google Scholar] [CrossRef]
- Smith, R.E.; Tran, K.; Smith, C.C.; McDonald, M.; Shejwalkar, P.; Hara, K. The role of the Nrf2/Are antioxidant system in preventing cardiovascular diseases. Diseases 2016, 4, 34. [Google Scholar] [CrossRef]
- Liu, S.; Jingbo, P.; Qiang, Z. Signal amplification in the Keap1-Nrf2-are antioxidant response pathway. Redox Biol. 2022, 54, 102389. [Google Scholar] [CrossRef]
- Ou, C.; Jiang, P.; Tian, Y.; Yao, Z.; Yang, Y.; Peng, J.; Zeng, M.; Song, H.; Peng, Q. Fructus Lycii and Salvia miltiorrhiza Bunge extract alleviate retinitis pigmentosa through Nrf2/HO-1 signaling pathway. J. Ethnopharmacol. 2021, 273, 113993. [Google Scholar] [CrossRef]
- Herrera-Bravo, J.; Beltrán-Lissabet, J.F.; Saavedra, K.; Saavedra, N.; Hevia, M.; Alvear, M.; Lanas, F.; Salazar, L.A. Protective effect of pinot noir pomace extract against the cytotoxicity induced by polycyclic aromatic hydrocarbons on endothelial cells. Food Chem. Toxicol. 2021, 148, 111947. [Google Scholar] [CrossRef]












| 1 | 2 | |||
|---|---|---|---|---|
| Position | 1H (Multiplicity, J in Hz) | 13C | 1H (Multiplicity, J in Hz) | 13C |
| 1 | 6.49 (d, 2.5) | 107.1 | - | 180.7 |
| 2 | - | 160.7 | - | 158.9 |
| 3 | 6.45 (d, 2.5) | 102.4 | 5.95 (s) | 108.0 |
| 4 | - | 155.6 | - | 185.1 |
| 4a | - | 114.8 | - | 142.1 |
| 4b | - | 123.2 | - | 112.6 |
| 5 | - | 146.8 | - | 160.4 |
| 6 | 6.88 (d, 8.5) | 116.1 | 6.43 (d, 2.0) | 99.5 |
| 7 | 6.84 (d, 8.5) | 111.7 | - | 161.5 |
| 8 | - | 150.9 | 6.41 (d, 2.0) | 108.2 |
| 8a | - | 129.3 | - | 143.9 |
| 9 | 2.58–2.65 (m) | 23.0 | 2.58 (t, 7.0) | 29.3 |
| 10 | 2.58–2.65 (m) | 31.6 | 2.46 (br s) | 20.6 |
| 10a | - | 143.4 | - | 137.6 |
| MeO-2 | 3.77 (s) | 55.4 | 3.82 (s) | 56.3 |
| MeO-5 | - | - | 3.69 (s) | 56.0 |
| MeO-8 | 3.78 (s) | 56.6 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panuthai, P.; Phumsuay, R.; Muangnoi, C.; Maitreesophone, P.; Kongkatitham, V.; Mekboonsonglarp, W.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Isolation and Identification of Dihydrophenanthrene Derivatives from Dendrobium virgineum with Protective Effects against Hydrogen-Peroxide-Induced Oxidative Stress of Human Retinal Pigment Epithelium ARPE-19 Cells. Antioxidants 2023, 12, 624. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12030624
Panuthai P, Phumsuay R, Muangnoi C, Maitreesophone P, Kongkatitham V, Mekboonsonglarp W, Rojsitthisak P, Likhitwitayawuid K, Sritularak B. Isolation and Identification of Dihydrophenanthrene Derivatives from Dendrobium virgineum with Protective Effects against Hydrogen-Peroxide-Induced Oxidative Stress of Human Retinal Pigment Epithelium ARPE-19 Cells. Antioxidants. 2023; 12(3):624. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12030624
Chicago/Turabian StylePanuthai, Pongsawat, Rianthong Phumsuay, Chawanphat Muangnoi, Porames Maitreesophone, Virunh Kongkatitham, Wanwimon Mekboonsonglarp, Pornchai Rojsitthisak, Kittisak Likhitwitayawuid, and Boonchoo Sritularak. 2023. "Isolation and Identification of Dihydrophenanthrene Derivatives from Dendrobium virgineum with Protective Effects against Hydrogen-Peroxide-Induced Oxidative Stress of Human Retinal Pigment Epithelium ARPE-19 Cells" Antioxidants 12, no. 3: 624. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12030624