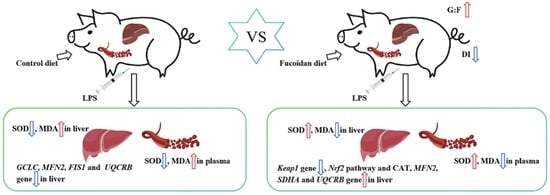
Fucoidan Supplementation Improves Antioxidant Capacity via Regulating the Keap1/Nrf2 Signaling Pathway and Mitochondrial Function in Low-Weaning Weight Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics Approval
2.2. Animals and Treatment
2.3. Sample Collection
2.4. Assay of Antioxidant Indices
2.5. Real-Time Quantitative PCR Analysis (qPCR)
2.6. Statistical Analysis
3. Results
3.1. Growth Performance and Diarrhea Incidence
3.2. Plasma IGF-1 Concentrations
3.3. Plasma Antioxidant Enzyme Activity
3.4. Hepatic Antioxidant Enzyme Activity
3.5. Hepatic Antioxidant Genes mRNA Expression
3.6. Hepatic Mitochondrial Genes mRNA Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Items | Experiment Treatments | |||
|---|---|---|---|---|
| FC 0 | FC 150 | FC 300 | FC 600 | |
| Ingredients | ||||
| Corn | 16.45 | 16.45 | 16.45 | 16.45 |
| Extruded corn | 32.00 | 32.00 | 32.00 | 32.00 |
| Soybean meal, 46%CP | 14.00 | 14.00 | 14.00 | 14.00 |
| Extruded soybean | 11.50 | 11.50 | 11.50 | 11.50 |
| Fish meal | 5.60 | 5.60 | 5.60 | 5.60 |
| Whey | 15.00 | 15.00 | 15.00 | 15.00 |
| Soybean oil | 1.00 | 1.00 | 1.00 | 1.00 |
| Dicalcium phosphate | 0.40 | 0.40 | 0.40 | 0.40 |
| Limestone (CaCO3) | 0.75 | 0.75 | 0.75 | 0.75 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 |
| Choline chloride (60%) | 0.05 | 0.05 | 0.05 | 0.05 |
| L-Lysine HCl | 1.20 | 1.20 | 1.20 | 1.20 |
| DL-Methionine | 0.09 | 0.09 | 0.09 | 0.09 |
| Threonine | 0.27 | 0.27 | 0.27 | 0.27 |
| Tryptophan | 0.02 | 0.02 | 0.02 | 0.02 |
| Phytase | 0.02 | 0.02 | 0.02 | 0.02 |
| Acidifier | 0.35 | 0.35 | 0.35 | 0.35 |
| Zinc oxide | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin and mineral premix 1 | 0.80 | 0.80 | 0.80 | 0.80 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrition composition (Analyzed value) | ||||
| GE, KJ/g | 16.84 | 16.80 | 16.85 | 16.84 |
| Crude protein | 19.45 | 19.48 | 19.45 | 19.46 |
| Calcium | 0.77 | 0.76 | 0.78 | 0.77 |
| Phosphorus | 0.65 | 0.66 | 0.64 | 0.65 |
| Ether extract | 4.22 | 4.21 | 4.21 | 4.22 |
| Crude Ash | 6.21 | 6.19 | 6.18 | 6.21 |
| Nutrition composition (Calculated value) | ||||
| ME, MJ/kg | 14.23 | 14.23 | 14.23 | 14.23 |
| Lysine | 1.30 | 1.30 | 1.30 | 1.30 |
| Methionine | 0.38 | 0.38 | 0.38 | 0.38 |
| Threonine | 0.76 | 0.76 | 0.76 | 0.76 |
| Tryptophan | 0.21 | 0.21 | 0.21 | 0.21 |
| Items | Experiment Treatments | |||
|---|---|---|---|---|
| CON | FC | LPS | FC + LPS | |
| Ingredients | ||||
| Corn | 16.45 | 16.45 | 16.45 | 16.45 |
| Extruded corn | 32.00 | 32.00 | 32.00 | 32.00 |
| Soybean meal, 46%CP | 14.00 | 14.00 | 14.00 | 14.00 |
| Extruded soybean | 11.50 | 11.50 | 11.50 | 11.50 |
| Fish meal | 5.60 | 5.60 | 5.60 | 5.60 |
| Whey | 15.00 | 15.00 | 15.00 | 15.00 |
| Soybean oil | 1.00 | 1.00 | 1.00 | 1.00 |
| Dicalcium phosphate | 0.40 | 0.40 | 0.40 | 0.40 |
| Limestone (CaCO3) | 0.75 | 0.75 | 0.75 | 0.75 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 |
| Choline chloride (60%) | 0.05 | 0.05 | 0.05 | 0.05 |
| L-Lysine HCl | 1.20 | 1.20 | 1.20 | 1.20 |
| DL-Methionine | 0.09 | 0.09 | 0.09 | 0.09 |
| Threonine | 0.27 | 0.27 | 0.27 | 0.27 |
| Tryptophan | 0.02 | 0.02 | 0.02 | 0.02 |
| Phytase | 0.02 | 0.02 | 0.02 | 0.02 |
| Acidifier | 0.35 | 0.35 | 0.35 | 0.35 |
| Zinc oxide | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin and mineral premix 1 | 0.80 | 0.80 | 0.80 | 0.80 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrition composition (Analyzed value) | ||||
| GE, KJ/g | 17.00 | 16.89 | 17.00 | 16.89 |
| Crude protein | 19.29 | 19.31 | 19.29 | 19.31 |
| Calcium | 0.79 | 0.78 | 0.79 | 0.78 |
| Phosphorus | 0.67 | 0.65 | 0.67 | 0.65 |
| Ether extract | 4.32 | 4.29 | 4.32 | 4.29 |
| Crude Ash | 6.23 | 6.13 | 6.23 | 6.13 |
| Nutrition composition (Calculated value) | ||||
| ME, MJ/kg | 14.23 | 14.23 | 14.23 | 14.23 |
| Lysine | 1.30 | 1.30 | 1.30 | 1.30 |
| Methionine | 0.38 | 0.38 | 0.38 | 0.38 |
| Threonine | 0.76 | 0.76 | 0.76 | 0.76 |
| Tryptophan | 0.21 | 0.21 | 0.21 | 0.21 |
Appendix B
| Gene | Primer Sequence (5′→3′) | Product Length, bp | Accession No. |
|---|---|---|---|
| ATP5H | F: CATTGACTGGGTAGCCTTTG | 115 | XM_021066093.1 |
| R: CTTCTCAGGTAGAGCAGCCA | |||
| CAT | F: CCTGCAACGTTCTGTAAGGC | 72 | NM_214301.2 |
| R: GCTTCATCTGGTCACTGGCT | |||
| DRP1 | F:GTAAACCGAAGCCAGAAGGACA | 102 | XM_021069575.1 |
| R: CAAGTGGCGATAGGAAGGGTGG | |||
| FIS1 | F:CCAAAGGGAGCAAAGAGGAGCA | 132 | XM_021086263.1 |
| R: CCTGGTTGTTCTGTGGCTCTGT | |||
| GAPDH | F: GCTTGTCATCAATGGAAAGG | 86 | NM_001206359.1 |
| R: CATACGTAGCACCAGCATCA | |||
| GCLC | F: GGAGAGGGGAGAAAGTTGTC | 103 | XM_021098556.1 |
| R: GCCTTCGCTGCTTCATCATC | |||
| GCLM | F: GCTTCGAGACTGTATCCAAA | 132 | XM_001926378.4 |
| R: CTTTCATCGGGATTTATTTT | |||
| GPX2 | F: TCTCCAGTGTGTCGCAATGA | 104 | NM_214201.1 |
| R: TCGATGGTCAGAAAGCGACG | |||
| GPX4 | F: GATTCTGGCCTTCCCTTGC | 173 | NM_214407.1 |
| R: TCCCCTTGGGCTGGACTTT | |||
| HO1 | F: GAGAAGGCTTTAAGCTGGTG | 74 | NM_001004027.1 |
| R: GTTGTGCTCAATCTCCTCCT | |||
| Keap1 | F: AGCTGGGATGCCTCAGTGTT | 100 | NM_001114671.1 |
| R: AGGCAAGTTCTCCCAGACATTC | |||
| MFN1 | F:CAATAGAAGAGAGGGAAGACC | 117 | NM_001315732.1 |
| R: TATTTGCCACCTCCTCTGTAA | |||
| MFN2 | F:AGAGGAGAAGAGGAGCGTCAAGA | 95 | XM_021095370.1 |
| R: ACATCACACTCACCAGGCTGC | |||
| NDUFS2 | F: CTAAACGCGCAGAGATGAAGA | 108 | XM_005663166.3 |
| R: CCTCAATGGCAGTGTATGTGG | |||
| NDUFV2 | F:CCCAGATACTCCATTTGATTTCA | 169 | NM_001097475.2 |
| R: AATTTCTGCCACCTTGTTCATG | |||
| Nrf2 | F: GAGAAGGCTTTAAGCTGGTG | 103 | XM_005671981.3 |
| R: GTTGTGCTCAATCTCCTCCT | |||
| OPA1 | F:CAGAGGATGGTGCTTGTTGAC | 128 | XM_021070065.1 |
| R: AGTATGATGGCGTTGGGATTC | |||
| SDHA | F:TCTCTGAGGCCGGGTTTAACACA | 124 | XM_021076930.1 |
| R: CACCTCCAGTTGTCCTCCTCCAT | |||
| SOD1 | F: GAAGACAGTGTTAGTAACGG | 93 | NM_001190422.1 |
| R: CAGCCTTGTGTATTATCTCC | |||
| UQCRB | F: GGATGACGATGTAAAAGAAGCCA | 141 | NM_001185172.1 |
| R: TCCTCCTCATATTTTGTCCACTG |
References
- Wang, L.; Zhang, S.; Johnston, L.; Levesque, C.; Yin, J.; Dong, B. A systematic review and meta-analysis of dietary fat effects on reproductive performance of sows and growth performance of piglets. J. Anim. Sci. Biotechnol. 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Peng, J.; Cui, C.; Gu, Q.; Zhou, L.; Chao, W.; Sun, H.; Peng, J.; Wei, H. Effect of gestation dietary methionine-to-lysine ratio on methionine metabolism and antioxidant ability of high-prolific sows. Anim. Nutr. 2021, 7, 849–858. [Google Scholar]
- Craig, A.; Muns, R.; Gordon, A.; Magowan, E. Extended nursing and/or increased starter diet allowances for low weaning weight pigs. Asian-Australas. J. Anim. Sci. 2020, 33, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Magowan, E.; Ball, M.; McCracken, K.; Beattie, V.; Bradford, R.; Robinson, M.; Scott, M.; Gordon, F.; Mayne, C. The performance response of pigs of different wean weights to ‘high’ or ‘low’ input dietary regimes between weaning and 20 weeks of age. Livest. Sci. 2011, 136, 232–239. [Google Scholar] [CrossRef]
- Collins, C.; Pluske, J.; Morrison, R.; McDonald, T.; Smits, R.; Henman, D.; Stensland, I.; Dunshea, F. Post-weaning and whole-of-life performance of pigs is determined by live weight at weaning and the complexity of the diet fed after weaning. Anim. Nutr. 2017, 3, 372–379. [Google Scholar] [CrossRef]
- St-Pierre, B.; Palencia, J.; Samuel, R. Impact of early weaning on development of the swine gut microbiome. Microorganisms 2023, 11, 1753. [Google Scholar] [CrossRef]
- Yin, C.; Comi, M.; Cai, L.; Chen, W.; Perricone, V.; Xiao, J.; Agazzi, A.; Li, X.; Jiang, X. Hydrolysed yeast from Kluyveromyces fragilis improves plasma antioxidant efficiency and immunoglobulin concentration, and faecal microbiota of weaned piglets. Ital. J. Anim. Sci. 2023, 22, 578–588. [Google Scholar] [CrossRef]
- Cai, L.; Gao, G.; Yin, C.; Bai, R.; Li, Y.; Sun, W.; Pi, Y.; Jiang, X.; Li, X. The effects of dietary silybin supplementation on the growth performance and regulation of intestinal oxidative injury and microflora dysbiosis in weaned piglets. Antioxidants 2023, 12, 1975. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, X.; Cai, L.; Zhang, Y.; Ding, H.; Yin, J.; Li, X. Effects of daidzein on antioxidant capacity in weaned pigs and IPEC-J2 cells. Anim. Nutr. 2022, 11, 48–59. [Google Scholar] [CrossRef]
- Novais, A.; Deschene, K.; Martel-Kennes, Y.; Roy, C.; Laforest, J.; Lessard, M.; Matte, J.; Lapointe, J. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PLoS ONE 2021, 16, e0247188. [Google Scholar] [CrossRef]
- Yu, L.; Li, H.; Peng, Z.; Ge, Y.; Liu, J.; Wang, T.; Wang, H.; Dong, L. Early weaning affects liver antioxidant function in piglets. Animals 2021, 11, 2679. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning induced hepatic oxidative stress, apoptosis, and aminotransferases through MAPK signaling pathways in piglets. Oxid. Med. Cell. Longev. 2016, 2016, 4768541. [Google Scholar] [CrossRef] [PubMed]
- Guerbette, T.; Boudry, G.; Lan, A. Mitochondrial function in intestinal epithelium homeostasis and modulation in diet-induced obesity. Mol. Metab. 2022, 63, 101546. [Google Scholar] [CrossRef] [PubMed]
- Valera-Alberni, M.; Canto, C. Mitochondrial stress management: A dynamic journey. Cell Stress 2018, 2, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yuan, S.; Wu, C.; Lv, Z. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish. Aquat. Toxicol. 2016, 180, 36–44. [Google Scholar] [CrossRef]
- Cai, L.; Ming, D.; Chen, W.; Zhao, Y.; Li, Y.; Sun, W.; Pi, Y.; Jiang, X.; Li, X. Silybin alleviated hepatic injury by regulating redox balance, inflammatory response, and mitochondrial function in weaned piglets under paraquat-induced oxidative stress. Antioxidants 2024, 13, 324. [Google Scholar] [CrossRef]
- Zhou, W.; He, H.; Wei, Q.; Che, L.; Zhao, X.; Liu, W.; Yan, Y.; Hu, L.; Du, Y.; Yin, Z.; et al. Puerarin protects against acetaminophen-induced oxidative damage in liver through activation of the Keap1/Nrf2 signaling pathway. Food Sci. Nutr. 2023, 11, 6604–6615. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Wu, P.; Chu, Y.; Gui, S.; Zheng, Y.; Chen, X. Dietary selenium alleviated mouse liver oxidative stress and NAFLD induced by obesity by regulating the Keap1/Nrf2 Pathway. Antioxidants 2022, 11, 349. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.; Yang, S.; Yang, F.; Lu, J. Fucoidan extracted from undaria pinnatifida: Source for nutraceuticals/functional foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.; Hyun, J.; Wang, K.; Fu, X.; Xu, J.; Gao, X.; Park, Y.; Jeon, Y. Antioxidant and anti-photoaging effects of a fucoidan isolated from Turbinaria ornata. Int. J. Biol. Macromol. 2023, 225, 1021–1027. [Google Scholar] [CrossRef]
- Ji, Y.; Jin, D.; Qi, J.; Wang, X.; Zhang, C.; An, P.; Luo, Y.; Luo, J. Fucoidan protects against doxorubicin-induced cardiotoxicity by reducing oxidative stress and preventing mitochondrial function injury. Int. J. Mol. Sci. 2022, 23, 10685. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Sun, H.; Yang, L.; He, D.; Ren, C.; Zhu, C.; Lv, G. Effects of fucoidan on growth performance, immunity, antioxidant ability, digestive enzyme activity, and hepatic morphology in juvenile common carp (Cyprinus carpio). Front. Mar. Sci. 2023, 10, 1167400. [Google Scholar]
- Van Nieuwamerongen, S.; Bolhuis, J.; Van, P.; Kemp, B.; Soede, N. Effects of pre-weaning housing in a multi-suckling system on performance and carbohydrate absorption of relatively light and heavy piglets around weaning. Animal 2018, 12, 802–809. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Chen, J.; Liu, Y.; Wen, W.; Li, H.; Huang, X. Lactobacillus delbrueckii ameliorates intestinal integrity and antioxidant ability in weaned piglets after a lipopolysaccharide challenge. Oxid. Med. Cell. Longev. 2020, 2020, 6028606. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Awati, A.; Agazzi, A.; Vitari, F.; Ferrari, A.; Bento, H.; Crestani, M.; Domeneghini, C.; Bontempo, V. Effects of a blend of essential oils and an enzyme combination on nutrient digestibility, ileum histology and expression of inflammatory mediators in weaned piglets. Animal 2015, 9, 417–426. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Fang, R. Role of epidermal growth factor in regulation of intestinal health of weaned piglets. Chin. J. Anim. Nutr. 2021, 33, 614–621. [Google Scholar]
- Feng, Y.; An, Z.; Chen, H.; He, X.; Wang, W.; Zhang, H.; Li, F.; Liu, D. Ulva prolifera extract alleviates intestinal oxidative stress via Nrf2 signaling in weaned piglets challenged with hydrogen peroxide. Front. Immunol. 2020, 11, 599735. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, N.; Robert, C.; Guay, F. Feed supplementation with arginine and zinc on antioxidant status and inflammatory response in challenged weanling piglets. Anim. Nutr. 2017, 3, 236–246. [Google Scholar] [CrossRef]
- Larriestra, A.; Wattanaphansak, S.; Neumann, E.; Bradford, J.; Morrison, R.; Deen, J. Pig characteristics associated with mortality and light exit weight for the nursery phase. Can. Vet. J. 2006, 47, 560–566. [Google Scholar]
- O’Shea, C.; McAlpine, P.; Sweeney, T.; Varley, P.; O’Dohert, J. Effect of the interaction of seaweed extracts containing laminarin and fucoidan with zinc oxide on the growth performance, digestibility and faecal characteristics of growing piglets. Brit. J. Nutr. 2014, 111, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.; Meredith, H.; Vigors, S.; McDonnell, M.J.; Ryan, M.; Thornton, K.; O’Doherty, J. Extracts of laminarin and laminarin/fucoidan from the marine macroalgal species laminaria digitata improved growth rate and intestinal structure in young chicks, but does not influence Campylobacter jejuni colonisation. Anim. Feed Sci. Technol. 2017, 232, 71–79. [Google Scholar] [CrossRef]
- Yang, W.; Chen, J.; Guo, G.; Wang, S.; Peng, S.; Gao, Z.; Zhao, Z.; Lan, R.; Yin, F. The effects of fucoidan dietary supplementation on growth performance, serum antioxidant capacity, immune function indices and intestinal morphology in weaned kids. Animals 2022, 12, 574. [Google Scholar] [CrossRef]
- Tuller, J.; De, S.; Jerry, D. Dietary influence of fucoidan supplementation on growth of lates calcarifer. Aquac. Res. 2014, 45, 749–754. [Google Scholar] [CrossRef]
- Sivagnanavelmurugan, M.; Thaddaeus, B.; Palavesam, A.; Immanuel, G. Dietary effect of Sargassum wightii fucoidan to enhance growth, prophenoloxidase gene expression of Penaeus monodon and immune resistance to Vibrio parahaemolyticus. Fish Shellfish Immun. 2014, 39, 439–449. [Google Scholar] [CrossRef]
- Rattigan, R.; Sweeney, T.; Vigors, S.; Thornton, K.; Rajauria, G.; O’Doherty, J. The effect of increasing inclusion levels of a fucoidan-rich extract derived from Ascophyllum nodosum on growth performance and aspects of intestinal health of pigs post-weaning. Mar. Drugs 2019, 17, 680. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.; Sweeney, T.; O’Shea, C.; Doyle, D.; O’Doherty, J. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Br. J. Nutr. 2013, 110, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wei, Y.; Hua, H.; Jing, X.; Zhu, H.; Xiao, K.; Zhao, J.; Liu, Y. Polyphenols sourced from Ilex latifolia thunb. relieve intestinal injury via modulating ferroptosis in weanling piglets under oxidative stress. Antioxidants 2022, 11, 966. [Google Scholar] [CrossRef]
- Pardo, Z.; Fernandez-Figares, I.; Lachica, M.; Lara, L.; Nieto, R.; Seiquer, I. Impact of heat stress on meat quality and antioxidant markers in iberian pigs. Antioxidants 2021, 10, 1911. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, M.; Liu, Y.; Zhu, Z.; Fahad, S.; Qayyum, A.; Zhu, G. Vanadium stress alters sweet potato (Ipomoea batatas L.) growth, ros accumulation, antioxidant defense system, stomatal traits, and vanadium uptake. Antioxidants 2022, 11, 2407. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Reyes-Castro, L.; Rodriguez-Gonzalez, G.; Bautista, C.; Vazquez-Martinez, M.; Larrea, F.; Chamorro-Cevallos, G.; Nathanielsz, P.; Zambrano, E. Resveratrol partially prevents oxidative stress and metabolic dysfunction in pregnant rats fed a low protein diet and their offspring. J. Physiol. 2016, 594, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yin, Y.; Wang, F.; Bao, X.; Long, L.; Tan, B.; Yin, Y.; Chen, J. Effects of dietary rosemary extract supplementation on growth performance, nutrient digestibility, antioxidant capacity, intestinal morphology, and microbiota of weaning pigs. J. Anim. Sci. 2021, 99, skab237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Yu, J.; Chen, H.; He, J.; Luo, Y.; Zheng, P. Dietary ferulic acid supplementation improves antioxidant capacity and lipid metabolism in weaned piglets. Nutrients 2020, 12, 3811. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Bjrkman, S.; Soede, N.; Oliviero, C.; Peltoniemi, O. IGF-1 concentrations after weaning in young sows fed different pre-mating diets are positively associated with piglet mean birth weight at subsequent farrowing. Animal 2021, 15, 100029. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Meng, Q.; Feng, X.; Tang, X.; Jia, A.; Feng, J.; Zhang, H. Probing the molecular regulation of lipopolysaccharide stress in piglet liver by comparative proteomics analysis. Electrophoresis 2018, 39, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, Z.; He, M.; Pastor, J.; Tedo, G.; Liu, J.; Wang, H. Olive oil cake extract stabilizes the physiological condition of lipopolysaccharide-challenged piglets by reducing oxidative stress and inflammatory responses and modulating the ileal microbiome. Food Funct. 2021, 12, 10171–10183. [Google Scholar] [CrossRef] [PubMed]
- Rishi, G.; Subramaniam, V. The liver in regulation of iron homeostasis. Am. J. Physiol. Gastr. L. 2017, 313, 157–165. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, K.; Zheng, X.; Ahmad, H.; Zhang, L.; Wang, T. Effects of dietary supplementation with enzymatically treated artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018, 97, 430–437. [Google Scholar] [CrossRef]
- Ma, X.; McKeen, T.; Zhang, J.; Ding, W. Role and mechanisms of mitophagy in liver diseases. Cells 2020, 9, 837. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.; Mohamed, H.; Wikstrom, J.; Walzer, G.; Stiles, L.; Haigh, S.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008, 27, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Che, Z.; Meng, X.; Yu, Y.; Li, M.; Yu, Z.; Shi, H.; Yang, D.; Yu, M. MCU up-regulation contributes to myocardial ischemia-reperfusion injury through calpain/OPA-1-mediated mitochondrial fusion/mitophagy inhibition. Int. J. Implant. Dent. 2019, 5, 7830–7843. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Li, G.; Liu, Y.; Yang, L.; Zhang, Y.; Zhang, Y.; Ding, J.; Lu, M.; Yu, G.; Hu, G. Fucoidan from Fucus vesiculosus prevents the loss of dopaminergic neurons by alleviating mitochondrial dysfunction through targeting ATP5F1a. Carbohydr. Polym. 2023, 303, 120470. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, I.; Sazanov, L. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2021, 23, 141–161. [Google Scholar] [CrossRef]
- Protasoni, M.; Perez-Perez, R.; Lobo-Jarne, T.; Harbour, M.; Ding, S.; Penas, A.; Diaz, F.; Moraes, C.; Fearnley, I.; Zeviani, M.; et al. Respiratory supercomplexes act as a platform for complex III-mediated maturation of human mitochondrial complexes I and IV. Embo J. 2020, 39, e102817. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, C.; Bi, Q.; Chen, W.; Wang, C.; Castiglioni, B.; Li, Y.; Sun, W.; Pi, Y.; Bontempo, V.; Li, X.; et al. Fucoidan Supplementation Improves Antioxidant Capacity via Regulating the Keap1/Nrf2 Signaling Pathway and Mitochondrial Function in Low-Weaning Weight Piglets. Antioxidants 2024, 13, 407. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13040407
Yin C, Bi Q, Chen W, Wang C, Castiglioni B, Li Y, Sun W, Pi Y, Bontempo V, Li X, et al. Fucoidan Supplementation Improves Antioxidant Capacity via Regulating the Keap1/Nrf2 Signaling Pathway and Mitochondrial Function in Low-Weaning Weight Piglets. Antioxidants. 2024; 13(4):407. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13040407
Chicago/Turabian StyleYin, Chenggang, Qingyue Bi, Wenning Chen, Chengwei Wang, Bianca Castiglioni, Yanpin Li, Wenjuan Sun, Yu Pi, Valentino Bontempo, Xilong Li, and et al. 2024. "Fucoidan Supplementation Improves Antioxidant Capacity via Regulating the Keap1/Nrf2 Signaling Pathway and Mitochondrial Function in Low-Weaning Weight Piglets" Antioxidants 13, no. 4: 407. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13040407