Bilberry (Vaccinium myrtillus L.) Extracts Comparative Analysis Regarding Their Phytonutrient Profiles, Antioxidant Capacity along with the In Vivo Rescue Effects Tested on a Drosophila melanogaster High-Sugar Diet Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extracts Preparation
2.3. Phytonutrient Profile Determination Via UHPLC-ESI-MS/MS Analysis
2.4. Spectrophotometric Determication of the Total Polyphenol and Flavonoid Content, and the Antioxidant Activity of the Extracts
2.4.1. Total Polyphenol Content Determination
2.4.2. Total Flavonoid Content
2.4.3. Antioxidant Activity through DPPH Free Radical Scavenging Assay
2.5. Drosophila Melanogaster Strains and Feeding Design
2.6. Drosophila Melanogaster Two-Choice Feeding Preference Assay
2.7. Drosophila Melanogaster Hemolymph Glycemia Measurement
2.8. Analysis of mRNA Levels of Neurohormonal Drosophila Melanogaster Genes by qRT-PCR
2.8.1. Total RNA Extraction and cDNA Synthesis
2.8.2. Primer Design
2.8.3. Reverse Transcription qPCR
2.8.4. Data Mining and Selection of Reference Gene Candidates
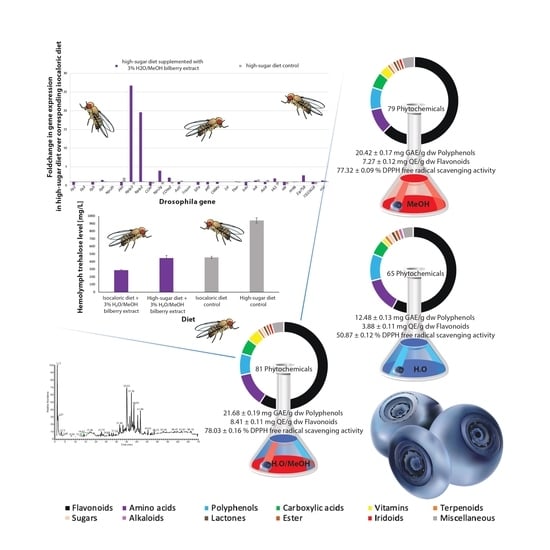
3. Results
3.1. Chemical Analysis of the Extracts
3.2. In Vivo Drosphila Melanogaster Studies of the Rescue-Effect of the Hydro-Methanolic Extract (E3)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘northblue’ blueberry (Vaccinium corymbosum × V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Directorate-General for Research and Innovation—European Commission. Publications Office of the European Union: Luxembourg, Luxembourg. Functional Foods. 2010. Available online: https://op.europa.eu/en/publication-detail/-/publication/238407ee-0301-4309-9fac-e180e33a3f89 (accessed on 10 June 2020).
- Fraisse, D.; Bred, A.; Felgines, C.; Senejoux, F. Screening and Characterization of Antiglycoxidant Anthocyanins from Vaccinium myrtillus Fruit Using DPPH and Methylglyoxal Pre-Column HPLC Assays. Antioxidants 2020, 9, 512. [Google Scholar] [CrossRef] [PubMed]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Iuhas, C.I.; Ayvaz, H.; Rugină, D.; Stanilă, A.; Dulf, F.; Bunea, A.; Socaci, S.A.; Socaciu, C.; Pintea, A. Phytochemical Characterization of Commercial Processed Blueberry, Blackberry, Blackcurrant, Cranberry, and Raspberry and Their Antioxidant Activity. Antioxidants 2019, 8, 540. [Google Scholar] [CrossRef] [Green Version]
- Ștefănescu, B.E.; Szabo, K.; Mocan, A.; Crişan, G. Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef] [Green Version]
- Muller, D.; Schantz, M.; Richling, E. High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), blueberries (Vaccinium corymbosum L.), and corresponding juices. J. Food Sci. 2012, 77, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Ştefănescu, R.E.; Eșianu, S.; Laczkó-Zöld, E.; Mare, A.; Tudor, B.; Dogaru, M.T. Short Period Storage Impact on Bioactive Constituents from Bilberries and Blueberries. Acta Med. Marisiensis 2017, 63, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Cifuentes-Gomez, T.; Tabatabaee, S.; Lecras, C.; Spencer, J.P. Procyanidin, anthocyanin, and chlorogenic acid contents of highbush and lowbush blueberries. J. Agric. Food Chem. 2012, 60, 5772–5778. [Google Scholar] [CrossRef]
- Åkerström, A. Factors affecting the Anthocyanidin Concentration in Fruits of Vaccinium myrtillus. Acta Uni Agric. Sued. Agrar. 2010, 52, 1652–6880. Available online: https://pub.epsilon.slu.se/2342/ (accessed on 29 September 2020).
- Seeram, N.P. Berry Fruits: Compositional Elements, Biochemical Activities, and the Impact of Their Intake on Human Health, Performance, and Disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef]
- Chan, S.W.; Tomlinson, B. Effects of Bilberry Supplementation on Metabolic and Cardiovascular Disease Risk. Molecules 2020, 25, 1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujor, O.-C.; Tanase, C.; Popa, M.E. Phenolic Antioxidants in Aerial Parts of Wild Vaccinium Species: Towards Pharmaceutical and Biological Properties. Antioxidants 2019, 8, 649. [Google Scholar] [CrossRef] [Green Version]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Brasanac-Vukanovic, S.; Mutic, J.; Stankovic, D.M.; Arsic, I.; Blagojevic, N.; Vukasinovic-Pesic, V.; Tadic, V.M. Wild Bilberry (Vaccinium myrtillus L.; Ericaceae) from Montenegro as a Source of Antioxidants for Use in the Production of Nutraceuticals. Molecules 2018, 23, 1864. [Google Scholar] [CrossRef] [Green Version]
- Musselman, L.P.; Fink, J.L.; Narzinski, K.; Ramachandran, P.V.; Hathiramani, S.S.; Cagan, R.L.; Baranski, T.J. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model. Mech. 2011, 4, 842–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musselman, L.P.; Fink, J.L.; Ramachandran, P.V.; Patterson, B.W.; Okunade, A.L.; Maier, E.; Baranski, T.J. Role of fat body lipogenesis in protection against the effects of caloric overload in Drosophila. J. Biol. Chem. 2013, 288, 8028–8042. [Google Scholar] [CrossRef] [Green Version]
- Graham, P.; Pick, L. Chapter Thirteen—Drosophila as a Model for Diabetes and Diseases of Insulin Resistance. Curr. Top. Dev. Biol. 2017, 121, 397–419. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-Y.; Walden, T.B.; Cai, D.; Ahl, D.; Bertilsson, S.; Phillipson, M.; Nyman, M.; Holm, L. Dietary Fiber in Bilberry Ameliorates Pre-Obesity Events in Rats by Regulating Lipid Depot, Cecal Short-Chain Fatty Acid Formation and Microbiota Composition. Nutrients 2019, 11, 1350. [Google Scholar] [CrossRef] [Green Version]
- Delli Bovi, A.P.; Di Michele, L.; Laino, G.; Vajro, P. Obesity and Obesity Related Diseases, Sugar Consumption and Bad Oral Health: A Fatal Epidemic Mixtures: The Pediatric and Odontologist Point of View. Transl. Med. UniSa 2017, 16, 11–16. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC5536157/pdf/tm-16-11.pdf (accessed on 18 September 2020).
- World Health Organization. Guidelines—Sugars Intake for Adults and Children. 2015. Available online: http://www.who.int/nutrition/publications/guidelines/sugars_intake/en/ (accessed on 18 September 2020).
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- National Center for Health Statistics, Division of Health Interview Statistics, Centers for Disease Control and Prevention, Division of Diabetes Translation. Crude and Age-adjusted Percentage of Civilian, Noninstitutionalized Adults with Diagnosed Diabetes, United States, 1980–2010; National Center for Chronic Disease Prevention and Health Promotion: Atlanta, GA, USA, 2012.
- Frum, A.; Georgescu, C.; Gligor, F.G.; Lengyel, E.; Stegarus, D.I.; Dobrea, C.M.; Tita, O. Identification and Quantification of Phenolic Compounds from Red Grape Pomace. Sci. Stud. Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 1–8. Available online: http://pubs.ub.ro/dwnl.php?id=CSCC6201801V01S01A0005 (accessed on 7 May 2020).
- Soroceanu, V. Romanian Pharmacopoeia, 10th ed.; Medical Publishing House Bucharest: Bucharest, Romania, 1993. [Google Scholar]
- Tylkowski, B.; Tsibranska, I.; Kochanov, R.; Peev, G.; Giamberini, M. Concentration of Biologically Active Compounds Extracted from Sideritis Ssp. L. by Nanofiltration. Food Bioprod. Process. 2011, 89, 307–314. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Blanc, E.; Leitão-Gonçalves, R.; Yang, M.; He, X.; Linford, N.J.; Hoddinott, M.P.; Hopfen, C.; Soultoukis, G.A.; Niemeyer, C.; et al. A holidic medium for Drosophila melanogaster. Nat. Methods 2014, 11, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche LifeScience—Universal ProbeLibrary Assay Design Center. Available online: https://lifescience.roche.com/en_gb/brands/universal-probe-library.html#assay-design-center (accessed on 5 May 2019).
- Walker, N. A Technique Whose Time Has Come. Science 2002, 296, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Lefever, S.; Hellemans, J.; Pattyn, F.; Przybylski, D.R.; Taylor, C.; Geurts, R. RDML Consortium. RDML: Structured language and reporting guidelines for real-time quantitative PCR data. Nucleic Acids Res. 2009, 37, 2065–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 34–41. Available online: https://0-link-springer-com.brum.beds.ac.uk/protocol/10.1385/1-59745-229-7:205 (accessed on 22 September 2020). [CrossRef] [Green Version]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal Variations of the Phenolic Constituents in Bilberry (Vaccinium Myrtillus L.) Leaves, Stems and Fruits, and Their Antioxidant Activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef]
- Saral, Ö.; Ölmez, Z.; Şahin, H. Comparison of Antioxidant Properties of Wild Blueberries (Vaccinium Arctostaphylos L. and Vaccinium Myrtillus L.) with Cultivated Blueberry Varieties (Vaccinium Corymbosum L.) in Artvin Region of Turkey. TURJAF 2014, 3, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Lima, E.C.; Baptista, J.B.; Albuquerque, L.M. Antioxidant Capacity versus Total Phenolic, Total Flavonoid and Anthocyanin Content of Endemic Azorean Vaccinium Cylindraceum: Comparison with Commercial Bilberry and Highbush Blueberry. Acta Hortic. 2009, 810, 901–910. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products. Assessment Report on Vaccinium myrtillus L., Fructus. Assessment Report of European Union; European Medicines Agency: London, UK, 2015; Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-vaccinium-myrtillus-l-fructus-recens_en.pdf (accessed on 23 September 2020).
- Morimoto, S.; Nonaka, G.; Nishioka, I. Tannins and Related Compounds. LX.: Isolation and Characterization of Proanthocyanidins with a Doubly-Linked Unit from Vaccinium vitis-idaea L. Chem. Pharm. Bull. 1988, 36, 33–38. Available online: https://www.jstage.jst.go.jp/article/cpb1958/36/1/36_1_33/_pdf (accessed on 9 September 2020). [CrossRef] [Green Version]
- Barizza, E.; Guzzo, F.; Fanton, P.; Lucchini, G.; Sacchi, G.; Lo Schiavo, F.; Nascimbene, J. Nutritional Profile and Productivity of Bilberry (Vaccinium myrtillus L.) in Different Habitats of a Protected Area of the Eastern Italian Alps. J. Food Sci. 2013, 78, C674–C678. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; Winterhalter, P. Isolation of two anthocyanin sambubiosides from bilberry (Vaccinium myrtillus) by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1045, 59–63. [Google Scholar] [CrossRef]
- Seeram, N.; Adams, L.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.; Heber, D. Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry Extracts Inhibit Growth and Stimulate Apoptosis of Human Cancer Cells In Vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, O.; Sandell, M.; Kallio, H. Chemical factors contributing to orosensory profiles of bilberry (Vaccinium myrtillus) fractions. Eur. Food Res. Technol. 2010, 231, 271–285. [Google Scholar] [CrossRef]
- Koskimäki, J.; Hokkanen, J.; Jaakola, L.; Suorsa, M.; Tolonen, A.; Mattila, S.; Hohtola, A. Flavonoid biosynthesis and degradation play a role in early defence responses of bilberry (Vaccinium myrtillus) against biotic stress. Eur. J. Plant Pathol. 2009, 125, 629–637. [Google Scholar] [CrossRef]
- Karppinen, K.; Hirvelä, E.; Nevala, T.; Sipari, N.; Suokas, M.; Jaakola, L. Changes in the abscisic acid levels and related gene expression during fruit development and ripening in bilberry (Vaccinium myrtillus L.). Phytochemistry 2013, 95, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, B.; Paszkiewicz, M.; Podsędek, A.; Redzynia, M.; Różalska, B. Vaccinium myrtillus leaves and Frangula alnus bark derived extracts as potential antistaphylococcal agents. Acta Biochim. Pol. 2016, 61. [Google Scholar] [CrossRef] [Green Version]
- Korus, A.; Jaworska, G.; Bernaś, E.; Juszczak, L. Characteristics of physico-chemical properties of bilberry (Vaccinium myrtillus L.) jams with added herbs. JFST 2015, 52, 2815–2823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhao, X.; Wan, J.; Ran, L.; Qin, Y.; Wang, X.; Zhang, Q. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial. Pharmacol. Res. 2015, 99, 74–81. [Google Scholar] [CrossRef]
- Xiong, D.; Zhu, J.; Liu, C. Effect of ampelopsis extract from young stems and leaves on antimicrobial activities. J. Food Sci. 2000, 21, 48–50. [Google Scholar]
- Lin, X.; Zhang, R. Effect of ampelopsin on anti-inflammation and analgesic. Fujian J. Med. 1995, 17, 39–41. [Google Scholar]
- Ni, F.; Gong, Y.; Li, L.; Abdolmaleky, H.; Zhou, J. Flavonoid Ampelopsin Inhibits the Growth and Metastasis of Prostate Cancer In Vitro and in Mice. PLoS ONE 2012, 7, e38802. [Google Scholar] [CrossRef] [PubMed]
- Xuefen, Z.; Guifen, Z. Hypoglycemic Effect of Ampelopsin on Diabetic Rats Induced by Steptozotocin. Guangxi Sci. 2000, 3, 203–205. Available online: https://europepmc.org/article/cba/338628 (accessed on 1 July 2020).
- Hou, X.; Zhang, J.; Ahma, H.; Zhang, H.; Xu, Z.; Wang, T. Evaluation of Antioxidant Activities of Ampelopsin and Its Protective Effect in Lipopolysaccharide-Induced Oxidative Stress Piglets. PLoS ONE 2014, 9, e108314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhou, T. A pharmacognostical study on “Tengcha”, bigdentate ampelopsis (Ampelopsis grossedentata). Zhong Cao Yao 1999, 30, 459–463. Available online: https://europepmc.org/article/cba/332003 (accessed on 1 July 2020).
- Zheng, Z.; Zeng, C.; Lin, Y. The protective effect of APS against acute chemical liver injury of the mouse. J. Guangxi Coll. TCM 2002, 5, 15–16. [Google Scholar]
- Ku, K.; Huang, Y.; Huang, Y.; Chiou, W. Miyabenol inhibits LPS-induced NO production via IKK/IkappaB inactivation in RAW 264.7 macrophages: Possible involvement of the p38 and PI3K pathways. J. Agric. Food Chem. 2008, 56, 8911–8918. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Zhou, C. Study of pharmacological function of ampelopsis composition. J. Hunan Norm. Univ. 2006, 3, 36–39. [Google Scholar]
- Fujimori, K.; Shibano, M. Avicularin, a Plant Flavonoid, Suppresses Lipid Accumulation through Repression of C/EBPα-Activated GLUT4-Mediated Glucose Uptake in 3T3-L1 Cells. J. Agric. Food Chem. 2013, 61, 5139–5147. [Google Scholar] [CrossRef]
- Feng, W. Antihistamine Composition. U.S. Patent 7022349, 4 April 2006. Available online: https://europepmc.org/article/pat/us7022349 (accessed on 1 July 2020).
- Guo, X.; Liu, J.; Ma, S.; Zhang, P.; Sun, W. Avicularin reversed multidrug-resistance in human gastric cancer through enhancing Bax and BOK expressions. Biomed. Pharmacother. 2018, 103, 67–74. [Google Scholar] [CrossRef]
- Dall’Agnola, R.; Ferraza, A.; Bernardia, A.; Albringa, D.; Nöra, C.; Sarmentob, L.; Schapoval, E. Antimicrobial activity of some Hypericum species. Phytomedicine 2003, 10, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Jürgenliemk, G.; Nahrstedt, A.; Winterhoff, H. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med. 2000, 66, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, K.; Jho, E.; Jung, Y.; Nho, C.; Um, B.; Pan, C. Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide. Phytother. Res. 2011, 25, 1011–1017. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y.; Kim, M.; Yang, S.; Lee, S.; Kim, Y. Protective effect of components isolated from Lindera erythrocarpa against oxidative stress-induced apoptosis of H9c2 cardiomyocytes. Phytother. Res. 2011, 25, 1612–1617. [Google Scholar] [CrossRef]
- Van Anh Vo, J.W.; Chang, J.E.; Kim, J.Y.; Kim, N.H.; Lee, H.J.; Kim, S.S.; Chun, W.; Kwon, Y.S. Avicularin Inhibits Lipopolysaccharide-Induced Inflammatory Response by Suppressing ERK Phosphorylation in RAW 264.7 Macrophages. Biomol. Ther. 2012, 20, 532–537. [Google Scholar] [CrossRef] [Green Version]
- Crespy, V.; Williamson, G. A Review of the Health Effects of Green Tea Catechins in In Vivo Animal Models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nonaka, G.; Nishioka, I.; Chang, J.; Lee, K. Tannins and relatedcompounds as selective cytotoxic agents. Antitumor Agents 1992, 129, 1033–1043. Available online: https://0-pubs-acs-org.brum.beds.ac.uk/doi/pdf/10.1021/np50086a002 (accessed on 2 July 2020).
- Taher, M.; Abdul Majid, F.; Sarmidi, M. Cinnamtannin B1 activity on adipocytes formation. Med. J. Malays. 2004, 59 (Suppl. SB), 97–98. Available online: https://europepmc.org/article/med/15468836 (accessed on 2 July 2020).
- Balde, A.; van Hoof, L.; Pieters, L.; Vanden Berghe, D.; Vlietinck, A. Plant antiviral agents. VII. Antiviral and antibacterial proanthocyanidins from the bark of Pavetta owariensis. Phytother. Res. 1990, 4, 182–188. [Google Scholar] [CrossRef]
- Ho, K.; Huang, J.; Tsai, C.; Lin, T.; Hsu, Y.; Lin, C. Antioxidant Activity of Tannin Components from Vaccinium vitis-idaea L. J. Pharm. Pharmacol. 1999, 51, 1075–1078. [Google Scholar] [CrossRef]
- Way, T.; Tsai, S.; Wang, C.; Jhan, Y.; Ho, C.; Chou, C. Cinnamtannin D1 from Rhododendron formosanum induces autophagy via the inhibition of Akt/mTOR and activation of ERK1/2 in non-small-cell lung carcinoma cells. J. Agric. Food Chem. 2015, 63, 10407–10417. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, T.; Chen, L.; Yu, B.W.; Jia, Q.; Chen, K.X.; Fan, H.M.; Li, Y.M.; Wang, H.Y. Trimer procyanidin oligomers contribute to the protective effects of cinnamon extracts on pancreatic β-cells in vitro. Acta Pharmacol. Sin. 2006, 37, 1083–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, G.; Park, J.; Kang, J.H.; Jeong, T. Flavonoids from Lindera glauca Blume as low-density lipoprotein oxidation inhibitors. Nat. Prod. Res. 2014, 28, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Yuan, P.; Yang, Y.; Chen, K.; Jia, Q.; Li, Y. Immunosuppressive Effects of A-Type Procyanidin Oligomers from Cinnamomum tamala. Evid. Based Complement. Altern. Med. 2014, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Serraino, I.; Dugo, L.; Dugo, P.; Mondello, L.; Mazzon, E.; Dugo, G.; Caputi, A.P.; Cuzzocrea, S. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003, 73, 1097–1114. [Google Scholar] [CrossRef]
- Guo, H.; Xia, M.; Zou, T.; Ling, W.; Zhong, R.; Zhang, W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J. Nutr. Biochem. 2012, 23, 349–360. [Google Scholar] [CrossRef]
- Tsuda, T.; Watanabe, M.; Ohshima, K.; Norinobu, S.; Choi, S.; Kawakishi, S.; Osawa, T. Antioxidative activity of the anthocyanin pigments cyanidin 3-O-beta-D-glucoside and cyanidin. J. Agric. Food Chem. 1994, 42, 2407–2410. Available online: https://0-pubs-acs-org.brum.beds.ac.uk/doi/pdf/10.1021/jf00047a009 (accessed on 3 July 2020). [CrossRef]
- Sun, C.; Zheng, Y.; Chen, Q.; Tang, X.; Jiang, M.; Zhang, J.; Li, X.; Chen, K. Purification and anti-tumour activity of cyanidin-3-O-glucoside from Chinese bayberry fruit. Food Chem. 2012, 131, 1287–1294. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Volti, G.L.; Galvano, F.; et al. Cyanidin-3-O-β-Glucoside and Protocatechuic Acid Exert Insulin-Like Effects by Upregulating PPARγ Activity in Human Omental Adipocytes. BMJ Open Diab. Res. CA 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Guo, H.; Shen, T.; Tang, X.; Yang, Y.; Ling, W. Cyanidin-3-O-β-glucoside Purified from Black Rice Protects Mice against Hepatic Fibrosis Induced by Carbon Tetrachloride via Inhibiting Hepatic Stellate Cell Activation. J. Agric. Food Chem. 2015, 63, 6221–6230. [Google Scholar] [CrossRef]
- Speciale, A.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside Protection against TNF-α-Induced Endothelial Dysfunction: Involvement of Nuclear Factor-κB Signaling. J. Agric. Food Chem. 2010, 58, 12048–12054. [Google Scholar] [CrossRef] [PubMed]
- Cimino, F.; Ambra, R.; Canali, R.; Saija, A.; Virgili, F. Effect of Cyanidin-3-O-glucoside on UVB-Induced Response in Human Keratinocytes. J. Agric. Food Chem. 2006, 54, 4041–4047. [Google Scholar] [CrossRef]
- Im, S.E.; Nam, T.G.; Lee, H.; Han, M.W.; Heo, H.J.; Koo, S.I.; Lee, C.Y.; Kim, D.O. Anthocyanins in the ripe fruits of Rubus coreanus Miquel and their protective effect on neuronal PC-12 cells. Food Chem. 2013, 139, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.R.; Fan, J.; Johnson, M.; Gonzalez de Mejia, E. Berry Phenolic Compounds Increase Expression of Hepatocyte Nuclear Factor-1α (HNF-1α) in Caco-2 and Normal Colon Cells Due to High Affinities with Transcription and Dimerization Domains of HNF-1α. PLoS ONE 2015, 10, e0138768. [Google Scholar] [CrossRef] [PubMed]
- Meiers, S.; Kemény, M.; Weyand, U.; Gastpar, R.; von Angerer, E.; Marko, D. The anthocyanidins cyanidin and delphinidin are potent inhibitors of the epidermal growth-factor receptor. J. Agric. Food Chem. 2001, 49, 958–962. [Google Scholar] [CrossRef]
- Inoue, H.; Maeda-Yamamoto, M.; Nesumi, A.; Murakami, A. Delphinidin-3-O-galactoside protects mouse hepatocytes from (-)-epigallocatechin-3-gallate-induced cytotoxicity via up-regulation of heme oxygenase-1 and heat shock protein 70. Nutr. Res. 2012, 32, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.; Potter, J. Vegetables, fruit, and cancer. Epidemiol. Cancer Causes Control 1991, 2, 325–357. [Google Scholar] [CrossRef]
- Chakravarthy, B.; Gupta, S.; Gode, K. Functional beta cell regeneration in the islets of pancreas in alloxan induced diabetic rats by (−)-epicatechin. Life Sci. 1982, 31, 2693–2697. [Google Scholar] [CrossRef]
- Terao, J.; Piskula, M.; Yao, Q. Protective Effect of Epicatechin, Epicatechin Gallate, and Quercetin on Lipid Peroxidation in Phospholipid Bilayers. Arch. Biochem. Biophys. 1994, 308, 278–284. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.; Hollenberg, N.; Kelm, M. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. PNAS 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [Green Version]
- Hertog, M.; Feskens, E.; Hollman, P.; Katan, M.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.K.; Milbury, P.; Paul, S.M.; Blumberg, J.; et al. Flavonoid-Rich Dark Chocolate Improves Endothelial Function and Increases Plasma Epicatechin Concentrations in Healthy Adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Feyes, D.; Agarwal, R.; Mukhtar, H.; Nieminen, A. Green Tea Constituent Epigallocatechin-3-Gallate and Induction of Apoptosis and Cell Cycle Arrest in Human Carcinoma Cells. JNCI 1997, 89, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.; McGillicuddy, F.; Harford, K.; Finucane, O.; Mills, K.; Roche, H. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol. Nutr. Food Res. 2012, 56, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Sharangi, A. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—A review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Xu, Y.; Feng, Y.; Che, J.; Wang, G.; Zheng, J. Combination of quercetin and hyperoside has anticancer effects on renal cancer cells through inhibition of oncogenic microRNA-27a. Oncol. Rep. 2014, 31, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Amresh, G.; Sahu, P.; Mishra, N.; Rao, C.; Singh, A. Pharmacological evaluation of hyperin for antihyperglycemic activity and effect on lipid profile in diabetic rats. Indian J. Exp. Biol. 2013, 51, 65–72. Available online: http://nopr.niscair.res.in/handle/123456789/15273 (accessed on 10 July 2020).
- Ku, S.; Kwak, S.; Kwon, O.; Bae, J. Hyperoside inhibits high-glucose-induced vascular inflammation in vitro and in vivo. Inflammation 2014, 37, 1389–1400. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, C.; Pan, F.; Shi, D.; Zhang, Y. Antidepressant- like effect of hyperoside isolated from Apocynum venetum leaves: Possible cellular mechanisms. Phytomedicine 2012, 19, 145–149. [Google Scholar] [CrossRef]
- Xing, H.; Liu, Y.; Chen, J.; Sun, F.; Shi, H.; Xia, P. Hyperoside attenuates hydrogen peroxide-induced L02 cell damage via MAPK-dependent Keap (1)-Nrf(2)-ARE signaling pathway. Biochem. Biophys. Res. Commun. 2011, 410, 759–765. [Google Scholar] [CrossRef]
- Geng, M.; Wang, J.; Chen, H.; Yang, X.; Huang, Z. Effects of hyperin on the cccDNA of duck hepatitis B virus and its immunological regulation. Acta Pharm. Sin. 2009, 44, 1440–1444. Available online: https://europepmc.org/article/med/21351483 (accessed on 10 July 2020).
- Li, Q.; Chou, G.; Chen, Z.; MA, C. Inhibitory mechanism of hyperin on the apoptosis in myocardial ischemia reperfusion in rats. Acta Pharm. Sin. 2002, 37, 849–852. Available online: https://europepmc.org/article/cba/379068 (accessed on 10 July 2020).
- Song, M.; Hong, M.; Lee, M.Y.; Jee, J.G.; Lee, Y.M.; Bae, J.S.; Jeong, T.C.; Lee, S. Selective inhibition of the cytochrome P450 isoform by hyperoside and its potent inhibition of CYP2D6. Food Chem. Toxicol. 2013, 59, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, Z.; Song, B.; Wang, Y.; Wang, Q.; Tang, M.; Jiang, Q. The protective effect of hyperin on gastric mucosal injury in mice and its mechanism. Acta Univ. Med. Nahui 1999, 34, 178–180. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-YIKE903.007.htm (accessed on 10 July 2020).
- Choi, J.; Kim, D.; Yun, N.; Choi, J.; Islam, M.; Kim, Y.; Lee, S. Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J. Nat. Prod. 2011, 74, 1055–1060. [Google Scholar] [CrossRef]
- Huang, K.; Yang, X.; Huang, Z.; Geng, M.; Chen, H.; Wang, J.; Yang, J. The effect of hyperin on immune function in normal mice. PJCPLA 2009, 25, 133–135. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-JFJN200902011.htm (accessed on 10 July 2020).
- Lee, S.; Son, K.; Chang, H.; Do, L.; Jung, Y.; Kang, S.; Kim, H. Antiinflammatory activity of naturally occurring flavone and fiavonol glycosides. APHRDQ 1993, 16, 25–28. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, X.; Hu, J.; Sun, L.; Du, G. Neuroprotective effects of hyperoside on sodium azide- induced apoptosis in pc12 cells. Chin. J. Nat. Med. 2011, 77, 450–455. [Google Scholar] [CrossRef]
- Zeng, K.; Wang, X.; Ko, H.; Kwon, H.; Cha, J.; Yang, H. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid beta-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2011, 672, 45–55. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 149–160. [Google Scholar] [CrossRef]
- Hou, D.; Douglas, L. Potential mechanisms of cancer chemopre- vention by anthocyanins. Curr. Mol. Med. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, C.; Andersen, Ø.M.; Gardner, P.T.; Morrice, P.C.; Wood, S.G.; Duthie, S.J.; Collins, A.R.; Duthie, G.G. Anthocyanin-rich extract decreases indices of lipid peroxidation and DNA damage in vitamin E-depleted rats. Free Radic. Biol. Med. 2001, 31, 1033–1037. [Google Scholar] [CrossRef]
- Joseph, J.; Denisova, N.; Arendash, G.; Gordon, M.; Diamond, D.; Shukitt-Hale, B.; Morgan, D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr. Neurosci. 2003, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Reyes, R.; Ponce, H.; Oropeza, M.; Vancalsteren, M.; Jankowski, C.; Campos, M. Isoquercitrin from Argemone platyceras inhibits carbachol and leukotriene D4-induced contraction in guinea-pig airways. Eur. J. Pharmacol. 2005, 522, 108–115. [Google Scholar] [CrossRef]
- Sumi, M.; Tateishi, N.; Shibata, H.; Ohki, T.; Sata, M. Quercetin glucosides promote ischemia-induced angiogenesis, but do not promote tumor growth. Life Sci. 2013, 93, 814–819. [Google Scholar] [CrossRef]
- Panda, S.; Kar, S. Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside. Biofactors 2007, 31, 201–210. [Google Scholar] [CrossRef]
- Li, R.; Yuan, C.; Dong, C.; Shuang, S.; Choi, M. In vivo antioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney. Naunyn Schmiedeberg. Arch. Pharmacol. 2011, 383, 437–445. [Google Scholar] [CrossRef]
- Junior, A.G.; Prando, T.B.; Leme, T.D.; Gasparotto, F.M.; Lourenço, E.L.; Rattmann, Y.D.; Da Silva-Santos, J.E.; Kassuya, C.A.; Marques, M.C. Mechanisms underlying the diuretic effects of Tropaeolum majus L. extracts and its main component isoquercitrin. J. Ethnopharmacol. 2012, 141, 501–509. [Google Scholar] [CrossRef]
- Morikawa, K.; Nonaka, M.; Narahara, M.; Torii, I.; Kawaguchi, K.; Yoshikawa, T.; Kumazawa, Y.; Morikawa, S. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci. 2003, 74, 709–721. [Google Scholar] [CrossRef]
- Loscalzo, L.; Wasowski, C.; Marder, M. Neuroactive flavonoid glycosides from Tilia petiolaris DC. extracts. Phytother. Res. 2009, 23, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.; Lee, H.; Kim, S.; Kwon, Y.; Chun, W. Isorhamnetin-3-O-Glucuronide Suppresses JNK and p38 Activation and Increases Heme-Oxygenase-1 in Lipopolysaccharide-Challenged RAW264.7 Cells. Drug Dev. Res. 2016, 77, 143–151. [Google Scholar] [CrossRef]
- Jimenez, R.; Lopez-Sepulveda, R.; Romero, M.; Toral, M.; Cogolludo, A.; Perez-Vizcaino, F.; Duarte, J. Quercetin and its metabolites inhibit the membrane NADPH oxidase activity in vascular smooth muscle cells from normotensive and spontaneously hypertensive rats. Food Funct. 2015, 6, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.; West, K.; Fernandez, M. Grape polyphenols decrease plasma triglycerides and cholesterol accumulation in the aorta of ovariectomized guinea pigs. J. Nutr. 2003, 133, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Kameda, K.; Takaku, T.; Okuda, H.; Kimura, Y.; Okuda, T.; Hatano, T.; Agata, I.; Arichi, S. Inhibitory effects of various flavonoids isolated from leaves of persimmon on angiotensin- converting enzyme activity. J. Nat. Prod. 1987, 50, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.W.; Chiu, Y.J.; Fan, M.J.; Lu, H.F.; Yeh, H.F.; Li, K.H.; Chen, P.Y.; Chung, J.G.; Yang, J.S. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol. Nutr. Food Res. 2010, 54, 1585–1595. [Google Scholar] [CrossRef]
- Jorge, A.; Horst, H.; de Sousa, E.; Pizzolatti, M.; Silva, F. Insulinomimetic effects of kaempferitrin on glycaemia and on 14C- glucose uptake in rat soleus muscle. Chem. Biol. Interact. 2004, 149, 89–96. [Google Scholar] [CrossRef]
- Kataoka, M.; Hirata, K.; Kunikata, T.; Ushio, S.; Iwaki, K.; Ohashi, K.; Ikeda, M.; Kurimoto, M. Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils. J. Gastroenterol. 2001, 36, 5–9. Available online: https://0-link-springer-com.brum.beds.ac.uk/content/pdf/10.1007/s005350170147.pdf (accessed on 12 July 2020). [CrossRef]
- Kampkotter, A.; Gombitang, N.; Zurawski, R.; Timpel, C.; Chovolou, Y.; Watjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef]
- Tu, Y.; Lian, T.; Yen, J.; Chen, Z.; Wu, M. Antiatherogenic effects of kaempferol and rhamnocitrin. J. Agric. Food Chem. 2007, 55, 9969–9976. [Google Scholar] [CrossRef]
- Chung, M.; Gan, K.; Lin, C.; Ko, F.; Teng, C. Antiplatelet effects and vasorelaxing action of some constituents of Formosan plants. J. Nat. Prod. 1993, 56, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tu, Y.; Lian, T.; Hung, J.; Yen, J.; Wu, M. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.; Laricitrin, A. Polyphenolic Compound Of Red Grape, Inhibits Lung Cancer-Mediated Dendritic Cell Suppression By Decreasing Signal Transducer And Activator Of Transcription 3 Pathway. Am. J. Resp. Crit. Care Med. 2016, 193, S2564. Available online: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A2564 (accessed on 12 July 2020).
- Mukhtar, H.; Das, M.; Khan, W.; Wang, Z.; Bik, D.; Bickers, D. Exceptional activity of tannic acid among naturally occurring plant phenols in protecting against 7,12-dimethylbenz(a)anthracene-, benzo(a)-pyrene, 3-methylcholanthrene-, and N-methyl-N-nitrosourea-induced skin tumorigenesis in mice. Cancer Res. 1988, 48, 2361–2365. Available online: https://cancerres.aacrjournals.org/content/48/9/2361.short (accessed on 12 July 2020). [PubMed]
- Chaudhry, P.; Cabrera, J.; Juliani, H.; Varma, S. Inhibition of human lens aldose reductase by flavonoids, sulindac and indomethacin. Biochem. Pharmcol. 1983, 32, 1995–1998. [Google Scholar] [CrossRef]
- Ong, K.K. Insulinomimetic effects of myricetin on lipogenesis and glucose transport in rat adipocytes but not glucose transporter translocation. Biochem. Pharmacol. 1996, 51, 423–429. [Google Scholar] [CrossRef]
- Robak, J.G. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Ono, K.; Nakane, H. Mechanisms of inhibition of various cellular DNA and RNA polymerases by several flavonoids. J. Biochem. 1990, 108, 609–613. Available online: https://www.jstage.jst.go.jp/article/biochemistry1922/108/4/108_4_609/_article/-char/ja/ (accessed on 12 July 2020). [CrossRef]
- Brown, J.; Dietrich, P. Mutagenicity of plant flavonols in the Salmonella/mammalian microsome test: Activation of flavonol glycosides by mixed glycosidases from rat cecal bacteria and other sources. MRGTEM 1979, 66, 223–240. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.; Menichini, F.; Bonesi, M.; Colica, C.; Menichini, F. In vitro cytotoxic activity of extracts and isolated constituents of Salvia leriifolia Benth. Against a panel of human cancer cell lines. Chem. Biodiv. 2011, 8, 1152–1162. [Google Scholar] [CrossRef]
- Sabarinathan, D.; Mahalakshmi, P.; Vanisree, A. Naringenin promote apoptosis in cerebrally implanted C6 glioma cells. Mol. Cell. Biochem. 2010, 345, 215–222. [Google Scholar] [CrossRef]
- Oršolić, N.; Gajski, G.; Garaj-Vrhovac, V.; Dikić, D.; Prskalo, Z.; Sirovina, D. DNA-protective effects of quercetin or naringenin in alloxan-induced diabetic mice. Eur. J. Pharmacol. 2011, 656, 110–118. [Google Scholar] [CrossRef]
- Yi, L.; Li, C.; Zhan, X.; Cui, C.; Xiao, F.; Zhou, L. Involvement of monoaminergic system in the antidepressant-like effect of the flavonoid naringenin in mice. Progr. Neuro Psychopharmacol. Biol. Psych. 2010, 34, 1223–1228. [Google Scholar] [CrossRef]
- Lakshmi, V.; Joseph, S.; Srivastava, S.; Verma, S.; Sahoo, M.; Dube, V. Antifilarial activity in vitro and in vivo of some flavonoids tested against Brugia malayi. Acta Tropica 2010, 116, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.; Shagirtha, K.; Renugadevi, J. Naringenin in combination with vitamins C and E potentially protects oxidative stress-mediated hepatic injury in cadmium-intoxicated rats. J. Nutr. Sci. Vitaminol. 2011, 57, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Salgado, J.; Castillo-España, P.; Ibarra-Barajas, M.; Villalobos-Molina, R.; Estrada-Soto, S. Cochlospermum vitifolium induces vasorelaxant and antihypertensive effects mainly by activation of NO/cGMP signaling pathway. J. Ethnopharmachol. 2010, 130, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Weng, C.; Chang, N.; Lin, J.; Kao, S.; Ho, F. Naringenin more effectively inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in macrophages than in microglia. Nutr. Res. 2010, 30, 858–864. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoshimura, M.; Yamaguchi, F.; Kouchi, T.; Tsuji, R.; Saito, M.; Obata, A.; Kikuchi, M. Anti-allergic Activity of Naringenin Chalcone from a Tomato Skin Extract. Biosci. Biotechnol. Biochem. 2014, 68, 1706–1711. [Google Scholar] [CrossRef] [Green Version]
- Yagura, T.; Motomiya, T.; Ito, M.; Honda, G.; Iida, A.; Kiuchi, F.; Tokuda, H.; Nishino, H. Anticarcinogenic compound in the Uzbek medicinal plant, Helichrysum maracandicum. J. Nat. Med. 2008, 62, 174–178. [Google Scholar] [CrossRef]
- Anto, R.; Sukumaran, K.; Kuttan, G.; Rao, M.; Subbaraju, V.; Kuttan, R. Anticancer and antioxidant activity of synthetic chalcones and related compounds. Cancer Lett. 1995, 97, 33–37. [Google Scholar] [CrossRef]
- Hirai, S.; Kim, Y.I.; Goto, T.; Kang, M.S.; Yoshimura, M.; Obata, A.; Yu, R.; Kawada, T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci. 2007, 81, 1272–1279. [Google Scholar] [CrossRef]
- Pereira, M.; Grubbs, C.; Barnes, L.; Li, H.; Olson, G.; Eto, I. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis 1996, 17, 1305–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayek, T.; Fuhrman, B.; Vaya, J.; Rosenblat, M.; Belinky, P.; Coleman, R. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. ATVB 1997, 17, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Pulcinelli, F.; Celestini, A.; Lenti, L.; Ghiselli, A.; Gazzaniga, P. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am. J. Clin. Nutr. 2000, 72, 1150–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Vizcaino, F.; Ibarra, M.; Cogolludo, A.; Duarte, J.; Zaragoza-Arnaez, F.; Moreno, L. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance resistance arteries. J. Pharmacol. Exp. Theor. 2002, 302, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Ferry, D.; Smith, A.; Malkhandi, J.; Fyfe, D.; de Takats, P.; Anderson, D. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. Available online: https://clincancerres.aacrjournals.org/content/2/4/659.short (accessed on 15 July 2020).
- Saint-Cricq de Gaulejac, N.; Provost, C.; Vivas, N. Comparative study of polyphenol scavenging activities assessed by different methods. J. Food Agric. Chem. 1999, 47, 425–431. [Google Scholar] [CrossRef]
- Jung, H.; Ali, M.; Bhakta, H.; Min, B.; Choi, J. Prunin is a highly potent flavonoid from Prunus davidiana stems that inhibits protein tyrosine phosphatase 1B and stimulates glucose uptake in insulin-resistant HepG2 cells. Acta Pharm. Res. 2017, 40, 37–48. [Google Scholar] [CrossRef]
- Klaman, L.D.; Boss, O.; Peroni, O.D.; Kim, J.K.; Martino, J.L.; Zabolotny, J.M.; Moghal, N.; Lubkin, M.; Kim, Y.B.; Sharpe, A.H.; et al. Increased energy expenditure, decreased adiposity, and tissue specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell Biol. 2000, 20, 5479–5489. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Gao, S.; Cheng, Y.; Sun, Y.; Liu, W.; Tang, L.; Ren, D. Protective effect of naringenin-7-O-glucoside against oxidative stress induced by doxorubicin in H9c2 cardiomyocytes. Biosci. Trends 2012, 6, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Ren, D.; Fan, P.; Shen, T.; Lou, H. Protective effects of naringenin-7-O-glucoside on doxorubicin-induced apoptosis in H9C2 cells. Eur. J. Pharmacol. 2007, 581, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Donnini, S.; Finetti, F.; Lusini, L.; Morbidelli, L.; Cheynier, V.; Barron, D. Divergent effects of quercetin conjugates on angiogenesis. Br. J. Nutr. 2006, 95, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, D.; Gao, X.; Parry, J.; Liu, K.; Liu, B.; Wang, M. Quercetin and quercetin-3-O-glucuronide are equally effective in ameliorating endothelial insulin resistance through inhibition of reactive oxygen species-associated inflammation. Mol. Nutr. Food Res. 2013, 57, 1037–1045. [Google Scholar] [CrossRef]
- Moon, J.T.; Nakahara, K.; Terao, J. Identification of quercetin 3-O-beta-d-glucuronide as an antioxi- dative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 2001, 30, 1274–1285. [Google Scholar] [CrossRef]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bylka, W.; Matlawska, I.; Pilewski, N. Natural flavonoids as antimicrobial agents. JANA 2004, 7, 24–31. Available online: https://www.researchgate.net/profile/Irena_Matlawska/publication/228800384_Natural_flavonoids_as_antimicrobial_agents/links/5452b81a0cf2bccc49094b17.pdf (accessed on 16 July 2020).
- Huber, G.; Rupasinghe, H.; Shahidi, F. Inhibition of oxidation of omega-3 polyunsaturated fatty acids and fish oil by quercetin glycosides. Food Chem. 2009, 117, 290–295. [Google Scholar] [CrossRef]
- Liu, H.; Yu, D.; Shin, S.; Park, H.; Park, J.; Bark, K. Spectroscopic Properties of Quercetin Derivatives, Quercetin-3-O-rhamnoside and Quercetin-3-O-rutinoside, in Hydro-organic Mixed Solvents. Photochem. Photobiol. 2009, 85, 934–942. [Google Scholar] [CrossRef]
- Wang, T.J.; Ngo, D.; Psychogios, N.; Dejam, A.; Larson, M.G.; Vasan, R.S.; Ghorbani, A.; O’Sullivan, J.; Cheng, S.; Rhee, E.P.; et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J. Clin. Investig. 2013, 123, 10. [Google Scholar] [CrossRef]
- Sell, D.; Strauch, M.; Shen, W.; Monnier, V. 2-Aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: Effects of diabetes, renal failure and sepsis. Biochem. J. 2007, 404, 269–277. [Google Scholar] [CrossRef]
- Da Silva, J.C.; Amaral, A.U.; Cecatto, C.; Wajner, A.; dos Santos Godoy, K.; Ribeiro, R.T.; de Mello Gonçalves, A.; Zanatta, Â.; da Rosa, M.S.; Loureiro, S.O.; et al. α-Ketoadipic Acid and α-Aminoadipic Acid Cause Disturbance of Glutamatergic Neurotransmission and Induction of Oxidative Stress In Vitro in Brain of Adolescent Rats. Neurotox. Res. 2017, 32, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Pieper, G.; Peltier, B. Amelioration by L-arginine of a dysfunctional arginine/nitric oxide pathway in diabetic endothelium. J. Cardiovasc. Pharmacol. 1995, 25, 397–403. [Google Scholar] [CrossRef]
- Giroux, I.; Kurowska, E.; Caroll, K. Role of dietary lysine, methionine and arginine in the regulation of hypercholesterolemia in rabbits. J. Nutr. Biochem. 1999, 10, 166–171. [Google Scholar] [CrossRef]
- Maxwell, A.; Cooke, J. Cardiovascular effects of L-arginine. Curr. Opin. Nephrol. Hypertens. 1998, 7, 63–70. Available online: https://journals.lww.com/co-nephrolhypertens/Abstract/1998/01000/Cardiovascular_effect_of_L_arginine.11.aspx (accessed on 18 July 2020). [CrossRef] [PubMed]
- Lewis, B.; Langkamp-Henken, B. Arginine enhances in vivo immune responses in young, adult and aged mice. J. Nutr. 2000, 130, 1827–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.; Brooks, M.; Wicha, M. Asparagine and Glutamine: Co-conspirators Fueling Metastasis. Cell Met. 2018, 27, 947–949. [Google Scholar] [CrossRef] [Green Version]
- Ruzzo, E.; Capo-Chichi, J.; Ben-Zeev, B.; Chitayat, D.; Mao, H.; Pappas, A.; Goldstein, D. Deficiency of Asparagine Synthetase Causes Congenital Microcephaly and a Progressive Form of Encephalopathy. Neuron 2013, 80, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Berl, S.; Takagaki, G.; Clarke, D.; Waelsch, H. Metabolic compartmentsin vitro. Ammonia and glutamic acid metabolism in brain and liver. J. Biol. Chem. 1962, 237, 2562–2569. [Google Scholar]
- Marks, N.; Datta, R.; Lajtha, A. Distribution of amino acids and of exo- and endo-peptidases along vertebrate and invertebrate nerves. J. Neurochem. 1970, 17, 53–63. [Google Scholar] [CrossRef]
- Sheiner, J.; Morris, P.; Anderson, G. Food intake suppression by histidine. Pharmacol. Biochem. Behav. 1985, 23, 721–726. [Google Scholar] [CrossRef]
- Gerber, D. Low free serum histidine concentration in rheumatoid arthritis. A measure of disease activity. J. Clin. Investig. 1975, 55, 1164–1173. Available online: https://www.jci.org/articles/view/108033 (accessed on 18 July 2020). [CrossRef] [PubMed] [Green Version]
- Frestedt, J.; Zenk, J.; Kuskowski, M. A whey-protein supplement increases fat loss and spares lean muscle in obese subjects: A randomized human clinical study. Nutr. Met. 2008, 5, 8–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieu, I.; Sornet, C.; Bayle, G. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J. Nutr. 2003, 133, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieu, I.; Balage, M.; Sornet, C. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575, 305–315. [Google Scholar] [CrossRef]
- Layman, D. The role of leucine in weight loss diets and glucose homeostasis. J. Nutr. 2003, 133, 261–267. [Google Scholar] [CrossRef]
- Papes, F.; Surpili, M.; Langone, F.; Trigo, J.; Arruda, P. The essential amino acid lysine acts as precursor of glutamate in the mammalian central nervous system. FEBS Lett. 2001, 488, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Van Spronsen, F.; Hoeksma, M.; Reijngoud, D. Brain dysfunction in phenylketonuria: Is phenylalanine toxicity the only possible cause? J. Inherit. Met. Dis. 2009, 32, 46–51. [Google Scholar] [CrossRef]
- Griffiths, P.; Paterson, L.; Harvie, A. Neuropsychological effects of subsequent exposure to phenylalanine in adolescents and young adults with eariy-treated phenylketonuria. J. Intellect. Disabil. Res. 1995, 39, 365–372. [Google Scholar] [CrossRef]
- Rasheed, A.; Kumar, C. Tyrosine and glycine derivatives as potential prodrugs: Design, synthesis, and pharmacological evaluation of amide derivatives of mefenamic acid. J. Enzyme Inhib. Med. Chem. 2010, 25, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Wurtman, R.; Hefti, F.; Melamed, E. Precursor control of neurotransmitter synthesis. Pharmacol. Rev. 1980, 32, 315–335. Available online: https://pharmrev.aspetjournals.org/content/32/4/315/tab-article-info (accessed on 19 July 2020).
- Deijen, J.; Wientjes, C.; Vullinghs, H.; Cloin, P.; Langefeld, J. Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain Res. Bull. 1999, 48, 203–209. [Google Scholar] [CrossRef]
- Korol, S.V.; Jin, Z.; Jin, Y.; Bhandage, A.K.; Tengholm, A.; Gandasi, N.R.; Barg, S.; Espes, D.; Carlsson, P.O.; Laver, D.; et al. Functional Characterization of Native, High-Affinity GABA Receptors in Human Pancreatic β Cells. EBioMedicine 2018, 30, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandage, A.K.; Jin, Z.; Korol, S.V.; Shen, Q.; Pei, Y.; Deng, Q.; Espes, D.; Carlsson, P.O.; Kamali-Moghaddam, M.; Birnir, B. GABA Regulates Release of Inflammatory Cytokines From Peripheral Blood Mononuclear Cells and CD4+ T Cells and Is Immunosuppressive in Type 1 Diabetes. EBioMedicine 2018, 30, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Scheel-Krüger, J. Dopamine-GABA interactions: Evidence that GABA transmits, modulates and mediates dopaminergic functions in the basal ganglia and the limbic system. Acta Neurol. Scand. 1986, 73 (Suppl. S107), 54–67. Available online: https://psycnet.apa.org/record/1987-27370-001 (accessed on 19 July 2020).
- Huang, K.; Liang, X.; Zhong, Y.; He, W.; Wang, Z. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. J. Sci. Food Agric. 2015, 95, 1903–1910. [Google Scholar] [CrossRef]
- Cho, A.; Jeon, S.; Kim, M.; Yeo, J.; Seo, K.; Choi, M.; Lee, M. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Yagasaki, K.; Miura, Y.; Okauchi, R.; Furuse, T. Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. Cytotechnology 2000, 33, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Crozier, T.; Stalmach, A.; Lean, M.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2012, 2, 30–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.; Hsu, A.; Tan, B. Antidiabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.; Bondonno, C.P.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: A randomized trial. J. Agric. Food Chem. 2012, 60, 9130–9136. [Google Scholar] [CrossRef]
- Sticha, H.; Rosina, M.; Wub, C.; Powrieb, W. A comparative genotoxicity study of chlorogenic acid (3-O-caffeoylquinic acid). MRGTEM 1981, 90, 201–212. [Google Scholar] [CrossRef]
- Raina, K.; Rajamanickam, S.; Deep, G.; Singh, M.; Agarwal, R.; Agarwal, C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol. Cancer Ther. 2008, 7, 1258–1267. [Google Scholar] [CrossRef] [Green Version]
- Gichner, T.; Pospísil, F.; Velemínský, J.; Volkeová, V.; Volke, J. Two Types of Antimutagenic Effects of Gallic and Tannic Acids towards N-nitroso-compounds-Induced Mutagenicity in the Ames Salmonella Assay. Folia Microbiol. 1987, 32, 55–62. Available online: https://0-link-springer-com.brum.beds.ac.uk/content/pdf/10.1007/BF02877259.pdf (accessed on 19 July 2020). [CrossRef]
- Kim, D.; Lee, K.; Lee, H.; Lee, C. Vitamin C equivalent antioxidant capacity of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Kroes, B.; van den Berg, A.; Quarles van Ufford, H.; van Dijk, H.; Labadie, R. Anti-inflammatory Activity of Gallic Acid. Planta Med. 1992, 58, 499–504. [Google Scholar] [CrossRef]
- Luae, Z.; Nieb, G.; Beltonc, P.; Tangd, H.; Zhao, B. Structure–Activity Relationship Analysis of Antioxidant Ability and Neuroprotective Effect of Gallic Acid Derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef]
- Lodovici, M.; Caldini, S.; Morbidelli, L.; Akpan, V.; Ziche, M.; Dolara, P. Protection against ultraviolet B-induced oxidative DNA damage in rabbit corneal-derived cells (SIRC) by 4-coumaric acid. Toxicology 2003, 184, 141–147. [Google Scholar] [CrossRef]
- Luceri, C.; Guglielmi, F.; Lodovici, M.; Giannini, L.; Messerini, L.; Dolara, P. Plant phenolic 4-coumaric acid protects against intestinal inflammation in rats. Scand. J. Gastroenterol. 2004, 39, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Kabir, S.; Pal, A.; Pakrashi, A. Modulation of luteinizing hormone receptors: Effect of an inhibitor of prolactin secretion, p-coumaric acid. J. Endocrinol. 1983, 98, 307–311. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Hwang, E.; Maeng, S.; Park, J. p-Coumaric Acid Enhances Long-term Potentiation and Recovers Scopolamine-Induced Learning and Memory Impairments. Biochem. Biophys. Res. Commun. 2017, 492, 493–499. [Google Scholar] [CrossRef]
- Symes, A.; Shavandi, A.; Zhang, H.; Mohamed Ahmed, I.; Al-Juhaimi, F.; Bekhit, A. Antioxidant Activities and Caffeic Acid Content in New Zealand Asparagus (Asparagus officinalis) Roots Extracts. Antioxidants 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kırmızıbekmez, H.; İnan, Y.; Reis, R.; Sipahi, H.; Gören, A.; Yeşilada, E. Phenolic compounds from the aerial parts of Clematis viticella L. and their in vitro anti-inflammatory activities. Nat. Prod. Res. 2018, 33, 2541–2544. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.J.; Jin, X.L.; Qian, Y.P.; Wang, Q.; Yang, R.T.; Dai, F.; Tang, J.J.; Shang, Y.J.; Cheng, L.X.; Yang, J.; et al. Hydroxycinnamic acids as DNA-cleaving agents in the presence of Cu(II) ions: Mechanism, structure-activity relationship, and biological implications. Chemistry 2009, 15, 12889–12899. [Google Scholar] [CrossRef]
- Yun, K.J.; Koh, D.J.; Kim, S.H.; Park, S.J.; Ryu, J.H.; Kim, D.G.; Lee, J.Y.; Lee, K.T. Anti-Inflammatory Effects of Sinapic Acid through the Suppression of Inducible Nitric Oxide Synthase, Cyclooxygase-2, and Proinflammatory Cytokines Expressions via Nuclear Factor-κB Inactivation. J. Agric. Food Chem. 2008, 56, 10265–10272. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant Potential of Ferulic Acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Stewart, E. Adenine Compounds, Their Chemical, Physiological, and Therapeutic Properties. JAPhA 1948, 1, 3–9. [Google Scholar] [CrossRef]
- Changyuan, W.; Zhendong, S.; Haiqing, Y.; Kexin, L.; Xiaodong, M. Adenine: An important drug scaffold for the design of antiviral agents. Acta Pharm. Sin. B 2015, 5, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wan, T.; Ye, M.; Qiu, Y.; Pei, L.; Jiang, R.; Pang, N.; Huang, Y.; Liang, B.; Ling, W.; et al. Nicotinamide riboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1α/mitochondrial biosynthesis pathway. Redox Biol. 2018, 17, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Medenica, M. Response of Generalized Granuloma Annulare to High-Dose Niacinamide. Arch. Dermatol. 1983, 119, 836–839. [Google Scholar] [CrossRef]
- Handfield-Jones, S.; Jones, S.; Peachey, R. High-dose nicotinamide in the treatment of necrobiosis lipoidica. Br. J. Dermatol. 1988, 118, 693–696. [Google Scholar] [CrossRef]
- Reiche, L.; Wojnarowska, F.; Mallon, E. Combination therapy with nicotinamide and tetracyclines for cicatricial pemphigoid: Further support for its efficacy. Clin. Exp. Dermatol. 1998, 23, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Neumann, R.; Rappold, E.; Pohl-Mark, L. Treatmentof polymorphous light eruption with nicotinamide: A pilot study. Br. J. Dermatol. 1986, 115, 77–80. [Google Scholar] [CrossRef]
- Lampeter, E.; Klinghammer, A.; Scherbaum, W.; Heinze, E.; Haastert, B.; Giani, G.; Kolb, H. The Deutsche Nicotinamide Intervention Study: An attempt to prevent type 1 diabetes. DENIS Group. Diabetes 1998, 47, 980–984. [Google Scholar] [CrossRef]
- Hoffer, A. Safety, Side Effects and Relative Lack of Toxicity of Nicotinic Acid and Nicotinamide. Schizophrenia 1969, 1, 78–87. [Google Scholar]
- Gianfrilli, D.; Lauretta, R.; Di Dato, C.; Graziadio, C.; Pozza, C.; De Larichaudy, J.; Giannetta, E.; Isidori, A.M.; Lenzi, A. Propionyl-L-carnitine, L-arginine and niacin in sexual medicine: A nutraceutical approach to erectile dysfunction. Andrologia 2012, 44 (Suppl. S1), 600–604. [Google Scholar] [CrossRef]
- Adams, G.G.; Imran, S.; Wang, S.; Mohammad, A.; Kok, M.S.; Gray, D.A.; Channell, G.A.; Harding, S.E. The hypoglycemic effect of pumpkin seeds, Trigonelline (TRG), Nicotinic acid (NA), and D-Chiro-inositol (DCI) in controlling glycemic levels in diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2014, 54, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, S.; Karas, R.; Kuvin, J. Present-day uses of niacin: Effects on lipid and non-lipid parameters. Expert Opin. Pharmacother. 2007, 8, 1711–1717. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Scherr, P.A.; Tangney, C.C.; Hebert, L.E.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N. Dietary niacin and the risk of incident Alzheimer’s disease and of cognitive decline. JNNP 2004, 75, 1093–1099. Available online: https://0-jnnp-bmj-com.brum.beds.ac.uk/content/75/8/1093 (accessed on 30 July 2020). [CrossRef] [Green Version]
- Shi, H.; Enriquez, A.; Rapadas, M.; Martin, E.M.; Wang, R.; Moreau, J.; Lim, C.K.; Szot, J.O.; Ip, E.; Hughes, J.N.; et al. NAD Deficiency, Congenital Malformations, and Niacin Supplementation. N. Engl. J. Med. 2017, 377, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Doreswamy, V.; Prakash, R. The biochemical pathways of central nervous system neural degeneration in niacin deficiency. NRR 2014, 9, 1509–1513. [Google Scholar] [CrossRef]
- Nemazannikova, N.; Mikkelsen, K.; Stojanovska, L.; Blatch, G.; Apostolopoulos, V. Is there a Link between Vitamin B and Multiple Sclerosis? Med. Chem. 2018, 14, 170–180. [Google Scholar] [CrossRef]
- Dumont, M. Clinical, pathogenic and therapeutic study of muscular cramps in pregnant women; peculiar action of panthenol. Gynecol. Obs. 1951, 50, 501–509. Available online: https://europepmc.org/article/med/14917312 (accessed on 30 July 2020).
- Naruta, E.; Egorov, A.; Omel’ianchik, C.; Buko, V. The influence of panthotenic acid mitochondrial oxidation and oxidative phosphorylation in liver of rats with alimentary obesity. Vopr. Pitan. 2004, 73, 3–7. Available online: https://europepmc.org/article/med/15460980 (accessed on 3 August 2020). [PubMed]
- Zhang, Y.M.; Chohnan, S.; Virga, K.G.; Stevens, R.D.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Newgard, C.B.; Lee, R.E.; Rock, C.O.; et al. Chemical knockout of pantothenate kinase reveals the metabolic and genetic program responsible for hepatic coenzyme A homeostasis. Chem. Biol. 2007, 14, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Voelkel, A. Mechanism of action of panthothenic acid in insulin shock therapy. Arch. Int. Pharmacodyn. Ther. 1956, 107, 271–274. [Google Scholar]
- Schutte, A.; van Rooyen, J.; Huisman, H.; Kruger, H.; Malan, N.; De Ridder, J. Dietary risk markers that contribute to the aetiology of hypertension in black South African children: The THUSA BANA study. J. Hum. Hypertens. 2003, 17, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gominak, S. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med. Hypotheses 2016, 94, 103–107. [Google Scholar] [CrossRef]
- Stettler, H.; Kurka, P.; Kandzora, J.; Pavel, V.; Breuer, M.; Macura-Biegun, A. A new topical panthenol-containing emollient for maintenance treatment of childhood atopic dermatitis: Results from a multicenter prospective study. J. Dermatol. Treatm. 2017, 28, 774–779. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Skoneczka, J.; Kingston, D.G.; Krishnan, A.; Misyak, S.A.; Guri, A.J.; Pereira, A.; Carter, A.B.; Minorsky, P.; Tumarkin, R.; et al. Mechanisms of Action and Medicinal Applications of Abscisic Acid. Curr. Med. Chem. 2010, 17, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sarasúa, S.; Moustafa, S.; García-Avilés, Á.; López-Climent, M.; Gómez-Cadenas, A.; Olucha-Bordonau, F.; Sánchez-Pérez, A. The effect of abscisic acid chronic treatment on neuroinflammatory markers and memory in a rat model of high-fat diet induced neuroinflammation. Nutr. Metab. 2016, 13, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilar Herrero, M.; Shafer, W. Pharmaceutical and Nutraceutical Compositions of Abscisic Acid. U.S. Patent 8,536,224, 19 September 2011. Available online: https://patents.google.com/patent/US8536224B2/en (accessed on 3 August 2020).
- Fernández-Ponce, M.; López-Biedma, A.; Sánchez-Quesada, C.; Casas, L.; Mantell, C.; Gaforio, J.; Martínez de la Ossa, E. Selective antitumoural action of pressurized mango leaf extracts against minimally and highly invasive breast cancer. Food Funct. 2017, 8, 3610–3620. [Google Scholar] [CrossRef] [PubMed]
- Whang, W.K.; Park, H.S.; Ham, I.; Oh, M.; Namkoong, H.; Kim, H.K.; Hwang, D.W.; Hur, S.Y.; Kim, T.E.; Park, Y.G.; et al. Methyl gallate and chemicals structurally related to methyl gallate protect human umbilical vein endothelial cells from oxidative stress. Exp. Mol. Med. 2005, 37, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, L.B.; Pádua, T.A.; Seito, L.N.; Costa, T.E.; Silva, M.A.; Candéa, A.L.; Rosas, E.C.; Henriques, M.G. Anti-inflammatory Effect of Methyl Gallate on Experimental Arthritis: Inhibition of Neutrophil Recruitment, Production of Inflammatory Mediators, and Activation of Macrophages. J. Nat. Prod. 2016, 79, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S. Dietary choline: Biochemistry, Physiology, and Pharmacology. Annu Rev. Nutr. 1981, 1, 95–121. Available online: https://0-www-annualreviews-org.brum.beds.ac.uk/doi/pdf/10.1146/annurev.nu.01.070181.000523 (accessed on 7 August 2020). [CrossRef]
- Dronkers, N.; Baldo, J. Encyclopedia of Neuroscience; Squire, L., Ed.; Academic Press: La Jolla, CA, USA, 2009; Volume 10. [Google Scholar]
- Porteiro, B.; Fondevila, M.F.; Buque, X.; Gonzalez-Rellan, M.J.; Fernandez, U.; Mora, A.; Beiroa, D.; Senra, A.; Gallego, R.; Fernø, J.; et al. Pharmacological stimulation of p53 with low-dose doxorubicin ameliorates diet-induced nonalcoholic steatosis and steatohepatitis. Mol. Metab. 2018, 8, 132–143. [Google Scholar] [CrossRef]
- Best, C.; Huntsman, M. Effect of choline on liver fat of rats in various states of nutrition. J. Physiol. 1935, 83, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Dai, W.; Yuan, S.; Lu, Y.; Chen, D.; Wang, Q. Iridoids from Pedicularis verticillata and their anti-complementary activity. Chem. Biodivers. 2018, 15, e1800033. [Google Scholar] [CrossRef]
- Wang, K.; Chang, J.; Chiang, L.; Lin, C. 4-Methoxycinnamaldehyde inhibited human respiratory syncytial virus in a human larynx carcinoma cell line. Phytomedicine 2009, 16, 882–886. [Google Scholar] [CrossRef]
- Li, X.Q.; Liu, X.X.; Wang, X.Y.; Xie, Y.H.; Yang, Q.; Liu, X.X.; Ding, Y.Y.; Cao, W.; Wang, S.W. Cinnamaldehyde Derivatives Inhibit Coxsackievirus B3-Induced Viral Myocarditis. Biomol. Ther. 2017, 25, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Srisook, E.; Palachot, M.; Mankhong, S.; Srisook, K. Anti-inflammatory effect of Etlingera pavieana (Pierre ex Gagnep.) R.M.Sm. rhizomal extract and its phenolic compounds in lipopolysaccharide-stimulated macrophages. Pharmacogn. Mag. 2017, 13, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Germond, J.; Philippossian, G.; Richli, U.; Bracco, I.; Arnaud, M. Rapid and complete urinary elimination of [14C]-5-hydroxymethyl-2-furaldehyde administered orally or intravenously to rats. J. Toxicol. Environ. Health 1987, 22, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Son, H.S.; Cui, Y.H.; Youn, B.; Son, B.; Kaushik, N.K.; Uddin, N.; Lee, J.S.; Song, J.Y.; Kaushik, N.; et al. Phytosphingosine exhibits an anti-epithelial-mesenchymal transition function by the inhibition of EGFR signaling in human breast cancer cells. Oncotarget 2017, 8, 77794–77808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, E.J.; Shin, Y.; Park, H.J.; Kim, D.; Jung, C.; Hong, J.Y.; Kim, S.; Lee, S.K. Anti-melanogenic activity of phytosphingosine via the modulation of the microphthalmia-associated transcription factor signaling pathway. J. Dermatol. Sci. 2017, 87, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Lee, J.; Jung, Y.; Kim, S.; Kim, T. Phytosphingosine Derivatives Ameliorate Skin Inflammation by Inhibiting NF-κB and JAK/STAT Signaling in Keratincoytes and Mice. J. Investig. Dermatol. 2014, 134, 1023–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Sharma, B. Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington’s disease. Pharmacol. Biochem. Behav. 2014, 122, 122–135. [Google Scholar] [CrossRef]
- Iannuzzi, C.; Borriello, M.; Irace, G.; Cammarota, M.; Di Maro, A.; Sirangelo, I. Vanillin Affects Amyloid Aggregation and Non-Enzymatic Glycation in Human Insulin. Sci. Rep. 2017, 7, 15086–15100. [Google Scholar] [CrossRef] [Green Version]
- Hannemann, A.; Cytlak, U.; Gbotosho, O.; Rees, D.; Tewari, S.; Gibson, J. Effects of o-vanillin on K+ transport of red blood cells from patients with sickle cell disease. Blood Cells Mol. Dis. 2014, 53, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Abraham, D.; Mehanna, A.; Wireko, F.; Whitney, J.; Thomas, R.; Orringer, E. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood 1991, 77, 1334–1341. Available online: https://pubmed.ncbi.nlm.nih.gov/2001455/ (accessed on 9 August 2020). [CrossRef] [Green Version]
- Lirdprapamongkol, K.; Kramb, J.; Suthiphongchai, T.; Surarit, R.; Srisomsap, C.; Dannhardt, G.; Svasti, J. Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. J. Agric. Food Chem. 2009, 57, 3055–3063. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Makni, M.; Chtourou, Y.; Fetoui, H.; Garoui el, M.; Boudawara, T.; Zeghal, N. Evaluation of the antioxidant, anti-inflammatory and hepatoprotective properties of vanillin in carbon tetrachloride-treated rats. Eur. J. Pharmacol. 2011, 668, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Belagali, Y.; Ullal, S.; Shoeb, A.; Bhagwath, V.; Ramya, K.; Maskeri, R. Effect of vanillin on lipid profile in a model of hyperlipidemia, a preliminary study. Indian J. Exp. Biol. 2013, 51, 288–291. Available online: http://nopr.niscair.res.in/handle/123456789/16551 (accessed on 9 August 2020). [PubMed]
- Colombani, J.; Raisin, S.; Pantalacci, S.; Radimerski, T.; Montagne, J.; Leopold, P. A nutrient sensor mechanism controls Drosophila growth. Cell 2003, 114, 739–749. [Google Scholar] [CrossRef] [Green Version]
- Haselton, A.; Sharmin, E.; Schrader, J.; Sah, M.; Poon, P.; Fridell, Y.W. Partial ablation of adult Drosophila insulin-producing neurons modulates glucose homeostasis and extends life span without insulin resistance. Cell Cycle 2010, 9, 3063–3071. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Rulifson, E.J. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 2004, 431, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Park, J.H. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 2004, 167, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Kang, P.; Tatar, M. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell 2012, 11, 978–985. [Google Scholar] [CrossRef]
- Chatterjee, D.; Katewa, S.D.; Qi, Y.; Jackson, S.A.; Kapahi, P.; Jasper, H. Control of metabolic adaptation to fasting by dILP6-induced insulin signaling in Drosophila oenocytes. PNAS 2014, 111, 17959–17964. [Google Scholar] [CrossRef] [Green Version]
- Doupé, D.P.; Marshall, O.J.; Dayton, H.; Brand, A.H.; Perrimon, N. Drosophila intestinal stem and progenitor cells are major sources and regulators of homeostatic niche signals. PNAS 2018, 115, 12218–12223. [Google Scholar] [CrossRef] [Green Version]
- Padmanabha, D.; Baker, K.D. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol. Metab. 2014, 25, 518–527. [Google Scholar] [CrossRef]
- Alfa, R.W.; Kim, S.K. Using Drosophila to discover mechanisms underlying type 2 diabetes. Dis. Model. Mech. 2016, 9, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Seol-min, K.; Kwon, J.Y. A Systematic Analysis of Drosophila Regulatory Peptide Expression in Enteroendocrine Cells. Mol. Cell. 2016, 39, 358–366. [Google Scholar] [CrossRef]
- Lahr, E.C.; Dean, D.; Ewer, J. Genetic analysis of ecdysis behavior in Drosophila reveals partially overlapping functions of two unrelated neuropeptides. J. Neurosci. 2012, 32, 6819–6829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.C.; del Campo, M.L.; Ewer, J. Neuroendocrine control of larval ecdysis behavior in Drosophila: Complex regulation by partially redundant neuropeptides. J. Neurosci. 2004, 24, 4283–4292. [Google Scholar] [CrossRef]
- Williams, M.J.; Akram, M.; Barkauskaite, D.; Patil, S.; Kotsidou, E.; Kheder, S.; Vitale, G.; Filaferro, M.; Blemings, S.W.; Maestri, G.; et al. CCAP regulates feeding behavior via the NPF pathway in Drosophila adults. PNAS 2020, 117, 7401–7408. [Google Scholar] [CrossRef]
- Sieber, M.H.; Thummel, C.S. The DHR96 Nuclear Receptor Controls Triacylglycerol Homeostasis in Drosophila. Cell Metab. 2009, 10, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Horner, M.A.; Pardee, K.; Liu, S.; King-Jones, K.; Lajoie, G.; Edwards, A.; Krause, H.M.; Thummel, C.S. The Drosophila DHR96 nuclear receptor binds cholesterol and regulates cholesterol homeostasis. Genes Dev. 2009, 23, 2711–2716. [Google Scholar] [CrossRef] [Green Version]
- Martelli, C.; Pech, U.; Kobbenbring, S.; Pauls, D.; Bahl, B.; Sommer, M.V.; Pooryasin, A.; Barth, J.; Arias, C.W.P.; Vassiliou, C.; et al. SIFamide Translates Hunger Signals into Appetitive and Feeding Behavior in Drosophila. Cell Rep. 2017, 20, 464–478. [Google Scholar] [CrossRef] [Green Version]
- Hermann-Luibl, C.; Helfrich-Förster, C. Clock network in Drosophila. Curr. Opin. Insect Sci. 2015, 7, 65–70. [Google Scholar] [CrossRef]
- Xu, K.; Zheng, X.; Sehgal, A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 2008, 8, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Parisky, K.M.; Agosto, J.; Pulver, S.R.; Shang, Y.; Kuklin, E.; Hodge, J.J.; Kang, K.; Liu, X.; Garrity, P.A.; Rosbash, M.; et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 2008, 60, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Reiher, W.; Hermann-Luibl, C.; Sellami, A.; Cognigni, P.; Kondo, S.; Helfrich-Förster, C.; Veenstra, J.A.; Wegener, C. Allatostatin A signalling in Drosophila regulates feeding and sleep and is modulated by PDF. PLoS Genet. 2016, 12, e1006346. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.-H.; Lee, J.-H.; Chae, H.-S.; Seong, J.Y.; Park, Y.; Park, Z.-Y.; Kim, Y.-J. Identification of a novel insect neuropeptide, CNMa and its receptor. FEBS Lett. 2014, 588, 2037–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gáliková, M.; Klepsatel, P.; Xu, Y.; Kühnlein, R.P. The obesity-related Adipokinetic hormone controls feeding and expression of neuropeptide regulators of Drosophila metabolism. Eur. J. Lipid Sci Technol. 2016, 119, 1600138. [Google Scholar] [CrossRef]
- Puig, O.; Marr, M.T.; Ruhf, M.L.; Tjian, R. Control of cell number by Drosophila FOXO: Downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003, 17, 2006–2020. Available online: http://www.genesdev.org/cgi/doi/10.1101/gad.1098703 (accessed on 9 August 2020). [CrossRef] [PubMed] [Green Version]
- Jünger, M.A.; Rintelen, F.; Stocker, H.; Wasserman, J.D.; Végh, M.; Radimerski, T.; Greenberg, M.E.; Hafen, E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2003, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Baum, M.; Huang, C.-L.; Rodan, A.R. Two inwardly rectifying potassium channels, Irk1 and Irk2, play redundant roles in Drosophila renal tubule function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R747–R756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duckworth, W.C.; Bennett, R.G.; Hamel, F.G. Insulin degradation: Progress and potential. Endocr. Rev. 1998, 19, 608–624. [Google Scholar] [CrossRef] [Green Version]
- Valera Mora, M.E.; Scarfone, A.; Calvani, M.; Greco, A.V.; Mingrone, G. Insulin clearance in obesity. J. Am. Coll. Nutr. 2003, 22, 487–493. [Google Scholar] [CrossRef]
- Rudovich, N.; Pivovarova, O.; Fisher, E.; Fischer-Rosinsky, A.; Spranger, J.; Möhlig, M.; Schulze, M.B.; Boeing, H.; Pfeiffer, A.F. Polymorphisms within insulin-degrading enzyme (IDE) gene determine insulin metabolism and risk of type 2 diabetes. J. Mol. Med. 2009, 87, 1145. [Google Scholar] [CrossRef]
- Maianti, J.P.; McFedries, A.; Foda, Z.H.; Kleiner, R.E.; Du, X.Q.; Leissring, M.A.; Tang, W.J.; Charron, M.J.; Seeliger, M.A.; Saghatelian, A.; et al. Anti-diabetic activity of insulin-degrading enzyme inhibitors mediated by multiple hormones. Nature 2014, 511, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Tundo, G.R.; Sbardella, D.; Ciaccio, C.; Grasso, G.; Gioia, M.; Coletta, A.; Polticelli, F.; Di Pierro, D.; Milardi, D.; Van Endert, P.; et al. Multiple functions of insulin-degrading enzyme: A metabolic crosslight? Crit. Rev. Biochem. Mol. Biol. 2017, 52, 554–582. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chen, D.; Jang, C.; Nall, A.; Zheng, X.; Sehgal, A. An ecdysone-responsive nuclear receptor regulates circadian rhythms in Drosophila. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Moraru, A.; Cakan-Akdogan, G.; Strassburger, K.; Males, M.; Mueller, S.; Jabs, M.; Muelleder, M.; Frejno, M.; Braeckman, B.P.; Ralser, M.; et al. THADA regulates the organismal balance between energy storage and heat production. Dev. Cell 2017, 41, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Zeggini, E.; Scott, L.J.; Saxena, R.; Voight, B.F.; Marchini, J.L.; Hu, T.; de Bakker, P.I.; Abecasis, G.R.; Almgren, P.; Andersen, G.; et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 2008, 40, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Sasorith, S.; Billas, I.M.; Iwema, T.; Moras, D.; Wurtz, J.M. Structure-based analysis of the ultraspiracle protein and docking studies of putative ligands. J. Insect Sci. 2002, 2. [Google Scholar] [CrossRef]
- Oro, A.E.; McKeown, M.; Evans, R.M. Relationship between the product of the Drosophila ultraspiracle locus and the vertebrate retinoid X receptor. Nature 1990, 347, 298–301. [Google Scholar] [CrossRef]
- Riddiford, L.M.; Ajami, A.M. Juvenile hormone: Its assay and effects on pupae of Manduca sexta. J. Insect Physiol. 1973, 19, 749–762. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer—World Health Organization. Colorectal Cancer—Source: Globocan 2018; The Global Cancer Observatory: Lyon, France, 2019. [Google Scholar]
- Chu, F.; Cui, Y.; Li, K.; Xiao, X.; Zhang, L.; Zhang, L.; Wang, L.; Gao, L.; Yin, N.; Wu, H. Long noncoding RNA THOR is highly expressed in colorectal cancer and predicts a poor prognosis. Future Oncol. 2020, 16, 1911–1920. [Google Scholar] [CrossRef]






| Chemical Classification | Putative Identification | E1 | E2 | E3 |
|---|---|---|---|---|
| Flavonoids | Ampelopsin (Ampeloptin, Dihydromyricetin) | ✗ | ✗ | ✓ |
| Avicularin (Quercetin-3-O-arabinofuranoside, Fenicularin) | ✓ | ✓ | ✓ | |
| Catechin or Epicatechin-O-hexoside | ✓ | ✓ | ✓ | |
| Cinnamtannin B1 | ✓ | ✓ | ✓ | |
| Cinnamtannin D1 | ✓ | ✓ | ✓ | |
| Cyanidin-3-O-arabinoside | ✓ | ✓ | ✓ | |
| Cyanidin-3-O-glucoside (Kuromanin, Asterin, Chrysanthemin) | ✓ | ✓ | ✓ | |
| Cyanidin-3-O-sambubioside (Sambicyanin, Gossypicyanin) | ✓ | ✓ | ✓ | |
| Cyanidin-O-(coumaroyl)hexoside | ✗ | ✓ | ✓ | |
| Delphinidin-3-O-arabinoside | ✓ | ✓ | ✓ | |
| Delphinidin-3-O-galactoside (Empetrin) | ✓ | ✓ | ✓ | |
| Delphinidin-O-(pentosyl)hexoside | ✗ | ✓ | ✓ | |
| Epicatechin | ✓ | ✓ | ✓ | |
| Epigallocatechin | ✓ | ✓ | ✓ | |
| Gallocatechin | ✓ | ✓ | ✓ | |
| Hyperoside (Quercetin-3-O-galactoside, Hyperin) | ✓ | ✓ | ✓ | |
| Idaein (Idein, Cyanidin-3-O-galactoside) | ✓ | ✓ | ✓ | |
| Isoquercitrin (Hirsutrin, Quercetin-3-O-glucoside) | ✓ | ✓ | ✓ | |
| Isorhamnetin-O-glucuronide | ✓ | ✓ | ✓ | |
| Kaempferol-3-O-glucuronide | ✓ | ✓ | ✓ | |
| Laricitrin (Myricetin-3′-O-methyl ether) | ✓ | ✓ | ✓ | |
| Laricitrin-O-hexoside | ✓ | ✓ | ✓ | |
| Malvidin-3-O-arabinoside | ✓ | ✓ | ✓ | |
| Malvidin-O-(coumaroyl)hexoside | ✗ | ✓ | ✓ | |
| Malvidin-O-hexoside | ✓ | ✓ | ✓ | |
| Myricetin | ✗ | ✓ | ✓ | |
| Myricetin-3-O-arabinoside | ✓ | ✓ | ✓ | |
| Myricetin-O-hexoside | ✓ | ✓ | ✓ | |
| Myricetin-O-pentoside isomer | ✓ | ✓ | ✓ | |
| Naringenin | ✓ | ✓ | ✓ | |
| Naringenin chalcone | ✗ | ✓ | ✓ | |
| Pentahydroxyflavone (Hypolaetin, Quercetin, Tricetin) | ✓ | ✓ | ✓ | |
| Peonidin-3-O-arabinoside | ✓ | ✓ | ✓ | |
| Peonidin-O-(coumaroyl)hexoside | ✗ | ✓ | ✓ | |
| Peonidin-O-(pentosyl)hexoside | ✗ | ✓ | ✗ | |
| Peonidin-O-hexoside | ✓ | ✓ | ✓ | |
| Peonidin-O-pentoside isomer | ✗ | ✓ | ✗ | |
| Petunidin-3-O-arabinoside | ✓ | ✓ | ✓ | |
| Petunidin-3-O-galactoside | ✓ | ✓ | ✓ | |
| Petunidin-O-(pentosyl)hexoside | ✗ | ✓ | ✗ | |
| Procyanidin B1 or B3 | ✓ | ✓ | ✓ | |
| Prunin (Naringenin 7-O-glucoside) | ✓ | ✓ | ✓ | |
| Quercetin-3-O-[3-Hydroxy-3-methylglutaroyl-(→4)-rhamnoside] | ✓ | ✓ | ✓ | |
| Quercetin-3-O-glucuronide | ✓ | ✓ | ✓ | |
| Quercetin-O-(coumaroyl)hexoside | ✗ | ✓ | ✓ | |
| Quercetin-O-rhamnoside-O-pentoside | ✓ | ✓ | ✓ | |
| Quercitrin (Quercetin-3-O-rhamnoside) | ✓ | ✓ | ✓ | |
| Syringetin-O-hexoside | ✓ | ✓ | ✓ | |
| Trihydroxyflavanone | ✗ | ✓ | ✓ | |
| Amino acids | 2-Aminoadipic acid | ✗ | ✓ | ✓ |
| Arginine | ✓ | ✓ | ✓ | |
| Asparagine | ✗ | ✓ | ✓ | |
| Glutamic acid | ✓ | ✗ | ✓ | |
| Histidine | ✓ | ✗ | ✓ | |
| Isoleucine or Leucine | ✗ | ✓ | ✓ | |
| Lysine | ✓ | ✗ | ✓ | |
| Phenylalanine | ✓ | ✓ | ✓ | |
| Threonine | ✓ | ✓ | ✓ | |
| Tryptophan | ✓ | ✓ | ✓ | |
| Tyrosine | ✓ | ✓ | ✓ | |
| γ-Aminobutyric acid (GABA) | ✓ | ✗ | ✗ | |
| Polyphenols | Caffeoylshikimic acid | ✓ | ✓ | ✓ |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | ✓ | ✓ | ✓ | |
| Coumaroylquinic acid | ✓ | ✓ | ✓ | |
| Coumaroyl-shikimate | ✓ | ✓ | ✓ | |
| Feruloylquinic acid | ✓ | ✓ | ✓ | |
| Gallic acid (3,4,5-Trihydroxybenzoic acid) | ✓ | ✓ | ✓ | |
| Carboxylic acids | 4-Coumaric acid | ✓ | ✓ | ✓ |
| Caffeic acid | ✓ | ✓ | ✓ | |
| Dihydroxy-methoxybenzoic acid | ✗ | ✓ | ✓ | |
| Dimethoxy-hidroxycinnamic acid (Sinapic acid) | ✓ | ✓ | ✓ | |
| Ferulic acid | ✓ | ✓ | ✓ | |
| Vitamins | Adenine | ✗ | ✓ | ✓ |
| Nicotinamide | ✓ | ✓ | ✓ | |
| Nicotinic acid (B3) | ✗ | ✓ | ✓ | |
| Pantothenic acid (B5) | ✗ | ✗ | ✓ | |
| Terpenoids | Abscisic acid (ABA) | ✓ | ✓ | ✓ |
| Sugars | Saccharic acid | ✓ | ✓ | ✓ |
| Lactones | Gulonic acid γ-lactone or δ-Gluconic acid δ-lactone | ✓ | ✓ | ✓ |
| Esters | Methyl gallate | ✓ | ✓ | ✓ |
| Alkaloids | Choline | ✓ | ✗ | ✗ |
| Iridoids | 7-Deoxyloganic acid | ✗ | ✓ | ✓ |
| Miscellaneous | 4-Methoxycinnamaldehyde | ✗ | ✗ | ✓ |
| 5-Hydroxymethyl-2-furaldehyde | ✓ | ✓ | ✓ | |
| 5-Methyl-2-furaldehyde | ✓ | ✓ | ✓ | |
| N-(2-Phenylethyl)acetamide | ✓ | ✗ | ✗ | |
| Phytosphingosine | ✗ | ✓ | ✗ | |
| Vanillin | ✗ | ✓ | ✓ |
| Extract Formulation | Total Polyphenols [mg GAE/g dw] | Total Flavonoids [mg QE/g dw] | Antioxidant Activity [%] |
|---|---|---|---|
| Aqueous extract (E1) | 12.48 ± 0.13 | 3.88 ± 0.11 | 50.87 ± 0.12 |
| Methanolic extract (E2) | 20.42 ± 0.17 | 7.27 ± 0.12 | 77.32 ± 0.09 |
| Hydro-methanolic extract (E3) | 21.68 ± 0.19 | 8.41 ± 0.11 | 78.03 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neamtu, A.-A.; Szoke-Kovacs, R.; Mihok, E.; Georgescu, C.; Turcus, V.; Olah, N.K.; Frum, A.; Tita, O.; Neamtu, C.; Szoke-Kovacs, Z.; et al. Bilberry (Vaccinium myrtillus L.) Extracts Comparative Analysis Regarding Their Phytonutrient Profiles, Antioxidant Capacity along with the In Vivo Rescue Effects Tested on a Drosophila melanogaster High-Sugar Diet Model. Antioxidants 2020, 9, 1067. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111067
Neamtu A-A, Szoke-Kovacs R, Mihok E, Georgescu C, Turcus V, Olah NK, Frum A, Tita O, Neamtu C, Szoke-Kovacs Z, et al. Bilberry (Vaccinium myrtillus L.) Extracts Comparative Analysis Regarding Their Phytonutrient Profiles, Antioxidant Capacity along with the In Vivo Rescue Effects Tested on a Drosophila melanogaster High-Sugar Diet Model. Antioxidants. 2020; 9(11):1067. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111067
Chicago/Turabian StyleNeamtu, Andreea-Adriana, Rita Szoke-Kovacs, Emoke Mihok, Cecilia Georgescu, Violeta Turcus, Neli Kinga Olah, Adina Frum, Ovidiu Tita, Carmen Neamtu, Zsombor Szoke-Kovacs, and et al. 2020. "Bilberry (Vaccinium myrtillus L.) Extracts Comparative Analysis Regarding Their Phytonutrient Profiles, Antioxidant Capacity along with the In Vivo Rescue Effects Tested on a Drosophila melanogaster High-Sugar Diet Model" Antioxidants 9, no. 11: 1067. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111067