Modeling of Vaccination and Contact Tracing as Tools to Control the COVID-19 Outbreak in Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. PDP COVID-19 Model
2.2. Statistical Study and Sensitivity Analysis
2.3. Ethical Statement
3. Results
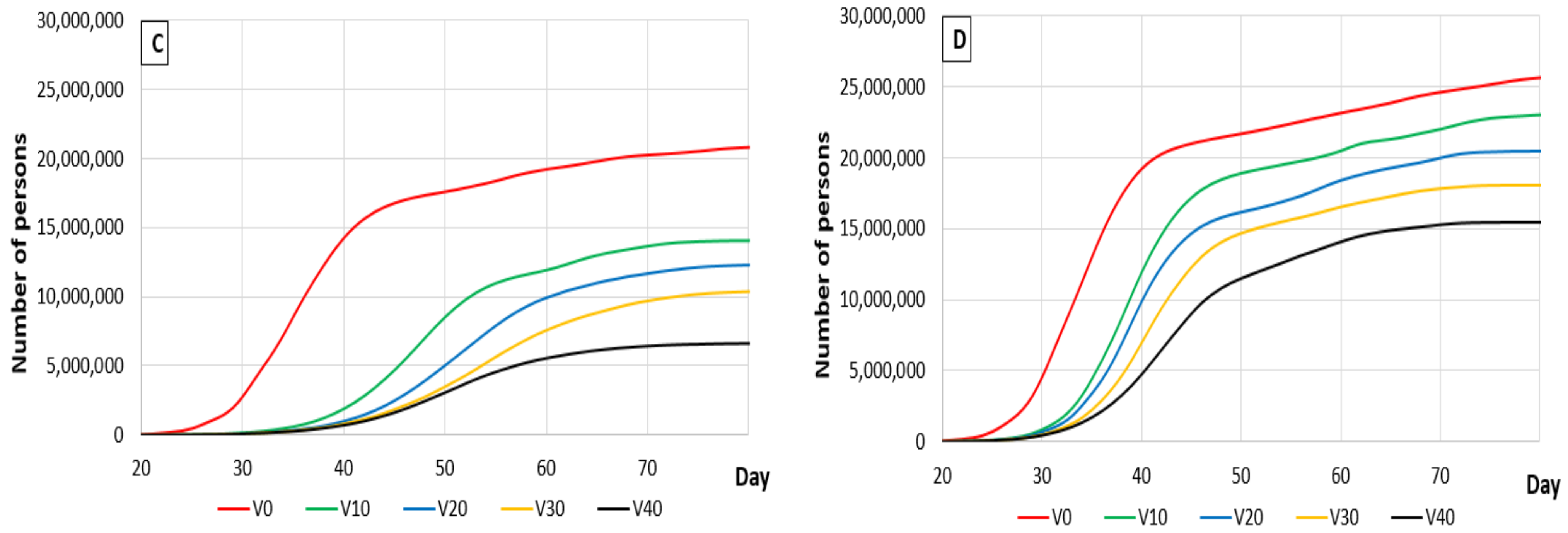
3.1. The Effects of Vaccination
3.1.1. Response Surface
3.1.2. Sensitivity Analysis
3.1.3. Vaccination Activity
3.2. Tracing Activity
3.2.1. Response Surface
3.2.2. Sensitivity Analysis
3.2.3. Effect of Tracing Measures
3.3. Analyzing Simultaneous Contact Tracing and Vaccine Protection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Chen, X.; Zhang, Z.; Roy, A.; Shen, Y. Strategies to trace back the origin of COVID-19. J. Infect. 2020, 80, e39–e40. [Google Scholar] [CrossRef]
- Mizumoto, K.; Kagaya, K.; Chowell, G. Effect of a wet market on coronavirus disease (COVID-19) transmission dynamics in China, 2019–2020. Int. J. Infect. Dis. 2020, 97, 96–101. [Google Scholar] [CrossRef]
- Kui, L.K.; Fang, Y.; Deng, Y.; Liu, W.; Wang, M.-F.; Jing-Ping, M.; Wei, X.; Ying-Nan, W.; Min-Hua, Z.; Cheng-Hong, L.; et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020, 133, 1025–1031. [Google Scholar]
- Casas-Rojo, J.M.; Antón-Santos, J.M.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.M.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.M.; Vargas-Núñez, J.A.; et al. Características clínicas de los pacientes hospitalizados con COVID-19 en España: Resultados del Registro SEMI-COVID-19. Revista Clínica Española 2020, 220, 480–494. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Real Time Learning Network. Available online: https://www.idsociety.org/covid-19-real-time-learning-network/disease-manifestations--complications/ (accessed on 31 December 2020).
- Mortality Risk of COVID-19. Available online: https://ourworldindata.org/mortality-risk-covid#the-current-case-fatality-rate-of-covid-19 (accessed on 9 April 2021).
- Coronavirus: The Economic impact—10 July 2020. Available online: https://www.unido.org/stories/coronavirus-economic-impact-10-july-2020#story-start (accessed on 9 April 2021).
- Sarkar, S. Pandemic and the state. J. Soc. Econ. Dev. 2020, 25, 1–7. [Google Scholar]
- Bonaccorsi, G.; Pierri, F.; Cinelli, M.; Flori, A.; Galezzi, A.; Porcelli, F.; Schmidt, A.L.; Valensise, C.M.; Scala, A.; Quattrociocchi, W.; et al. Economic and social consequences of human mobility restrictions under COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 15530–15535. [Google Scholar] [CrossRef]
- Laing, T. The economic impact of the Coronavirus 2019 (Covid-2019): Implications for the mining industry. Extrac. Indust. Soc. 2020, 7, 580–582. [Google Scholar] [CrossRef]
- Han, E.; Tan, M.M.; Turk, E.; Sridhar, D.; Leung, G.M.; Shibuya, K.; Asgari, N.; Oh, J.; García-Basteiro, A.L.; Hanefeld, J.; et al. Lessons learnt from easing COVID-19 restrictions: An analysis of countries and regions in Asia Pacific and Europe. Lancet 2020, 396, 1525–1534. [Google Scholar] [CrossRef]
- Rahman, M.; Thill, J.-C.; Paul, K.C. COVID-19 Pandemic severity, lockdown regimes, and people’s mobility: Early evidence from 88 countries. Sustainability 2020, 12, 9101. [Google Scholar] [CrossRef]
- Pillai, S.; Siddika, N.; Hoque Apu, E.; Kabir, R. COVID-19: Situation of European countries so far. Arch. Med. Res. 2020, 5, 723–725. [Google Scholar] [CrossRef]
- Lai, J.; Ma, S.; Wang, Y.; Cai, Z.; Hu, J.; Wei, N.; Wu, J.; Du, H.; Chen, T.; Li, R.; et al. Factors associated with mental health outcomes among health care workers exposed to Coronavirus disease 2019. JAMA 2020, 3, e203976. [Google Scholar]
- Pan, K.Y.; Kok, A.A.L.; Eikelnboom, M.; Horsfall, M.; Jörg, F.; Luteijn, R.A.; Rhebegen, D.; van Oppen, P.; Giltay, E.J.; Penninx, B.W.J.H. The mental health impact of the COVID-19 pandemic on people with and without depressive, anxiety, or obsessive-compulsive disorders: A longitudinal study of three Dutch case-control cohorts. Lancet Psych. 2020, 8, 121–129. [Google Scholar] [CrossRef]
- Brett, M.; Vera, V.; Reed, M. Mental health during the COVID-19 pandemic: Effects of stay-at-home policies, social distancing behavior, and social resources. Psych. Res. J. 2020, 293, 113419. [Google Scholar]
- World Health Organization. Mask Use in the Context of COVID-19: Interim Guidance, 1 December 2020. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/337199 (accessed on 30 September 2020).
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Phil. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [Green Version]
- Lahariya, C. “Health system approach” for improving immunization program performance. J. Fam. Medic. Primary Care 2015, 4, 487–494. [Google Scholar] [CrossRef]
- Aschwanden, C. The false promise of herd immunity for COVID-19. Nature 2020, 587, 26–28. [Google Scholar] [CrossRef]
- Böhm, R.; Meier, N.W.; Groß, M.; Korn, L.; Betsch, C. The willingness to vaccinate increases when vaccination protects others who have low responsibility for not being vaccinated. J. Behav. Med. 2019, 42, 381–391. [Google Scholar] [CrossRef]
- Bitsouni, V.; Lycett, S.; Opriessnig, T.; Doeschl-Wilson, A. Predicting vaccine effectiveness in livestock populations: A theoretical framework applied to PRRS virus infections in pigs. PLoS ONE 2019, 14, e0220738. [Google Scholar]
- Clinical Trials. Available online: https://www.clinicaltrials.gov/ (accessed on 9 April 2020).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez, M.G.; Moreira, E.D.; Zerbini, C.; et al. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2020, 397, 72–74. [Google Scholar] [CrossRef]
- Colomer, M.À.; Margalida, A.; Pèrez-Jimenez, M.J. Population Dynamics P system (PDP) models: A standardized protocol for describing and applying novel bio-inspired computing tools. PLoS ONE 2013, 8, e60698. [Google Scholar] [CrossRef] [Green Version]
- Colomer, M.À.; Margalida, A.; Fraile, L. Improving the management procedures in farms infected with the Porcine Reproductive and Respiratory Syndrome virus using PDP models. Sci. Rep. 2019, 9, 9959. [Google Scholar] [CrossRef]
- Colomer, M.À.; Margalida, A.; Fraile, L. Vaccination is a suitable tool in the control of Aujeszky's disease outbreaks in pigs using a Population Dynamics P Systems model. Animals 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Colomer, M.A.; Margalida, A.; Alòs, F.; Oliva-Vidal, P.; Vilella, A.; Fraile, L. Modelling the SARS-COV2 outbreak: Assessing the useful of protective measures to reduce the pandemic at population level. Under review.
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2013. Available online: http://www.R-project.org/ (accessed on 2 February 2021).
- Spain Hopes to Have Pfizer’s Coronavirus Vaccine for 10 Million People by Early 2021. Available online: https://english.elpais.com/society/2020-11-10/spain-hopes-to-have-pfizer-vaccine-for-10-million-people-by-early-2021.html (accessed on 9 April 2021).
- Dr. Fauci Said up to 90% of Population Needs to Get Vaccinated for Herd Immunity against Virus. Available online: https://www.businessinsider.com/fauci-up-to-90-population-needs-vaccine-for-herd-immunity-2020-12 (accessed on 9 April 2021).
- World Health Organization. Coronavirus Disease (COVID-19): Herd Immunity, Lockdowns and COVID-19. World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Thunström, L.; Newbold, S.C.; Finnoff, D.; Ashworth, M.; Shogren, J.F. The Benefits and Costs of Using Social Distancing to Flatten the Curve for COVID-19. J. Benefit-Cost Anal. 2020, 11, 179–195. [Google Scholar] [CrossRef]
- Koren, M.; Pető, R. Business disruptions from social distancing Business disruptions from social distancing. PLoS ONE 2020, 15, e0239113. [Google Scholar] [CrossRef]
- Hoertel, N.; Blachier, M.; Blanco, C.; Olfson, M.; Massetti, M.; Sánchez Rico, M.; Limosin, F.; Leleu, H. Lockdown exit strategies and risk of a second epidemic peak: A stochastic agent-based model of SARS-CoV-2 epidemic in France. medRxiv 2020. [Google Scholar] [CrossRef]
- Aleta, A.; Martín-Corral, D.; Pastore y Piontti, A.; Ajelli, M.; Litvinova, M.; Chinazzi, M.; Dean, N.E.; Halloran, M.E.; Longini, I.M., Jr.; Merler, S.; et al. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nature Hum. Behav. 2020, 4, 964–971. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Liu, X.; Liu, C.; Lian, X.; Huang, Z.; Chou, J.; Li, X.; Yang, K.; Wang, J.; et al. Global prediction system for COVID-19 pandemic. Sci. Bull. 2020, 65, 1884–1887. [Google Scholar] [CrossRef] [PubMed]
- Press, W.H.; Levin, R.C. Modeling, post COVID-19. Science 2020, 370, 1015. [Google Scholar] [CrossRef] [PubMed]
- Jewell, N.P.; Lewnard, J.A.; Jewell, B.L. Predictive Mathematical Models of the COVID-19 Pandemic: Underlying Principles and Value of Projections. JAMA 2020, 323, 1893–1894. [Google Scholar] [CrossRef] [PubMed]
- Poletto, C.; Scarpino, S.V.; Volz, E.M. Applications of predictive modelling early in the COVID-19 epidemic. Lancet Digit. Health 2020, 2, e498–e499. [Google Scholar] [CrossRef]
- COVID-19 Coronavirus: Top Ten Most-Affected Countries. Available online: https://www.pharmaceutical-technology.com/features/covid-19-coronavirus-top-ten-most-affected-countries/ (accessed on 9 April 2021).
- Casini, L.; Roccetti, M.A. Cross-regional analysis of the COVID-19 spread during the 2020 Italian vacation period: Results from three computational models are compared. Sensors 2020, 20, 319. [Google Scholar] [CrossRef] [PubMed]
- Prather, K.A.; Marr, L.C.; Schooley, R.T.; McDiarmid, M.A.; Wilson, M.E.; Milton, D.K. Airborne transmission of SARS-CoV-2. Science 2020, 370, 303–304. [Google Scholar]
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A review of viral, host, and environmental factors. Ann. Internal Med. 2020, 174, 69–79. [Google Scholar] [CrossRef]
- Muge, C.; Krutika, K.; Jason, K.; Malik, P. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 2020, 371, m3862. [Google Scholar]
- Madewe, Z.J.; Yang, Y.; Longini, I.M., Jr.; Halloran, M.E.; Household, N.E.D. Household Transmission of SARS-CoV-2A Systematic Review and Meta-analysis. JAMA 2020, 3, e2031756. [Google Scholar]
- Davies, N.G.; Kucharski, A.J.; Eggo, R.M.; Gimma, A.; Edmunds, W.J. Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: A modelling study. Lancet Public Health 2020, 5, e375–e385. [Google Scholar] [CrossRef]
- Sorci, G.; Faivre, B.; Morand, S. Explaining among-country variation in COVID-19 case fatality rate. Sci. Rep. 2020, 10, 18909. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ghosh, P. Factors affecting COVID-19 infected and death rates inform lockdown-related policymaking. PLoS ONE 2020, 15, e0241165. [Google Scholar] [CrossRef]
- Anderson, R.M.; Heesterbeek, H.; Klinkenberg, D.; Hollingsworth, T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 2020, 395, 931–934. [Google Scholar] [CrossRef]
- Martin, G.P.; Hanna, E.; Dingwall, R. Urgency and uncertainty: Covid-19, face masks, and evidence informed policy. BMJ 2020, 369, m2017. [Google Scholar] [CrossRef]
- Ollila, H.M.; Partinen, M.; Koskela, J.; Savolainen, R.; Rotkirch, A.; Laine, L.T. Face masks to prevent transmission of respiratory diseases: Systematic review and meta-analysis of randomized controlled trials. medRxiv 2020. [Google Scholar] [CrossRef]
- Li, J.; Giabbanelli, P.J. Identifying Synergistic Interventions to Address COVID-19 Using a Large Scale Agent-Based Model. medRxiv 2020. [Google Scholar] [CrossRef]
- Kretzschmar, M.E.; Rozhnova, G.; Bootsma, M.C.J.; van Boven, M.; van de Wijgert, J.H.; Bonten, M.J. Impact of delays on effectiveness of contact tracing strategies for COVID-19: A modelling study. Lancet Public Health 2020, 5, e452–e459. [Google Scholar] [CrossRef]
- Chang, S.; Pierson, E.; Koh, P.W.; Gerardin, J.; Redbird, B.; Grusky, D.; Leskovec, J. Mobility network models of COVID-19 explain inequities and inform reopening. Nature 2021, 589, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Baum, F.; Freeman, T.; Musolino, C.; Abramovitz, M.; De Ceukelaire, W.; Flavel, J.; Friel, S.; Giugliani, C.; Howden-Chapman, P.; Huong, N.T.; et al. Explaining covid-19 performance: What factors might predict national responses? Br. Med. J. 2021, 372, n91. [Google Scholar]




| Parameter | ||||
|---|---|---|---|---|
| Value (*) | p-Value | Value (*) | p-Value | |
| (Intercept) | 118,259.5 | <0.001 | 17,077,712 | <0.001 |
| Probability of disease transmission (Pd) | 27,333.1 | <0.001 | 4,390,755 | <0.001 |
| % of the population protected by vaccination (V) | −37,108.5 | <0.001 | −4,674,854 | <0.001 |
| Number of people infected at time 0 (F) | 5560.6 | 0.06991 | −19,057 | 0.9136 |
| Pd × V | −3146.7 | 0.41215 | −318,566 | 0.2296 |
| Pd × F | −1788 | 0.63444 | 64,945 | 0.7943 |
| V × F | 3927.8 | 0.31357 | −13,346 | 0.9571 |
| Pd2 | −6782.2 | 0.1063 | −915,654 | 0.0085 |
| V2 | 3031 | 0.42859 | −303,586 | 0.2497 |
| F2 | −3087.3 | 0.42054 | 331,471 | 0.2135 |
| Dead people | Probability of disease transmission from infected to non-infected people | 10,933 | People who die if the probability of transmission of the disease is increased by 1% |
| Percentage of people protected by vaccination | −1855 | Decrease in the number of people who died if the number of vaccinated people is increased by 1% | |
| Recovered people | Probability of disease transmission from infected to non-infected people | 1,756,302 | Number of people who recovered due to a 1% increase in the probability of disease transmission. |
| Percentage of people protected by vaccination | −233,743 | Number of people who recovered due to a 1% increase in the population protected by vaccination. |
| Social Measures | Population Protected by Vaccination (%) | |||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | ||
| With (p = 0.05) | Died | 0.33% | 0.22% (0.11%) | 0.17% (0.16%) | 0.17% (0.16%) | 0.10% (0.23%) |
| Recovered | 45.34% | 30.73% (14.61%) | 26.93% (18.41%) | 22.75% (22.59%) | 14.46% (30.88%) | |
| Without (p = 0.1) | Died | 0.41% | 0.32% (0.09%) | 0.29% (0.12%) | 0.22% (0.19%) | 0.16% (0.25%) |
| Recovered | 56.11% | 50.43% (5.68%) | 44.55% (11.56%) | 39.29% (16.82%) | 33.55% (22.56%) | |
| Parameter | Dead People | Recovered People | Number of Diagnostics Tests Required for Adequate Tracing | |||
|---|---|---|---|---|---|---|
| Value (*) | p-Value | Value (*) | p-Value | Value (*) | p-Value | |
| (Intercept) | 48,039 | <0.001 | 13,511,678 | <0.001 | 27,364,890 | <0.001 |
| Probability of disease transmission (Pd) | 17,049.88 | <0.001 | 3,660,762 | <0.001 | 5,478,335 | <0.001 |
| Percentage of people being traced (T) | −61,096 | <0.001 | −5,373,225 | <0.001 | 20,264,444 | <0.001 |
| Number of people infected at time 0 (F) | 433.12 | 0.8205 | −32,519 | 0.8367 | −92,972 | 0.8493 |
| Pd × T | −14,757 | 0.0012 | −1,025,858 | 0.0030 | 4,683,341 | <0.001 |
| Pd × F | 195.25 | 0.9422 | −365,194 | 0.1383 | −811,020 | 0.2671 |
| T × F | −78 | 0.9769 | −17,971 | 0.9357 | 24,372 | 0.9719 |
| Pd2 | −1053.62 | 0.6976 | −590,357 | 0.0327 | −954,013 | 0.2002 |
| T2 | 43,716.62 | <0.001 | 2,515,438 | <0.001 | −6,884,809 | <0.001 |
| F2 | 2329.38 | 0.402 | 88,975 | 0.692 | 522,739 | 0.460 |
| Dead people | Probability of disease transmission from infected to non-infected people | 6820 | Increase in the number of people who died by increasing the probability of disease transmission by 1% |
| Percentage of people being traced | −3055 | Decrease in the number of people who died by increasing tracing contacts by 1% | |
| Recovered people | Probability of disease transmission from infected to non-infected people | 1,464,305 | Increase in the number of people who recovered due to a 1% increase in the probability of disease transmission. |
| Percentage of people being traced | −268,661 | Decrease in the number of people who recovered due to a 1% increase in tracing contacts. | |
| Diagnostic tests | Probability of disease transmission from infected to non-infected people | 2,191,334 | Increase in the number of tests by increasing the probability of disease transmission by 1% |
| Percentage of people being traced | 1,013,222 | Increase in the number of tests by increasing the tracing contacts by 1% |
| Social Measures | People with Positive Contacts That Have Been Traced (%) | |||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | ||
| With (p = 0.05) | Died | 0.33 | 0.12 (0.21) | 0.08 (0.25) | 0.06 (0.27) | 0.06 (0.27) |
| Recovered | 45.34 | 25.88% (19.46) | 20.15 (25.19) | 18.26 (27.08) | 16.45 (28.89) | |
| Without (p = 0.1) | Died | 0.41 | 0.21 (0.20) | 0.14 (0.27) | 0.10 (0.31) | 0.07 (0.34) |
| Recovered | 56.11 | 44.15 (11.96) | 37.14 (18.97) | 32.24 (23.87) | 27.48 (28.63) | |
| Probability of Transmission of the Disease | Contacts Traced (%) | Number of People Traced | People Traced against the Total Population (%) |
|---|---|---|---|
| With social measures p = 0.05 | 0 | 0 | 0.00 |
| 10 | 13,106,761 | 28.48 | |
| 20 | 19,831,667 | 43.10 | |
| 30 | 26,495,337 | 57.58 | |
| 40 | 31,767,937 | 69.04 | |
| Without social measures p = 0.1 | 0 | 0 | 0.00 |
| 10 | 21,943,371 | 47.69 | |
| 20 | 34,913,187 | 75.87 | |
| 30 | 43,462,608 | 94.45 | |
| 40 | 47,673,047 | 103.60 |
| Percentage of the Population | No Vaccine | 19.56% of the Population Protected by Vaccination | |||||
|---|---|---|---|---|---|---|---|
| Positive Contact Tracing (%) | |||||||
| 0 | 10 | 20 | 30 | 40 | |||
| With social measures (p = 0.05) | Died | 0.17 | 0.09 | 0.06 | 0.06 | 0.05 | |
| Recovered | 26.96 | 19.60 | 14.46 | 13.26 | 10.62 | ||
| Without social measures (p = 0.1) | Died | 0.4 | 0.31 | 0.16 | 0.11 | 0.08 | 0.08 |
| Recovered | 56.1 | 45.06 | 36.06 | 30.37 | 26.83 | 28.68 | |
| Probability of Disease Transmission | Number of Diagnostic Tests Performed with Respect to Population Size (%) | |||
|---|---|---|---|---|
| 10% Positive Contact Tracing (%) | 20% Positive Contact Tracing (%) | 30% Positive Contact Tracing (%) | 40% Positive Contact Tracing (%) | |
| With social measures (p = 0.05) | 19.3 (8,876,461) | 27.7 (12,750,293) | 36.8 (16,927,530) | 39.0 (17,930,132) |
| Without social measures p = 0.10 | 33.4 (15,346,800) | 52.5 (24,166,442) | 68.0 (31,294,462) | 78.8 (36,247,483) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomer, M.À.; Margalida, A.; Alòs, F.; Oliva-Vidal, P.; Vilella, A.; Fraile, L. Modeling of Vaccination and Contact Tracing as Tools to Control the COVID-19 Outbreak in Spain. Vaccines 2021, 9, 386. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9040386
Colomer MÀ, Margalida A, Alòs F, Oliva-Vidal P, Vilella A, Fraile L. Modeling of Vaccination and Contact Tracing as Tools to Control the COVID-19 Outbreak in Spain. Vaccines. 2021; 9(4):386. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9040386
Chicago/Turabian StyleColomer, Mª Àngels, Antoni Margalida, Francesc Alòs, Pilar Oliva-Vidal, Anna Vilella, and Lorenzo Fraile. 2021. "Modeling of Vaccination and Contact Tracing as Tools to Control the COVID-19 Outbreak in Spain" Vaccines 9, no. 4: 386. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9040386






